Abstract
Background
Malignacies are still a major public concern worldwide and despite the intensive search for new chemotherapeutic agents, treatment still remains a challenging issue. This work was designed to assess the cytotoxicity of six selected Cameroonian medicinal plants, including Nauclea pobeguinii and its constituents 3-acetoxy-11-oxo-urs-12-ene (1), p-coumaric acid (2), citric acid trimethyl ester (3), resveratrol (4), resveratrol β-D-glucopyranoside (5) and strictosamide (6), against 8 drug-sensitive and multidrug-resistant (MDR) cancer cell lines.
Methods
The resazurin reduction assay was used to evaluate the cytotoxicity of the crude extracts and compounds, whilst column chromatography was used to isolate the constituents of Nauclea pobeguinii. Structural characterization of isolated compounds was performed using nuclear magnetic resonance (NMR) spectroscopic data.
Results
Preliminary experiments on leukemia CCRF-CEM cells at 40 μg/mL showed that the leaves and bark extracts from Tragia benthamii, Canarium schweinfurthii, Myrianthus arboreus, Dischistocalyx grandifolius and Fagara macrophylla induced more than 50 % growth of this cell line contrary to the leaves and bark extracts of N. pobeguinii. IC50 values below or around 30 μg/mL were obtained with leaves and bark extracts of N. pobeguinii towards two and five, respectively, of the 8 tested cancer cell lines. The lowest IC50 value was obtained with the bark extract of N. pobeguinii against HCT116 (p53−/−) colon cancer cells (8.70 μg/mL). Compounds 4 and 6 displayed selective activity on leukemia and carcinoma cells, whilst 1–3 were not active. IC50 values below 100 μM were recorded with compound 5 on all 9 tested cancer cell lines as well as with 4 against 7 out of 8 and 6 against 2 out of 8 cell lines.
Collateral sensitivity was observed in CEM/ADR5000 leukemia cells, MDA-MB-231-BCRP breast adenocarcinoma cells (0.53-fold), HCT116 (p53+/+) cells, human U87MG.ΔEGFR glioblastome multiforme cells to the methanolic bark extract of N. pobeguinii, as well as in MDA-MB-231-BCRP cells and HCT116 (p53+/+) cells and U87MG.ΔEGFR cells (0.86-fold) to compound 5.
Conclusions
The results of this study demonstrate the cytotoxicity of six Cameroonian medicinal plants, Canarium schweinfurthii, Dischistocalyx grandifolius, Tragia benthamii, Fagara macrophylla, Myrianthus arboreus and Nauclea pobeguinii. We also demonstrated the antiproliferative potential of Nauclea pobeguinii against drug-resistant cancer cell lines. Resveratrol and its glucoside are the major cytotoxic constituents in the bark of Nauclea pobeguinii.
Similar content being viewed by others
Background
Malignacies are still a major public concern worldwide and despite the intensive search for new chemotherapeutic agents, treatment still remains a challenging issue. The situation is more complicated by the spread of drug resistance in tumors. Continuous efforts are necessary to boost drug discovery to treat multidrug resistant (MDR) cancer cells. Many factors are involved in MDR, including the over-expression of ABC transporters, particularly breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp) [1], as well as the epidermal growth factor receptor (EGFR) [2–4] and mutations in the p53 tumor suppressor gene [5]. MDR cancer cells are resistant to a variety of chemically unrelated drugs [6–9]. In our previous studies, we documented the cytotoxicity of several secondary metabolites from selected Cameroonian plants against MDR cancer cells [10–15]. In our continous search for potentially antineoplastic agents from Cameroonian medicinal plants, the present study was designed at investigating the cytotoxicity of Canarium schweinfurthii Engl. (Burseraceae), Dischistocalyx grandifolius C.B. Clarke (Acanthaceae) and Tragia benthamii Bak. (Euphorbiaceae), Fagara macrophylla Engl. (Rutaceae), Myrianthus arboreus P. Beauv. (Moraceae) and Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Rubiaceae). The work was extended to the isolation of the active constituents of Nauclea pobeguinii. The above plants are used in Africa to treat many different ailments (Table 1). However, it has been recommended that ethnopharmacological usages such as immune and skin disorders, inflammatory, infectious, parasitic and viral diseases should be taken into account when selecting plants used to treat cancer, since these reflect disease states bearing relevance to cancer or cancer-like symptoms [16, 17].
Methods
General procedure
Vacuum liquid chromatography (VLC), column chromatography (CC) and thin layer chromatography (TLC) were performed on silica gel 60 (particle size 90 % <45 mm), 200–300 mesh silica gel, and silica gel GF254 (Merck), respectively. Melting points (m.p.) were measured by an Electro thermal IA 9000 digital melting point apparatus (Electro thermal) and are uncorrected. The NMR data were recorded with a Bruker DRX-400 MHz (Bruker). LR-EI-MS were recorded with JEOL mass spectrometer instrument (JEOL). The purity of the molecules was determined by HPLC (Shimadzu HPLC system), using a LiChrospher100 RP-18 (250 × 4 mm, 5 μM) column and MeOH-H2O (6:4 and 8:2)/0.1 TEA as mobile phase with detection at 273 nm.
Chemicals
Doxorubicin 98.0 % from Sigma-Aldrich was provided by the University Pharmacy of the Johannes Gutenberg University-Mainz and dissolved in PBS (Invitrogen) at a concentration of 10 mM. Geneticin >98 % (72.18 mM) was obtained from Sigma-Aldrich.
Plant material
The plant materials used in this study were the bark of Canarium schweinfurthii Engl. (Burseraceae), the whole plant of Dischistocalyx grandifolius C.B.Clarke (Acanthaceae) and Tragia benthamii Bak. (Euphorbiaceae), the bark and leaves of Fagara macrophylla Engl. (Rutaceae), Myrianthus arboreus P.Beauv. (Moraceae), Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Rubiaceae). The plant materials were collected in March and April 2013 in Bangangté and Mbouda (west region of Cameroon). They were identified at the National Herbarium in Yaoundé, Cameroon and compared with voucher specimens formerly kept under the registration number (Table 1).
Extraction
Air-dried plant material (3 kg for the bark of Nauclea pobeguinii and 1 kg for other samples) was powdered and extracted with methanol (MeOH; 10 L for the bark of Nauclea pobeguinii and 3 L for other samples) for two days. The organic solution was concentrated in vacuo to yield a paste (crude extract). The yield of each extract was determined (Table 1) and the samples were kept at 4 °C until further use.
Isolation of compounds from the bark of nauclea pobeguinii
The crude extract (80 g) was further poured onto distilled water and separated with dichloromethane (DCM) (A), ethyl acetate, EA (B), and n-butanol, n-BuOH (C) under the non-miscible liquid-liquid process. The concentration in vacuo of each organic portion afforded fractions A (42 g), B (12 g), and C (28 g), respectively. A column (5 × 60 cm) was used for the purification of fraction A. Silica gel (160 g) column chromatography was prepared and A was eluted under gradient conditions with pure (100 %) hexane (hex) and EA affording 75 fractions of 100 mL each. A colorless powder (1, 10 mg) was obtained from sub-fractions 15–20, while a brown oil (2, 3.5 mg) was isolated from sub-fractions 25–27. A colorless sticky gum (3, 11.2 mg) was further obtained from sub-fractions 50–63. Moreover, fraction B was loaded onto a silica gel (50 g) column (2 cm × 50 cm) and the column was eluted with DCM/EA (98:2, v/v) to give exclusively 2.1 mg of a brownish solid (4). Fraction C was also loaded onto the same column as A using 150 g of silica gel. The column was eluted with pure DCM/MeOH under gradient condition to afford 90 fractions. Sub-fractions 2–10 afforded 5 mg of compound 4, while sub-fractions 30–40 pooled together based on TLC profile gave a colorless solid (5, 5 mg). Similarly, a yellow solid (6, 20 mg) was obtained after filtration of sub-fractions 60–75. These sub-fractions were pooled together based on the TLC profile, after complete evaporation, the solid residue was recrystallized with acetone to give again 6 (15 mg).
Structural characterization of isolated compounds
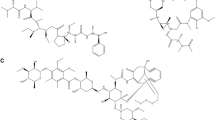
The structures of compounds (1–6) (Fig. 1) were established based on 1D (1H, and 13C) and 2D (HSQC, COSY and HMBC) NMR spectroscopy as well as mass spectrometry. After comparing the obtained data (Additional file 1: See Supporting information S1) with those reported in the literature, the compounds were identified as 3-acetoxy-11-oxo-urs-12-ene (1), p-coumaric acid (2), citric acid trimethyl ester (3), resveratrol (4), resveratrol β-D-glucopyranoside (5) and strictosamide (6).
Cell cultures
The cell lines used in the present work, their origins and their treatments were previously reported [18, 19]. They include drug-sensitive leukemia CCRF-CEM cells, its multidrug-resistant subline CEM/ADR5000 cells [3, 20, 21], breast cancer MDA-MB-231-pcDNA3 cells, its resistant subline MDA-MB-231-BCRP clone 23) cells [22], colon HCT116 (p53+/+) cancer cells, its knockout clones HCT116 (p53−/−), glioblastoma U87MG cells, its resistant subline U87MG.ΔEGFR cells and normal AML12 hepatocytes [14, 15, 19, 23]. The CCRF-CEM and CEM/ADR5000 leukemia cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10 % fetal calf serum in a humidified 5 % CO2 atm at 37 °C. Breast cancer cells, transduced with control vector (MDA-MB-231-pcDNA3) or with cDNA for the breast cancer resistance protein BCRP (MDA-MB-231-BCRP clone 23), were maintained under standard conditions as described above for CCRF-CEM cells. Human wild-type HCT116 (p53+/+) colon cancer cells as well as knockout clones HCT116 (p53−/−) derived by homologous recombination were a generous gift from Dr. B. Vogelstein and H. Hermeking (Howard Hughes Medical Institute, Baltimore, MD). Human glioblastoma multiforme U87MG cells (non-transduced) and U87MG cell line transduced with an expression vector harboring an epidermal growth factor receptor (EGFR) gene with a genomic deletion of exons 2 through 7 (U87MG.ΔEGFR) were kindly provided by Dr. W. K. Cavenee (Ludwig Institute for Cancer Research, San Diego, CA). MDA-MB-231-BCRP, U87MG.ΔEGFR and HCT116 (p53−/−) were maintained in DMEM medium containing 10 % FBS (Invitrogen) and 1 % penicillin (100 U/mL)-streptomycin (100 μg/mL) (Invitrogen) and were continuously treated with 800 ng/mL and 400 μg/mL geneticin, respectively. Normal AML12 heptocytes were obtained from the American Type Culture Collection (ATCC, USA). The above medium without geneticin was used to maintain MDA-MB-231, U87MG, HCT116 (p53+/+) and AML12 cell lines. The cells were passaged twice weekly. All experiments were performed with cells in the logarithmic growth phase.
Resazurin reduction assay
The cytotoxicity of the tested samples was performed by resazurin reduction assay as we previously described [14, 15, 18, 19, 24, 25]. Briefly, adherent cells at 1 × 104 cells were allowed to attach overnight and were then treated with different concentrations of the studied samples. For suspension cells, aliquots of 2 × 104 cells per well were seeded in 96-well-plates in a final volume of 200 μL. Extracts and compounds were prior diluted in DMSO and tested in a final concentration below 0.1 % (A final concentration of 0.1 % DMSO was used as negative control and did not show any effect on cell growth). After 72 h incubation and resazurin (Sigma-Aldrich, Schnelldorf, Germany) staining, fluorescence was measured on an Infinite M2000 Pro™ plate reader (Tecan, Crailsheim, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Each assay was done at least two times, with six replicates each. IC50 values represent the sample concentration required to inhibit 50 % of cell proliferation and were calculated from a calibration curve by linear regression using Microsoft Excel.
Results and discussion
Compounds were identified as 3-acetoxy-11-oxo-urs-12-ene C32H50O3 (1; m.p. 282.1-283.4 °C; m/z: 482.4; purity: 90 %)[26], p-coumaric acid C9H8O3 (2; m/z: 164.0; purity: 97 %)[27], citric acid trimethyl ester C9H14O7 (3; m/z: 234.0; purity: 97 %)[28], resveratrol C14H12O3 (4; m/z: 228.1; purity: 98 %)[29], resveratrol β-D-glucopyranoside C20H22O8 (5; m/z: 390.1; purity: 95 %)[30], and strictosamide C26H30N2O8 (6; m/z: 498.2; purity: 96 %) [31]. The irido-indole alkaloid strictosamide (6, 35 mg) was the major constituent of the bark extract. This is in accordance with previous studies reporting 6 as the main compound isolated from the bark methanolic extract of Nauclea pobeguinii harvested in Democratic Republic of Congo [32]. However, Xu et al. [32] also identified several other alkaloids as minor constituents of the bark extract, such as naucleidinic acid and 19-O-methyl-3,14-dihydroangustoline, naucleidinal, magniflorine, naucleofficine D, 3,14-dihydroangustoline, strictosidine, desoxycordifoline, 3a,5a-tetrahydrodeoxycordifoline lactam, and a phenol kelampayoside A. These compounds were not isolated in our sample from Cameroon. In addition, compounds 1–5 reported in this study were also not found in the plant harvested in Congo. This could either be due to the isolation techniques used or to the environmental variation that influences the concentration of the minor constituents synthesized by the Nauclea pobeguinii. strictosamide (6) was obtained as the major constituent isolated from the methanolic extract in both cases.
A preliminary cytotoxicity study was first carried out on leukemia CCRF-CEM cells with crude extracts at a single concentration of 40 μg/mL for crude extracts. Doxorubicin (10 μM) served as positive control. According to the National Cancer Institute (USA), 30 μg/mL is the upper IC50 limit considered promising for purification of a crude extract [33]. We tested a slightly higher concentration (of 40 μg/mL) in our preliminary assay. The results depicted in Fig. 2 show that more than 50 % growth was obtained if CCRF-CEM cells were treated with the methanol extracts from Tragia benthamii (63.7 %), Canarium schweinfurthii (59.96 %), Myrianthus arboreus (57.8 %), Dischistocalyx grandifolius (56.8 %) and Fagara macrophylla leaves (52.0 %). Only the crude extracts from the leaves (36.6 %) and bark (33.0 %) of Nauclea pobeguinii as well as doxorubicin (13.6 %) were able to induce more than 50 % inhibition of CCRF-CEM leukemia cell growth (Fig. 2). The IC50 values of extracts (bark and leaves) from Nauclea pobeguinii were further determined on eight cancer cell lines and values below 30 μg/mL were obtained both extracts towards two out of eight and five out of eight cell lines, respectively (Table 2). The lowest IC50 value of 8.70 μg/mL was obtained with the bark extract against HCT116 (p53−/−) cells. Consequently, this extract was subjected to purification from which six compounds (1–6) were obtained. Compounds 4 and 6 displayed selective activities on the studied cancer cell lines. IC50 values below 100 μM were recorded with compound 5 on all eight tested cancer cell lines. IC50 values below 175.36 μM and 80.29 μM (for 4 and 6 respectively) were also obtained against seven out of eight cell lines for 4 and two out of eight cell lines for 6. The IC50 values ranged from 25.08 μM (towards CCRF-CEM cells) to 97.64 (towards MDA-MB231 cells) for compound 5 and from 0.20 μM (against CCRF-CEM cells) and 195.12 μM (against CEM/ADR5000 cells) for doxorubicin. No IC50 values were obtainable, if compound 1 was tested at up to 82.92 μM. The same is true for compounds 2 (>243.90 μM) and 3 (>170.94 μM). The best compounds (4 and 5) were less toxic towards AML12 normal hepatocytes than against cancer cells. The IC50 threshold values of 20 μg/mL for crude extracts as well as 4 μg/mL or 10 μM for compounds [34, 35] after 48 and 72 h incubation have been set by the United States National Cancer Institute (USNCI) to identify good cytotoxic phytochemicals. None of the tested compounds displayed IC50 values below 10 μM. However, the bark extract of Nauclea pobeguinii (IC50 value below 20 μg/mL on four out of eight tested cancer cell lines) could be considered as a promising candidate to fight cancers. The best isolated compounds (4–6) displayed rather moderate activities, suggesting possible synergistic effects of constituents in the crude extract.
The development of MDR in cancer cells either through ABC transporters [36], the epidermal growth factor receptor (EGFR) [2–4], or the tumor suppressor p53 gene [5] represents a major hurdle in chemotherapy. The discovery of new compounds with activity against MDR is hence very important in the ongoing fight against malignancies. In the present study, we used cell lines possessing all these resistance mechanisms to investigate multi-factorial drug resistance. The degrees of resistance were determined as the ratio of IC50 value of the resistant cell line to that of the corresponding parental sensitive counterpart (Table 2). Compared to their corresponding sensitive cell lines, collateral sensitivity in resistant cells (hypersensitivity) was observed in P-glycoprotein-overexpressing CEM/ADR5000 cells (degree of resistance 0.80-fold), BCRP-overexpressing MDA-MB-231-BCRP cells (0.53-fold), p53 HCT116 (p53−/−) knockout cells (<0.54-fold) and epidermal growth factor receptor-overexpressing U87MG.ΔEGFR cells (0.47-fold) to the bark extract of N. pobeguinii. Collateral sensitivity was also found in HCT116 (p53−/−) cells (0.74-fold) and U87MG.ΔEGFR cells (0.86-fold) to compound 5.
The obtained data indicates that the stilbene resveratrol glucoside 5 and its aglycon 4 displayed slightly different degrees of activity on the cancer cell lines studied. This shows that glucosylation may positively (especially in leukemia cells) influence the cytotoxic activity. In fact, resveratrol glucoside 5 was more active than its aglcon 4 on the two tested leukemia cells. However, in carcinoma cell lines, the cytotoxicity of compounds 4 and 5 varied from one cell lines to other, none of two being more active than other one in all the solid cancer cell lines tested. To the best of our knowledge, this phytochemical and cytotoxicity study of the crude bark and leaf extracts as well as compounds 1–3 and 5 of Nauclea pobeguinii towards multifactorial drug resistant cancer lines is being reported here for the first time. Though strictosamide (6) is known to be the main constituent [32] of this plant, the present study suprisingly showed that it was not the most cytotoxic component of the extracts against the studied cancer cell lines. The best activities were reported with stilbenes namely resveratrol (4) and it glycoside resveratrol β-D-glucopyranoside (5). Compound 4 is a well known cytotoxic agent [37–39]. It is reported to suppress the proliferation of SKBR-3 breast cancer cells by inhibiting fatty acid synthase signaling pathway [38]. Compound 4 also alleviates the PI3K/Akt/mTOR signaling in breast cancer SKBR-3cells by down-regulation of Akt phosphorylation and up-regulation of PTEN expression [38]. Besides, compound 4 reportedly reverses MDR of the MCF-7/DOX breast cancer cells [39]. In the present study, compound 4 was most effective (IC50 < 23 μM) against MDA-MB231 breast adenocarcinoma cells and their drug-resistant, MDA-MB231/BCRP. These data are in accordance with previous reports and consolidates the potential cytotoxicity of 4 against breast cancer cells.
Conclusion
In conclusion, we demonstrate the cytotoxic potential of six Cameroonian medicinal plants, Canarium schweinfurthii, Dischistocalyx grandifolius, Tragia benthamii, Fagara macrophylla, Myrianthus arboreus and Nauclea pobeguinii. We also demonstrated the cytotoxic potential of leaves and bark of Nauclea pobeguinii against sensitive and MDR cancer cell lines. We further identified resveratrol and its β-glucoside as major cytotoxic constituents of the bark of Nauclea pobeguinii. The bark and leaves extracts of Nauclea pobeguinii are potential cytotoxic botanicals that deserves more investigations to develop novel cytotoxic phytomedicines against drug-resistant cancers.
References
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34.
Biedler JL, Spengler BA. Reverse transformation of multidrug-resistant cells. Cancer Metastasis Rev. 1994;13:191–207.
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64:382–94.
Efferth T, Sauerbrey A, Halatsch ME, Ross DD, Gebhart E. Molecular modes of action of cephalotaxine and homoharringtonine from the coniferous tree Cephalotaxus hainanensis in human tumor cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:56–67.
el-Deiry WS. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr Opin Oncol. 1997;9:79–87.
Efferth T. The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr Mol Med. 2001;1:45–65.
Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009.
Gillet JP, Efferth T, Remacle J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim Biophys Acta. 2007;1775:237–62.
Zaharia V, Ignat A, Palibroda N, Ngameni B, Kuete V, Fokunang CN, et al. Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and hydrazino-bis-thiazoles and their anticancer activity. Eur J Med Chem. 2010;45:5080–5.
Kuete V, Wabo HK, Eyong KO, Feussi MT, Wiench B, Krusche B, et al. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One. 2011;6, e21762.
Dzoyem JP, Nkuete AH, Kuete V, Tala MF, Wabo HK, Guru SK, et al. Cytotoxicity and antimicrobial activity of the methanol extract and compounds from Polygonum limbatum. Planta Med. 2012;78:787–92.
Kuete V, Ngameni B, Wiench B, Krusche B, Horwedel C, Ngadjui BT, et al. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011;77:1984–9.
Kuete V, Viertel K, Efferth T. 18 - Antiproliferative potential of African medicinal plants. In: Kuete V, editor. Medicinal Plant Research in Africa. Oxford: Elsevier; 2013.
Kuete V, Sandjo L, Nantchouang Ouete J, Fouotsa H, Wiench B, Efferth T. Cytotoxicity and modes of action of three naturally occuring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine. 2013;21:315–22.
Kuete V, Sandjo L, Wiench B, Efferth T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol. 2013;149:245–53.
Cordell G, Beecher C, Pezzut J. Can ethnopharmacology contribute to development of new anti-cancer? J Ethnopharmacol. 1991;32:117–33.
Popoca J, Aguilar A, Alonso D, Villarreal M. Cytotoxic activity of selected plants used as antitumorals in Mexican traditional medicine. J Ethnopharmacol. 1998;59:173–7.
O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–6.
Kuete V, Tchakam PD, Wiench B, Ngameni B, Wabo HK, Tala MF, et al. Cytotoxicity and modes of action of four naturally occuring benzophenones: 2,2',5,6'-tetrahydroxybenzophenone, guttiferone E, isogarcinol and isoxanthochymol. Phytomedicine. 2013;20:528–36.
Kimmig A, Gekeler V, Neumann M, Frese G, Handgretinger R, Kardos G, et al. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990;50:6793–9.
Gillet J, Efferth T, Steinbach D, Hamels J, de Longueville F, Bertholet V, et al. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64:8987–93.
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70.
Kuete V, Sandjo L, Seukep J, Maen Z, Ngadjui B, Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multi-factorial drug-resistant cancer cells. Planta Med. 2015;81:32–8.
Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-resistant tumor cells. Evid Based Complement Alternat Med. 2013;2013:285903.
Kuete V, Tankeo SB, Saeed ME, Wiench B, Tane P, Efferth T. Cytotoxicity and modes of action of five Cameroonian medicinal plants against multi-factorial drug resistance of tumor cells. J Ethnopharmacol. 2014;153:207–19.
Ogawa S, Wakatsuki Y, Makino M, Fujimoto Y, Yasukawa K, Kikuchi T, et al. Oxyfunctionalization of unactivated C-H bonds in triterpenoids with tert-butylhydroperoxide catalyzed by meso-5,10,15,20-tetramesitylporphyrinate osmium(II) carbonyl complex. Chem Phys Lipids. 2010;163:165–71.
Huang Y, Zeng W, Li G, Liu G, Zhao D, Wang J, et al. Characterization of a new sesquiterpene and antifungal activities of chemical constituents from Dryopteris fragrans (L.) Schott. Molecules. 2014;19:507–13.
Choi J, Lee D. A new citryl glycoside from Gastrodia elata and its inhibitory activity on GABA transaminase. Chem Pharm Bull. 2006;54:1720–1.
Aydin T, Cakir A, Kazaz C, Bayrak N, Bayir Y, Taskesenligil Y. Insecticidal metabolites from the rhizomes of Veratrum album against adults of Colorado potato beetle. Leptinotarsa decemlineata Chem Biodivers. 2014;11:1192–204.
Wei X, Yang S, Liang N, Hu D, Jin L, Xue W, et al. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules. 2013;18:1325–36.
Atta-ur-Rahman R, Zaman K, Perveen S, Habib-ur-Rehman R, Muzaffar A, Choudhary M, et al. Steroidal alkaloids from leaves of Buxus sempervirens. Phytochemistry. 1991;30:1298–3.
Xu Y-J, Foubert K, Dhooghe L, Lemière F, Cimanga K, Mesia K, et al. Chromatographic profiling and identification of two new iridoid-indole alkaloids by UPLC–MS and HPLC-SPE-NMR analysis of an antimalarial extract from Nauclea pobeguinii. Phytochem Lett. 2012;5:316–9.
Suffness M, Pezzuto J. Assays related to cancer drug discovery. London: Academic; 1990.
Boik J. Natural compounds in cancer therapy. Minnesota USA: Oregon Medical Press; 2001.
Brahemi G, Kona FR, Fiasella A, Buac D, Soukupova J, Brancale A, et al. Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. J Med Chem. 2010;53:2757–65.
Shen B, Li D, Dong P, Gao S. Expression of ABC transporters is an unfavorable prognostic factor in laryngeal squamous cell carcinoma. Ann Otol Rhinol Laryngol. 2011;120:820–7.
Zhou C, Ding J, Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR21 regulation of the Akt/Bcl2 signaling pathway. Mol Med Rep. 2014;9:1467–73.
Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;38:765–72.
Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–6.
Orwa C, Mutua A, Kindt R, Jamnadass R. Simons. A Agroforestree Database: a tree reference and selection guide version 4.0. World Agroforestry Centre: Nairobi-Kenya; 2009.
Kouambou C, Dimo T, Dzeufiet P, Ngueguim F, Tchamadeu M, Wembe E, et al. Antidiabetic and hypolipidemic effects of Canarium schweinfurthii hexane bark extract in streptozotocin-diabetic rats. PharmacologyOnline. 2007;1:209–19.
Berhaut J. Flore illustrée du Sénégal. Dicotylédones. Tome II, Balanophoracées. Dakar Senegal: Collation; 1974.
Koudou J, Abena AA, Ngaissona P, Bessiere JM. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia. 2005;76:700–3.
Tamboue H, Fotso S, Ngadjui B, Dongo E, Abegaz B. Phenolic metabolites from seeds of Canarium schweinfurthii. Bull Chem Soc Ethiop. 2000;14:155–9.
Atawodi S. Polyphenol composition and in vitro antioxydant potential of Nigerian Canarium schweinfurthii Engl. Oil Adv Biol Res. 2010;4:314–22.
Kamdem RS, Wafo P, Yousuf S, Ali Z, Adhikari A, Rasheed S, et al. Canarene: a triterpenoid with a unique carbon skeleton from Canarium schweinfurthii. Org Lett. 2011;13:5492–5.
Uzama D, Bwai DM, Oguntokun JO, Olutayo O O. Antioxidant and phytochemicals of hexane and ethanolic extracts of Canarium schweinfurthii Burseraceae. Asian J Phar Biol Res. 2012;2:188–90.
Nvau J, Gushit J, Orishadipe T, Kolo I. Antimycobacterial activity of the leaves extract of Canarium schweinfurthii Engl. Conti J Phar Sci. 2011;5:20–4.
Moshi MJ, Innocent E, Masimba PJ, Otieno DF, Weisheit A, Mbabazi P, et al. Antimicrobial and brine shrimp toxicity of some plants used in traditional medicine in Bukoba District, north-western Tanzania. Tanzan J Health Res. 2009;11:23–8.
Adjanohoun J, Aboubakar N, Dramane K, Ebot M, Ekpere J, Enow-Orock E, et al. Traditional medicine and pharmacopoeia: contribution to ethnobotanical and floristic studies in Cameroon. OUA/STRC: Lagos; 1996.
Fézan H, Trab G, Irié K, N’gaman C, Mohou C. Études de quelques plantes thérapeutiques utilisées dans le traitement de l’hypertension artérielle et du diabète : deux maladies émergentes en Côte d’Ivoire. Sci Nat. 2008;5:39–48.
Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, et al. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 2011;134:803–12.
Torto FG, Mensah IA. Alkaloids of Fagara macrophylla. Phytochemistry. 1970;9:911–4.
Tringali C, Spatafora C, Cali V, Simmonds MS. Antifeedant constituents from Fagara macrophylla. Fitoterapia. 2001;72:538–43.
Zirihi G, Yao D, Kra-adou K, Grellier P. Phytochemical and pharmacological studies of alcoholic extract of Fagara macrophylla (Oliv) Engl (Rutaceae): chemical structure of active compound inducing antipaludic activity. J Chin Clini Med. 2007;2:205–10.
Wansi JD, Nwozo SO, Mbaze LM, Devkota KP, Donkwe Moladje SM, Fomum ZT, et al. Amides from the stem bark of Fagara macrophylla. Planta Med. 2009;75:517–21.
Wall ME, Wani MC, Taylor H. Plant antitumor agents, 27. Isolation, structure, and structure activity relationships of alkaloids from Fagara macrophylla. J Nat Prod. 1987;50:1095–9.
Agwa O, Chuku W, Obichi E. The in vitro effect of Myrianthus arboreus leaf extract on some pathogenic bacteria of clinical origin. J Microbiol Biotechnol Res. 2011;1:77–85.
Uzodimma D. Medico-Ethnobotanical inventory of Ogii, Okigwe Imo State, South Eastern Nigeria - I. Glob Adv Res J Med Plant. 2013;2:030–44.
Otitoju G, Nwamarah J, Otitoju O, Odoh E, Iyeghe L. Phytochemical composition of some underutilsed green leafy vegetables in nsukka urban Lga of Enugu State. J Biodiv Env Sci. 2014;4:208–17.
Akinkurolere R, Adedire C, Odeyemi O, Raji J, Owoeye J. Bioefficacy of Extracts of some indigenous Nigerian plants on the developmental stages of mosquito (Anopheles gambiae). Jordan J Biol Sci. 2011;4:237–42.
Karou SD, Tchacondo T, Ilboudo DP, Simpore J. Sub-Saharan Rubiaceae: a review of their traditional uses, phytochemistry and biological activities. Pak J Biol Sci. 2011;14:149–69.
Kadiri H, Adegor E, Asagba S. Effect of aqueous Nauclea pobeguinii leaf extract on rats induced with hepatic injury. Res J Med Plant. 2007;1:139–43.
Mesia GK, Tona GL, Penge O, Lusakibanza M, Nanga TM, Cimanga RK, et al. Antimalarial activities and toxicities of three plants used as traditional remedies for malaria in the Democratic Republic of Congo: Croton mubango, Nauclea pobeguinii and Pyrenacantha staudtii. Ann Trop Med Parasitol. 2005;99:345–57.
Zeches M, Richard B, Gueye-M’Bahia L, LeMen-Olivier L, Delaude C. Constituants des écorces de racine de Nauclea pobeguinii. J Nat Prod. 1985;48:42–6.
Oladosu IA, Balogun SO, Ademowo GO. Phytochemical screening, antimalarial and histopathological studies of Allophylus africanus and Tragia benthamii. Chin J Nat Med. 2013;11:371–6.
Acknowledgments
The authors acknowledge the Cameroon National Herbarium (Yaoundé) for the plant identification. VK is very grateful to the Alexander von Humboldt Foundation for 18 months fellowship in Germany through the ''Georg Foster Research Fellowship for Experienced Researcher'' program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
VK and ATM carried out the experiments; VK, ATM, LPS and JAS contributed to plants collection, compound’s isolation and/or identification. VK wrote the manuscript. VK, BTN and TE designed the experiments; TE supervised the work and provided the facilities for the study. All authors read the manuscript and approved the final version.
Additional files
Additional file 1:
Supporting information S1. Data on compounds isolated from Nauclea pobeguiinii (DOC 860 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kuete, V., Sandjo, L.P., Mbaveng, A.T. et al. Cytotoxicity of selected Cameroonian medicinal plants and Nauclea pobeguinii towards multi-factorial drug-resistant cancer cells. BMC Complement Altern Med 15, 309 (2015). https://doi.org/10.1186/s12906-015-0841-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-015-0841-y