Abstract
Background
Olea africana leaves are used by Bapedi people to treat different ailments. The use of these leaves is not validated, therefore the aim of this study is to validate antimicrobial properties of this plant.
Methods
The ground leaves were extracted using solvents of varying polarity (hexane, chloroform, dichloromethane (DCM), ethyl acetate, acetone, ethanol, methanol, butanol and water). Thin layer chromatography (TLC) was used to analyse the chemical constituents of the extracts. The TLC plates were developed in three different solvent systems, namely, benzene/ethanol/ammonium solution (BEA), chloroform/ethyl acetate/formic acid (CEF) and ethyl acetate/methanol/water (EMW). The micro-dilution assay and bioautography method were used to evaluate the antibacterial activity of the extracts against Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis and Staphylococcus aureus and the antifungal activity against Candida albicans and Cryptococcus neoformans.
Results
Methanol was the best extractant, yielding a larger amount of plant material whereas hexane yielded the least amount. In phytochemical analyses, more compounds were observed in BEA, followed by EMW and CEF. Qualitative 2, 2- diphenylpacryl-1-hydrazyl (DPPH) assay displayed that all the extracts had antioxidant activity. Antioxidant compounds could not be separated using BEA solvent system while with CEF and EMW enabled antioxidant compounds separation. The minimum inhibitory concentrations (MIC) values against test bacteria ranged between 0.16 and 2.50 mg/mL whereas against fungi, MIC ranged from 0.16 to 0.63 mg/mL. Bioautography results demonstrated that more than one compound was responsible for antimicrobial activity in the microdilution assay as the compounds were located at different Rf values.
Conclusions
The results indicate that leaf extracts of Olea africana contain compounds with antioxidant, antibacterial and antifungal activities. Therefore, further studies are required to isolate the active compounds and perform other tests such as cytotoxicity. Olea africana may be a potential source of antimicrobial compounds.
Similar content being viewed by others
Background
The treatment of diseases began long time ago with the use of herbs. They were the only medicinal system before modern or orthodox medicine could be invented [1]. According to the World Health Organisation [2], up to 80 % of the population in Africa use traditional medicine to serve their health needs. Traditional remedies can be derived from any part of the plant, such as barks, leaves, flowers and seeds. These remedies can be prepared from a single plant or a combination of numerous plant species [3]. The mixture could be simple, for example, common known remedies used to treat minor illnesses such as colds, headaches, stomach and menstrual pains. Complex preparations are often used for life threatening diseases [4].
Medicinal plants contain bioactive compounds with the ability to heal. These include saponins, tannins, essential oils, flavonoids, alkaloids and other chemical compounds found as secondary metabolites in plants [5]. Most commercial drugs currently used are of plant origin. The pharmacological history has an endless list of examples such as picrotoxin, aspirin, etc. Potential drugs may be discovered from plants which are recommended regularly and are observed to be effective [6].
Plant secondary metabolites are largely viewed as potential source of novel antibiotics, insecticides and herbicides. This is because of their biological significance and potential health benefits such as antioxidant, anti-aging, anti-atherosclerotic, antimicrobial and anti-inflammatory activities [7]. Regular intake of plant products rich in phenolics have been reported to reduce risks of developing chronic diseases such as cancer, heart diseases and diabetes [8].
When people develop novel drugs to combat diseases, microorganisms also develop new ways to strengthen themselves in order to survive. Nevertheless, plants are capable of developing new natural antimicrobials than mankind remedies [9]. Therefore, plants remain promising for discovery of new bioactive compounds. Although a lot of species have been examined for antimicrobial properties, the vast majority of them have not yet been analysed [10]. Information gathered from ethnic groups or indigenous traditional medicine has played a significant part in the discovery of novel chemotherapeutic agents from plants [11].
The African wild olive, previously known as Olea africana subspecies Cuspidata is now regarded as O. europaea subspecies africana. The names depend on which taxonomy and nomenclature are followed. Its common name is African wild olive and vernacular names are umquma (Zulu, Xhosa and Ndebele) and motlhware (Tswana and Sotho). The plant belongs to the family Oleaceae [12]. It is geographically distributed throughout the Southern Africa and Northwards through east Tropical African into Eritrea. It has a variety of habitats, from forest, riverside, open grassveld, bush, mountain kloops and rocky ledges [13].
The O. europaea subspecies africana plant leaves are used in folk medicine as a remedy for eye infections, sore throat, urinary tract infections, kidney problems and backaches or headaches. It is also used as a hypotensive, emollient, febrifuge and styptic [14]. The leaves of the tree were reported to be potent for the treatment of malaria in 1854 [15].
In this study we are reporting the antibacterial, antifungal and antioxidant activities of O. europaea subspecies Africana. We have also reported that this species have potential to be used as traditional medicine and furthermore, it can be developed into antimicrobial drugs.
Methods
Plant collection
The leaves of Olea africana were collected from the University of Limpopo (Turfloop campus), South Africa in Summer, 2013. Plant identity was confirmed by Dr Brownyn Egan (University of Limpopo Herbarium) and the herbarium voucher number was UNIN 11938. The leaves were dried at room temperature. The dried leave material were ground to fine powder using an electric grinder and stored in an air-tight container in a dark place until extraction procedure to prevent oxidation.
Extraction procedure
The leaves of O. africana were dried at room temperature (25 ± 2 °C) for two weeks and grounded to fine powder. The ground leaves (1 g) were extracted with 10 mL of different solvents of varying polarity (hexane, chloroform, dichloromethane, ethyl acetate, acetone, ethanol, methanol, butanol and water) using 50 mL centrifuge tubes. Extraction was performed three times with the same volume of solvent added repeatedly. The extracts were filtered into pre-weighed universal bottles. The filtrates were placed under a fan to evaporate the solvents and the quantity of plant material extracted was determined. The dried crude extract was stored at 4 °C.
Microorganisms used in the study
Bacterial species
The test organisms were supplied by the Department of Biochemistry, Microbiology and Biotechnology section at the University of Limpopo (Turfloop campus). Two Gram positive bacteria (Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212) and two Gram negative bacteria (Escherichia coli ATCC 28922 and Pseudomonas aeruginosa ATCC 27853) were used as test microorganisms. These are the major cause of nosocomial infections in hospitals [16] and are primarily the strains recommended for use by the National Committee for Clinical Laboratory Standards [17]. The bacterial species were maintained on nutrient agar at 4 °C. The cells were inoculated and incubated at 37 °C in nutrient broth for 12 h prior to screening tests.
Fungal species
The isolates of Candida albicans and Cryptococcus neoformans were used in this study. The isolates were obtained as a donation from University of Pretoria, Faculty of Health Sciences. The fungal species were maintained on sabouraud dextrose agar at 4 °C. The fungal species were inoculated in sabouraud dextrose broth and incubated at 35 °C prior to screening tests.
Thin layer chromatography (TLC) for Phytochemical analysis
The chemical constituents of the plant extracts were analysed by thin layer chromatography (TLC) using aluminium-backed TLC plates (Merck, silica gel 60 F254). The extracted plant material was re-dissolved in acetone to a final concentration of 10 mg/mL and 10 μl of the extract was spotted onto a TLC plate. The TLC plates were developed in solvent systems of varying polarity, i.e. ethyl acetate: methanol: water (10:5.4:4), [EMW] (polar/neutral); chloroform: ethyl acetate: formic acid (10:8:2), [CEF] (intermediate polarity/acidic) and benzene: ethanol: ammonium hydroxide (18:2:0.2) [BEA] (non-polar/basic) [18]. The plates were removed from the tanks, air dried under a fumehood cabinet and observed under ultra-violet (UV) light (254 and 365 nm). To detect compounds which were not visible or fluorescing under UV light, vanillin sulphuric acid reagent (0.1 g vanillin (Sigma ®): 28 methanol: 1 mL sulphuric acid) was used and chromatograms were heated at 110 °C for optimal colour development.
Phytochemical analysis test of extracts
The following photochemical analysis were performed; reducing sugars [19], anthraquinones [19], terpenoids (Salkowski test) [6], flavonoids [6], saponins [20], tannins [21], alkaloids [22], cardiac glycosides (Keller- Killiani test) [6]. Steroids [6] and phlobatannin [6].
Qualitative 2, 2-diphenyl-1-pacrylhydrazyl (DPPH) assay on TLC
The plant extracts were separated using TLC as described in Thin layer chromatography (TLC) for Phytochemical analysis. The chromatograms were air dried and sprayed with 0.2 % 2, 2-diphenyl-2-picryl-hyrazyl (DPPH) (Sigma®) to detect any antioxidant compounds present in the separated plant extracts. The presence of antioxidant activity was detected by yellow spots against a purple background on TLC plates sprayed with 0.2 % DPPH in methanol [23].
Antimicrobial activity
Quantitative antibacterial and antifungal activity assay by Minimum Inhibitory Concentration (MIC)
The MIC values for bacterial strain were determined using the serial microplate dilution method developed by Eloff [24] while the MIC for fungi was determined using the microplate serial dilution modified by Masoko et al. [25]. Ampicillin (Sigma®) and Amphotericin B (Sigma®) were used as positive control for bacterial strains, and fungal strains respectively while acetone was used as the negative control for both. Total activity of the extracts was calculated by dividing the MIC values with the mass extracted from 1 g of the plant material. The resultant value indicate the volume to which the extract can be diluted and still inhibit the growth of the test organism [26].
Qualitative antibacterial and antifungal activity assay by Bioautography
The bioautography procedure for bacterial strain was done according to Begue and Kline [27], modified by Masoko et al. [28] to screen for compounds with antifungal activity. Clear zones indicated growth inhibition by compounds with antibacterial or antifungal activity.
Results and discussion
The plant material was extracted using different solvents of varying polarity (hexane, chloroform, dichloromethane (DCM), ethyl acetate, acetone, ethanol, methanol, butanol and water). Majority of traditional healers use water to prepare their decoctions because water is not harmful and is the only solvent available. A major challenge of using water for extraction is that non-polar bioactive compounds cannot be extracted. The type of solvent used in the extraction procedure determines the success of isolating compounds from plant material [29]. Therefore, to extract all compounds it is important to extract using different solvents of varying polarity to cover the polarity range. After evaporating the solvents used for extraction, the extracts were re-dissolved in acetone because acetone has been reported to be harmless towards fungi [30] and bacteria [24].
Of the nine solvents used, methanol was the best extractant, extracting greater quantity of plant material than the other solvents used (Fig. 1). The chemical constituents of the plant extracts were analysed using Thin Layer Chromatography (TLC). More bands were observed on chromatograms developed in BEA, followed by EMW and CEF (Fig. 2). This shows that the leaves of O. africana contain mostly non-polar compounds.
This study demonstrated that the leaves of O. africana contain compounds with antioxidant activity. This was demonstrated by numerous yellow bands which appeared on chromatograms against a purple DPPH background. Antioxidant compounds were not separated with the BEA solvent system as the yellow bands were observed at the spotted area (Fig. 3). This was probably because the compounds were highly polar to be separated with the non-polar BEA solvent system. The antioxidant compounds were well separated with CEF and EMW solvent systems (Fig. 3). O. africana extracts of ethyl acetate, acetone, ethanol, methanol and butanol had compounds with prominent antioxidant activity at retention factor (Rf) values of 0.13 (CEF) and 0.53 (EMW) respectively. Furthermore, researchers have reported that olive leaf extracts had radical scavenging activity more than twice that of Camelia sinensis (green tea) [31].
Table 1 shows the presence of flavonoids, reducing sugar, steroids, tannins and terpenoids and absence of alkaloids, anthraquinones, cardiac glycosides, phlobatanin and saponins.
The MIC values of O. africana extracts against E. coli, P. aeruginosa, E. faecalis and S. aureus are presented in Table 2. DCM and ethyl acetate extracts were more active compared to the other extracts with MIC values of 0.16 mg/mL against E. faecalis. Ethyl acetate and acetone extracts showed prominent activity against all test bacteria with overall average MIC values of 0.30 and 0.32 mg/mL respectively. The water extract had the highest MIC overall average value of 1.74 mg/mL and this shows that the water extract was less active against the four test bacteria. Of all the bacterial pathogens used in this study E. faecalis (Gram positive) was the most sensitive test organism with an overall average MIC value of 0.31 mg/mL, followed by E. coli (Gram negative) with an overall average MIC value of 0.35 mg/mL. The least sensitive organisms were P. aeruginosa (Gram negative) and S. aureus (Gram positive) with overall average MIC values of 0.90 and 0.67 mg/mL respectively. The results indicate that activity of O. africana extracts is not by targeting the cell membrane. The sensitivity between Gram positive and Gram negative bacteria is frequently attributed to difference in membrane morphology between these organisms [32].
To determine the total activity of the extracts, the quantity extracted in mg from 1 g of leaf material was divided by the MIC value in mg/mL. Total activity indicate the volume to which the bioactive compounds in 1 g of ground plant material can be diluted and still kill the bacteria or fungi. The extracts with high total activity are considered to be the best for isolation [26]. The results of total activity of O. africana extracts against E. coli, P. aeruginosa, E. faecalis and S. aureus are presented in Table 3. The results show that the highest total activity was found in methanol extract with the value of 1068 mL against E. faecalis while the lowest total activity was displayed by the hexane extract with the values of 25 mL against both P. aeruginosa and S. aureus. This means that the quantity present in methanol extract can be diluted to 1068 mL but would still inhibit the growth of E. faecalis.
The MIC values of O. africana extracts against C. albicans and C. neoformans are presented in Table 4. The extracts of chloroform, DCM, ethyl acetate, ethanol and methanol had the same overall average MIC values of 0.16 mg/mL, which was the lowest compared to the other extracts. The water extract had the highest average MIC value of 1.10 mg/mL and this shows that it was less active against the two fungal pathogens used. Overall, both C. albicans and C. neoformans were sensitive to most of the plant extracts with average MIC values of 0.37 and 0.30 respectively.
Other scientist studied the antimicrobial activities of O. africana. Battinellia et al. [33] reported antifungal activity of some aliphatic aldehydes from olive fruit against Tricophyton mentagrophytes, Microsporum canis and Candida spp. Aldehydes tested, inhibited the growth of T. mentagrophytes and M. canis in the range of concentration between <1.9 and 125 μg/ml. None of the aldehydes exhibited activity against Candida spp. Oleuropein isolated from O. Africana inhibited the growth of Salmonella spp., Vibrio spp. and Staphylococcus aureus with minimum inhibitory concentration (MIC) between 62.5 and 125 μg/ml for ATCC strains and between 31.25 and 250 μg/ml for clinical isolates. Hydroxytyrosol, derived from oleuropein by enzymatic hydrolysis was reported to have a more broad spectrum and a higher potency by inhibiting Haemophilus influenzae and Moraxella catharralis; its MIC values were between 0.24 and 7.85 μg/ml for ATCC strains and between 0.97 and 31.25 μg/ml for clinical isolates [34]. Dried leaf extracts (ethanol:water 1:1) at concentrations of 500 mg/ml, were found to be inactive in vitro against Aspergillus fumigatus, A. niger, Fusarium oxysporum, Penicillium digitatum, Rhizopus nigricans, Trichophyton mentagrophytes, Candida albicans and Saccharomyces pastorianus [35]. Furthermore, oleuropein showed activity against several species of Mycoplasma [36].
The results of total activity of O. africana extracts against C. albicans and C. neoformans are presented in Table 5. This table shows that the highest total activity was found in butanol extract with the value of 1375 mL against both test fungi. This means that the compound(s) present in the butanol crude extract can be diluted to 1375 mL but would still inhibit the growth of C. albicans and C. neoformans.
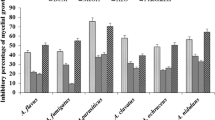
The bioautography assay was used to determine the number of compounds with antibacterial and antifungal activity in the different extracts of O. africana. Zones of inhibition were observed by white bands on a purple-red background. These white bands indicated where reduction of INT to the coloured formazan did not take place due to presence of compounds that inhibited the growth of the test bacteria or fungi. The white bands were located at different Rf values and this suggested that in the quantitative antimicrobial assays more than one compound was responsible for antimicrobial activity. Bioautography results demonstrated prominent inhibition zones by most extracts of O. africana against the growth of the test bacteria (Figs. 4 and 5). The Rf values of compounds with antibacterial activity ranged from 0.19 to 0.93. Only the chloroform and DCM extracts separated with BEA displayed antifungal activity against C. neoformans (Fig. 6a) and C. albicans (Fig. 6b) with Rf values of 0.18 and 0.17 respectively. Extracts separated with BEA demonstrated a total of 67 compounds with antimicrobial activity against the test organisms, followed by EMW with a total of 31 compounds and CEF with a total of 25 compounds. This shows that most compounds with antimicrobial activity from O. africana leaves are non-polar.
Conclusion
The results indicate that the leaf extracts of O. africana contain compounds with antioxidant activity, antibacterial and antifungal activity. C. neoformans and E. faecalis were the most sensitive test organisms with overall average MIC values of 0.30 and 0.31 mg/mL respectively. This study indicates that O. africana may be a potential source of antibacterial and antifungal compounds. Further studies are required to isolate the active compounds and perform other tests such as cytotoxicity test.
Abbreviations
- TLC:
-
Thin layer chromatography
- BEA:
-
Benzene/ethanol/ammonium solution
- CEF:
-
Chloroform/ethyl acetate/formic acid
- EMW:
-
Ethyl acetate/methanol/water
- DPPH:
-
Qualitative 2, 2- diphenylpacryl-1-hydrazyl
- MIC:
-
Minimum inhibitory concentrations
- ATCC:
-
America type culture collection
- INT:
-
ρ-iodonitrotetrazolium violet
References
Dyubeni L, Buwa LV. An ethnobotanical study of plants used for the treatment of ear, nose and throat (ENT) infections in Nkonkobe Municipality. South Africa J Med Plants Res. 2012;6(14):2721–6.
World Health Organisation. WHO traditional medicine strategy 2002- 2005. Geneva: World Health Organisation; 2002. p. 1.
Savithramma N, Linga-Rao M, Ankana S. Screening of traditional medicinal plants for secondary metabolites. Int J Res Pharm Sci. 2011;2(4):643–7.
Ndhlala AR, Stafford GI, Finnie JF, van Staden J. Commercial herbal preparations in KwaZulu-Natal, South Africa: The urban face of traditional medicine. S Afri J Bot. 2011;77:830–43.
Pavarini DP, Pavarinib SP, Niehuesa M, Lopesa NP. Exogenous influences on plant secondary metabolite levels. Ani Feed Sci Tech. 2012;176:5–16.
Borokini TI, Omotayo TO. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plants Res. 2012;6(7):1106–18.
Mulaudzi RB, Ndhlala AR, Kulkarni MG, van Staden J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. J Ethnopharma. 2012;143:185–19.
Zhu F, Cai Y, Sun M, Corke H. Effect of phytochemical extracts on the pasting, thermal, and gelling properties of wheat starch. Food Chem. 2009;112:919–23.
Emad MA. Plants: An alternative source for antimicrobials. J Appl Pharm Sci. 2011;1(6):16–20.
Viji M, Murugesan S. Phytochemical analysis and antibacterial activity of medicinal plant Cardiospermum halicacabum Linn. J Phyt Phytopharm. 2010;2(1):68–77.
Gul F, Shinwari ZK, Afzal I. Screening of indigenous knowledge of herbal remedies for skin diseases among local communities of north west Punjab, Pakistan. Pakistan J Bot. 2012;44(5):1609–16.
Long HS, Tilney PM, van Wyk BE. The ethnobotany and pharmacognosy of Olea europaea subsp. africana (Oleaceae). S Afri J Bot. 2010;76:324–31.
Green PS, Kupicha FK. Notes on the genus Olea. Kew Bulletin. 1979;34:69.
Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharm. 2003;84:299–305.
Altinyay C, Güvenç A, Altun ML. Antioxidant activities of oleuropein and the aqueous extracts of Olea europaea L. varieties growing in Turkey. Turk J Pharm Sci. 2011;8(1):23–30.
Sacho H, Schoub DB. Current perspectives on Nosocomial Infections. Pietermaritzburg: Natal Witness Printing and Publishing; 1993.
Quality Control. National Committee for Clinical Laboratory Standards. 1992; http://www.doctorfungus.org/thelabor/sec15.pdf (accessed 15 July 2013).
Kotze M, Eloff JN. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae). S Afri J Bot. 2002;68:62–7.
Ayoola GA, Coker HAB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and antioxidant activities of some medicinal plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res. 2008;7(3):1019–24.
Odebiyi OO, Sofowara EA. Phytochemical screening of Nigerian medicinal plants. LIodydia. 1978;41(3):234–46.
Trease GE, Evans WC. Text of Pharmacognosy. 14th ed. London: W.B. Sanders; 1989.
Harborne JB. Phytochemical methods. 3rd ed. London: Chapman and hall Ltd; 1973.
Deby C, Margotteaux G. Relationship between essential fatty acids and tissue antioxidant levels in mice. Cr Soc Bio. 1970;165:2675–81.
Eloff JN. A sensitive and quick microplate method to determine the minimum inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–3.
Masoko P, Eloff JN, Picard J. Antifungal activities of six South African Terminalia species (Combretaceae). J Ethnopharm. 2005;99:301–8.
Eloff JN. Quatification of the bioactivity of plant extracts during screening and bioassay guided fractionation. J Phytomed. 2004;11:370–1.
Begue WJ, Kline RM. The use of tetrazolium salts in bioautographic procedures. J Chromatogr. 1972;64:182–4.
Masoko P, Eloff JN. The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by Bioautography. Afri J Biotechnol. 2005;4(12):1425–31.
Masoko P, Mokgotho MP, Mbazima VG, Mampuru LJ. Biological activity of Typha capensis (Typhaceae) from Limpopo Province (South Africa). Afri J Biotechnol. 2008;20:3743–8.
Masoko P, Eloff JN, Picard J. Resistance of animal fungal pathogens to solvents used in bioassays. S Afri J Bot. 2007;73:667–9.
Wojcikowski K, Stevenson L, Leach D, Wohlmuth H, Gobe G. Antioxidant capacity of 55 medicinal herbs traditionally used to treat the urinary system: a comparison using a sequential three-solvent extraction process. Afri J Trad Compl Altern Med. 2007;13:103–9.
Masoko P, Gololo SS, Mokgotho MP, Eloff JN, Howard RL, Mampuru LJ. Evaluation of the antioxidant, antibacterial and antiproliferatory activities of the acetone extracts of the roots of Senna italica (Fabaceae). Afri J Trad Compl Altern Med. 2010;7(2):138–48.
Battinellia L, Danielea C, Cristianib M, Bisignanob G, Saijab A, Mazzanti G. In vitro antifungal and anti-elastase activity of some aliphatic aldehydes from Olea europaea L. fruit. Phytomedicine. 2006;13(8):558–63.
Bisignano G, Tomaino A, Lo Cascio R, Crisafi G, Uccella N, Saija A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm Pharmacol. 1999;51(8):971–4.
Guerin JC, Reveillere HP. A study of 40 plant extracts against 9 fungal species. Ann Pharm Fr. 1985;43(1):77–81.
Furneri PM, Marino A, Saija A, Uccella N, Bisignano G. In vitro antimycoplasmal activity of oleuropein. Int J Antimicrob Agents. 2002;20(4):293–6.
Acknowledgements
We would like to thank the University of Limpopo and NRF for financial support. Dr Z Mbita for proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PM, conception and design of the study. MMD carried out the experiments and analysed the data. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Masoko, P., Makgapeetja, D.M. Antibacterial, antifungal and antioxidant activity of Olea africana against pathogenic yeast and nosocomial pathogens. BMC Complement Altern Med 15, 409 (2015). https://doi.org/10.1186/s12906-015-0941-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-015-0941-8