Abstract
Background
Increasing studies have shown that dyslipidemia and inflammatory responses play important roles in the progression of microvascular diabetic complications. Esculin (ES), a coumarin derivative, was extracted from Fraxinus rhynchophylla. The present study was to evaluate the potential effects of ES on lipid metabolism, inflammation responses and renal damage in streptozotocin (STZ)-induced experimental diabetic rats and explore the possible mechanism.
Methods
Diabetic rat model was established by administration high-glucose-fat diet and intraperitoneal injection of STZ 45 mg/kg. ES was administrated to diabetic rats intragastrically at 10, 30 and 90 mg/kg for 10 weeks respectively. The levels of triglycerides (TG), total cholesterol (T-CHO), low density lipoproteins (LDL), and high-density-cholesterol (HDL-C) in serum were measured. IL-1, IL-6, ICAM-1, NO, NAGL, and AGEs level in serum were detected by ELISA assay. The accumulation of AGEs in kidney tissue was examined by immunohistochemistry assay.
Results
The results showed that ES could decrease TG, T-CHO, LDL levels in serum of diabetic rats in a dose dependent manner. ES also decreased IL-1, IL-6, ICAM-1, NO and NGAL levels in serum of diabetic rats in a dose dependent manner. Furthermore, ES at 30 and 90 mg/kg significantly decreased AGEs level in serum and alleviated AGEs accumulation in renal in diabetic rats.
Conclusions
Our findings indicate that ES could improve dyslipidemia, inflammation responses, renal damage in STZ-induced diabetic rats and the possible mechanism might be associated with the inhibition of AGEs formation.
Similar content being viewed by others
Background
Diabetes mellitus is characterized by hyperglycaemia, hypercholesterolemia and hypertriglyceridemia, resulting from defects in insulin secretion followed by dysfunction and failure of organs especially the eyes, kidneys, nerves, heart and arteries [1]. Abnormalities in lipid profile are one of the most common complications in diabetes mellitus [2]. The key components of diabetic dyslipidemia are elevated plasma low density lipoproteins (LDL), triglycerides (TGs), and lowered high density lipoprotein (HDL-C) [3]. Several studies have also demonstrated that inflammatory processes play a crucial role in the development of diabetic complications. In the present study, in order to verify the effects of ES on anti-diabetic complications, we investigated the level of proinflammatory cytokines in the diabetic rats.
Diabetic nephropathy is a major microvascular complication of diabetes mellitus and the most common cause of renal failure. The renal injury in diabetic nephropathy is due to a series of complex pathophysiological changes such as glomerulosclerosis, vascular diseases and changes of the tubulointerstitium with tubular atrophy and interstitial fibrosis initiated by disturbed glucose homeostasis [4]. Neutrophil gelatinase-associated lipocalin (NGAL), a 25 kDa protein belonging to the lipocalin superfamily, was initially found in activated neutrophils. However, many other cells, like kidney tubular cells, may also produce NGAL in response to various insults [5]. Both serum and uric NGAL have been found to be reliable predictors of kidney injury [6].
Recent studies have suggested that advanced glycation end products (AGEs) are believed to play a crucial role in the pathogenesis of diabetic complications [7]. AGEs ligate to the receptor of AGEs (RAGE), facilitating activation of the transcription factor NFκB may be involved in the development of diabetic complications [8].
Esculin (Fig. 1), a coumarin derivative, was extracted from Fraxinus rhynchophylla, belonging to the Oleaceae family, which is widely distributed in the east of Asia, especially in the south of China. In diabetes-related studies, it has been demonstrated that esculin and its metabolite esculetin inhibit formation of advanced glycation end-products formation [9], inhibit the activities of α-glucosidase and protein tyrosine phosphatase 1B [10], and have the beneficial effect against diabetes and oxidative stress-related inflammatory processes in the kidney of mice [11]. However, studies on the effect and mechanism of esculin on diabetic rats are limited. Therefore, we investigated the effect and possible mechanism of esculin against dyslipidemia, inflammation responses and renal damage in high-glucose-fat diet and STZ-induced diabetic rats.
Methods
Reagents
Esculin was purchased from Nanjing Zelang Medical Technology Co., Ltd (Nanjing, China). Streptozotocin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Triglyceride, total cholesterol, LDL cholesterol and HDL cholesterol were assayed using kits from Applygen Coporation (Beijing, China). One touch gluco-meter (Accu-chek sensor) was Roche products. IL-1 and IL-6 ELISA kits were purchased from ExCell Bio (Genetimes Technology, Inc., Beijing, China), ICAM-1 and NAGL ELISA Kits were purchase from Wuhan Boster Bio-engineering limited company (Wuhan, China). NO level was assayed by Griess Regant System from Promega Corporation (Madison, USA). AGEs ELISA Kit was purchase from CELL BIOLABS INC. (San Diego, CA). High-sugar-fat chow was added 10 % sugar, 10 % lard, 2 % cholesterol, 0.2 % cholic acid in the standard of rat chow.
Animals
Adult male Sprague–Dawley rats (200–220 g) were purchased from the Animal Centre of Beijing Vitra Liver (Beijing, China). All animals were housed in a room with controlled temperature of 23 ± 2 °C and humidity of 55 ± 5 % with a regular 12 h light–dark cycle and allowed to have water and food ad libitum. All animal care and experimental procedures regarding the animals were approved by the ethic committees of Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College.
STZ-induced experimental diabetic rats and experimental design
Diabetes was induced in all the rats except a group of ten animals which were treated as normal control group by intraperitoneal administration of STZ (45 mg/kg) dissolved in freshly prepared citrate buffer (pH 4.5). The animals were fasted for 12 h before STZ administration and blood glucose was estimated after 72 hours of STZ administration. The animals with blood glucose more than 16.7 mmol/l were treated as diabetic and the animals were divided into 6 groups (n = 10): one group was served as diabetic control; ES-L, ES-M, and ES-H groups were received esculin 10 mg/kg, 30 mg/kg and 90 mg/kg by per oral administration respectively for a period of 10 weeks; AG group was served as positive group by per oral administration. After administration, rats were anesthetized and killed, serum was collected. The kidney was separated and fixed in 4 % polyformaldehyde for histopathology assay and immunochemistry staining.
Determination of lipid profile in serum
Triglyceride (TG), Total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) content in serum were assayed following the manufacturer’s protocol.
Determination of IL-1, IL-6, ICAM-1, NO, AGEs, and NAGL level in serum
IL-1, IL-6, ICAM-1, NO, AGEs, and NAGL level in serum were measured according to the manufacturer’s protocol.
Histological examination
After fixation, renal tissues were stained with hematoxylin and eosin (HE) reagents for histological examination and examined under the microscope.
AGEs level estimated in renal by immunochemistry
AGEs levels in renal were determined by immunochemistry staining on 4 % polyformalin-fixed paraffin sections (2-μm-thick). Briefly, the deparaffinized and rehydrated tissue sections were incubated with 3 % hydrogen peroxide to block endogenous peroxidase and then subjected to antigen retrieval through microwave oven heating in 0.1 M sodium citrate (pH 6.0) for 10 min. After that, tissue sections were incubated with 10 % normal goat serum for 10 min followed by an overnight incubation with anti-AGEs (1:100) antibody in 10 % normal goat serum at 4 °C. The sections were washed, further incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit/mouse polyclonal antibody for 20 min at 37 °C, and developed with 3,3-diaminobenzidine (DAB, Sigma) for color reaction. The sections were then counterstained with hematoxylin. All measurements were performed blindly.
Statistical analysis
All data are expressed as the mean ± SD. Statistical analysis of the results was carried out by one-way analysis of variance (ANOVA), followed by t-test. Statistical significance was accepted at P < 0.05.
Results
Effect of ES on body weight and blood glucose in STZ-induced diabetic rats
The diabetic model rats exhibited reduced body weight and increased blood glucose levels compared with normal control rats (both P < 0.01). However, there were no effect on body weight (Fig. 2a) and blood glucose (Fig. 2b) observed in ES or AG-treated rats.
Effect of ES on biochemical parameters in STZ-induced diabetic rats
In diabetic control rats, TG, T-CHO, and LDL levels were all significantly increased when compared to normal rats (all P < 0.01). However, treatment with ES dose-dependently attenuated the increase in TG, T-CHO, and LDL from the diabetic rats (Fig. 3a, b, and c), but these levels were still higher than that observed in normal rats and AG-treated rats. These findings indicate that ES is able to ameliorate diabetes signs to a certain extent.
Effect of ES on TG, T-CHO, LDL, and HDL levels in STZ-induced diabetic rats. a TG level; b T-CHO level; c LDL level; d HDL level. Data are expressed as means ± S.D (n = 6 ~ 8). Data are expressed as means ± S.D. (n = 6 ~ 8). ##P < 0.01 vs normal control group; *P < 0.05, **P < 0.01 vs diabetic model group
Rats in the diabetic model group demonstrated significant decrease of HDL compared to the control group (P < 0.01). However, after treatment with ES, there was no significant change in HDL level all of these parameters improved significantly. Similar significant improvements in these parameters occurred in the AG group as well (Fig. 3d).
Effect of ES on inflammatory factors in STZ-induced diabetic rats
In diabetic model rats, IL-1, IL-6, ICAM-1 and NO levels were all significantly increased compared with normal control rats (P < 0.05, P < 0.05, P < 0.01, P < 0.01, respectively). However, treatment with ES dose-dependently attenuated the increase in IL-1, IL-6, ICAM-1 and NO levels from the diabetic rats (Fig. 4). These findings indicate that ES is able to ameliorate inflammatory responses to a certain extent in diabetic rats.
Effect of ES on NAGL level in serum in STZ-induced diabetic rats
As shown in Fig. 5, the NAGL level in serum was significantly increased in STZ-induced diabetic rats compared with the normal control rats (P < 0.01). However, treatment with ES 30 and 90 mg/kg significantly decreased the NAGL levels compared with the diabetic model group (both P < 0.05), and the level was lower than that observed in AG-treated rats (Fig. 5). These results indicate that to some extent, ES is able to protect against diabetic kidney injury.
Effect of ES on AGEs level in serum in STZ-induced diabetic rats
As shown in Fig. 6, the AGEs level in serum was significantly increased in STZ-induced diabetic rats compared with the normal control rats (P < 0.01). In contrast, treatment with ES 30 and 90 mg/kg significantly decreased the AGEs levels compared with the diabetic model group (P < 0.05, P < 0.01, respectively). These results indicate that to some extent, ES is able to protect against AGEs level increase in diabetic rats.
Effect of ES against STZ-induced diabetic renal histopathological changes in rats
HE staining was performed on renal sections to measure tubular damage. As shown in the representative pictures of renal sections, severe tubulointerstitial injuries including tubular epithelial cell detachments, cystic dilatation of tubules, and inflammatory cell infiltration occurred in kidneys of STZ-treated diabetic rats. However, the increased tubular damage in STZ-treated mice was reduced by treatment with esculin (Fig. 7).
Effect of ES against STZ-induced AGEs accumulation in renal of rats
Accumulation of AGEs is observed in kidney of diabetic rats, for which these molecules contribute to diabetes complications. In the present study, an increase of AGEs accumulation was found in the renal of the diabetic model group; however, treatment with ES 30 and 90 mg/kg resulted in obviously reduced AGEs accumulation (Fig. 8). These results indicate that ES is able to inhibit the nonenzymatic glycation of protein in vivo and may help to attenuate renal injury in diabetic rats.
Discussion
Diabetes is increasing at epidemic proportions throughout the world. Most of the morbidity and mortality associated with diabetes results from macro and microvascular complications [12, 13]. Compared to macrovascular complications, microvascular complications, such as retinopathy, neuropathy, nephropathy, are much earlier and common in diabetes. Approximately 40 % of patients with diabetes may progress to nephropathy and a good metabolic control can prevent the development of diabetic renal injury [14]. Hyperglycaemia and dyslipidemia are two pathological characteristics of diabetes [15, 16]. Increased levels of glucose and lipids in serum and kidney can directly stimulate oxidative stress, inflammation, fibrosis and advanced glycation end products (AGEs) products formation in tubular and glomerular structures [17, 18].
Esculin, a coumarin derivative, was extracted from Fraxinus rhynchophylla, which belonging to a traditional medicinal plant from East Asia. Thuong PT, et al. [19] found that derivative coumarins fraxetin has direct protective properties against LDL oxidation at lower concentrations, and higher concentrations of fraxetin induce antioxidant enzymes via Nrf2/ARE activation. These effects suggest potential anti-atherosclerosis effects of Fraxinus rhynchophylla. Naaz F, et al. [20] demonstrated that the protective efficacy of esculin against pro-oxidant AFB1-induced nephrotoxicity in mice might be due to its antioxidants and free radical scavenging properties. Kang KS, et al. [11] found that esculin lessened the elevated blood creatinine levels in diabetic mice and ameliorated diabetes-induced renal dysfunction by reducing caspase-3 activation in the kidney. However, our results showed that ES could improve dyslipidemia, inflammation responses, renal damage in STZ-induced diabetic rats and the possible mechanism might be associated with the inhibition of AGEs formation. In this study, the results also showed that hyperglycaemia, dyslipidemia and systemic inflammatory responses were observed in STZ-induced diabetes rats. However, ES significantly improved dyslipidemia in diabetes, but did not significantly change the level of blood glucose.
Previous reports suggested that several humoral markers of inflammation are elevated in diabetic patients and inflammation is involved in the progression of diabetic nephropathy. Some pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α are involved in matrix expansion and mesangial cells proliferation leading to glomerular fibrosis [21]. In the present study, our results showed that ES could dose-dependently attenuate the increase of IL-1, IL-6, ICAM-1 and NO levels in STZ-induced diabetic rats. These findings indicate that ES is able to ameliorate inflammation responses to a certain extent in diabetic rats.
Diabetic nephropathy is associated with mesangial cell expansion, thickening of glomerulra and tubular basement membrane, glomerulosclerosis and tubular necrosis. Previous reports suggested that the presence of hyperglycaemia and dyslipidemia causes functional and morphological kidney alterations that can induce renal dysfunction [15, 16]. Dyslipidemia can induce a glomerular phenotype characterized by proliferative and pro-inflammatory response, monocyte infiltration, increased extracellular matrix and podocytes degeneration [18]. It has been demonstrated that diabetes-induced oxidative stress and lipid alteration, in addition to a marked glucose elevation, play a detrimental role in the onset of nephropathy in diabetic animals [22, 23]. In the present study, it was shown that ES could obviously improve renal damage in STZ-induced diabetes rats.
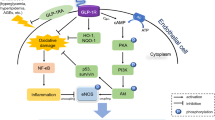
Due to prolonged high glucose in diabetes, reducing sugars can react non-enzymatically with the amino groups to initiate a complex series of rearrangements and dehydrations, and then to produce a class of irreversibly cross-linked moieties termed AGEs [24]. The accumulation of AGEs is an important feature in the development of diabetic complications [25, 26]. The formation and accumulation of AGEs in various tissues have been shown to progress at an accelerated rate under hyperglycemic conditions [27, 28]. There is accumulating evidence that AGEs and receptor for AGEs interaction induces oxidative stress generation and subsequently evokes inflammatory reactions, thereby causing progressive alteration in renal architecture and loss of renal function in diabetes [29–31]. In particular, AGEs are thought to be involved in the pathogenesis of diabetic nephropathy via multifactorial mechanisms such as oxidative stress generation and overproduction of various growth factors and cytokines [32]. It is reported that AGEs have been recently shown to increase leukocyte adhesion to cultured retinal microvascular endothelial cells by inducing intracellular cell adhesion molecule-1 (ICAM-1) expression [33].AGEs modification of proteins may produce in changes charge, solubility, and conformation leading to molecular dysfunction as well as disrupting interactions with other proteins. AGEs also interact with specific receptors and binding proteins to influence the renal expression of growth factors and cytokines, implicated in the progression of diabetic renal disease [34].
Investigations by others also demonstrated that formation of AGEs is an important pathophysiological mechanism in the development of diabetic nephropathy [35]. Jung HA, et al. have found that esculin may play a crucial role in AGE inhibition and may be as potential candidates for use as therapeutic or preventive agents for diabetic complications [9]. In agreement with this, our research firstly demonstrated the inhibitory effect of ES on AGE formation in diabetic rats. The results showed that in serum of normal rats, the level of AGEs was very low, but in diabetic rats, it was significantly increased. However, the increase of AGEs level in serum was obviously depressed by the administration of ES and AG. In this present study, we also found that the accumulation of AGEs in renal, especially in glomerulra, was increased in STZ-induced diabetic rats, which was correlated with renal damage. Moreover, ES administration reduced the accumulation of AGEs in diabetic rats. These observations suggested that ES could restrain the AGEs accumulation in kidney. Therefore, these results suggest that AGEs accumulation might play a considerable role in the diabetic nephropathy and it is likely that inhibiting the formation of AGEs or removing established AGEs modifications will form an important component part of future therapy in patients with diabetes, acting in concert with conventional approaches to prevent diabetic renal injury.
Conclusions
In conclusion, our research confirmed that ES had a protective effect against lipid metabolism disorders, inflammation responses, and renal injury in diabetic rats, the mechanism of which may be at least partly correlated with the amelioration of AGEs accumulation. Therefore, it can be concluded that ES may help to attenuate diabetes nephropathy.
References
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139
Kulkarni CR, Joglekar MM, Patil SB, Arvindekar AU (2012) Antihyperglycemic and antihyperlipidemic effect of Santalum album in streptozotocin induced diabetic rats. Pharm Biol 50:360–365
Ansar S, Koska J, Reaven PD (2011) Postprandial hyperlipidemia, endothelial dysfunction and cardiovascular risk: focus on incretins. Cardiovasc Diabetol 10:61
Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Fioretto P, Mauer M, Fioretto P (2000) Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes Metab 4:8–14
Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C (2010) NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol 42:141–150
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Takeuchi M, Yamagishi S (2009) Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer’s disease. J Alzheimers Dis 16:845–858
Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM (1999) N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 274:31740–31749
Jung HA, Park JJ, Islam MN, Jin SE, Min BS, Lee JH, Sohn HS, Choi JS (2012) Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch Pharm Res 35:1021–1035
Nurul Islam M, Jung HA, Sohn HS, Jin SE, Min BS, Lee JH, Sohn HS, Choi JS (2013) Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm Res 36:542–552
Kang KS, Lee W, Jung Y, Lee JH, Lee S, Eom DW, Jeon Y, Yoo HH, Jin MJ, Song KI, Kim WJ, Ham J, Kim HJ, Kim SN (2014) Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J Agric Food Chem 62:2069–2076
Shepherd J, Kastelein JJP, Bittner VA, Carmena R, Deedwania PC, Breazna A, Dobson S, Wilson DJ, Zuckerman AL, Wenger NK (2008) Intensive lipid lowering with atorvastatin in patients with coronary artery disease, diabetes, and chronic kidney disease. Mayo Clin Proc 83:870–879
Tiwari S, Riazi S, Elcebarger CA (2007) Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Ren Physiol 293:F974–F984
Louro TM, Matafome PN, Nunes EC, da Cunha FX, Seiça RM (2011) Insulin and metformin may prevent renal injury in young type 2 diabetic Goto-Kakizaki rats. Eur J Pharmacol 653:89–94
Balakumar P, Chakkarwar VA, Krishan P, Singh M (2009) Vascular endothelial dysfunction: a tug of war in diabetic nephropathy? Biomed Pharmacother 63:171–179
Early KA, Harry D, Zitouni K (2008) Circulating cholesterol as a modulator of risk for renal injury in patients with type 2 diabetes. Diab Res Clin Pract 79:68–73
Cahde AR, Lerman A, Lerman LO (2005) Kidney in early atherosclerosis. Hypertension 45:1042–1049
Grone EF, Grone HJ (2008) Does hyperlipidemia injure the kidney? Nat Clin Pract Nephrol 4:424–425
Pokharel YR, Lee MY, Kim SK, Bae K, Su ND, Oh WK, Kang KW (2009) Dual anti-oxidative effects of fraxetin isolated from fraxinus rhinchophylla. Biol Pharm Bul 32:1527–1532
Naaz F, Abdin MZ, Javed S (2014) Protective effect of esculin against prooxidant aflatoxin B1 -induced nephrotoxicity in mice. Mycotoxin Res 30:25–32
Cardellini M, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Perticone F, Federici M, Lauro D, Sesti G (2007) Carotid artery intima-media thickness is associated with insulin-mediated glucose disposal in nondiabetic normotensive offspring of type 2 diabetic patients. Am J Physiol Endocrinol Metab 292:E347–E352
Kadian S, Mahadevan N, Balakumar P (2013) Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy. Eur J Pharmacol 698:388–396
Pei F, Li BY, Zhang Z, Yu F, Li XL, Lu WD, Cai Q, Gao HQ, Shen L (2014) Beneficial effects of phlorizin on diabetic nephropathy in diabetic db/db mice. J Diabetes Complications 28:596–603
Grandhee SK, Monnier VM (1991) Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem 266:11649–11653
Ahmed N, Babaei-Jadidi R, Howell SK, Thornalley PJ, Beisswenger PJ (2005) Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes. Diabetes Care 28:2465–2471
Jandeleit-Dahm K, Watson A, Soro-Paavonen A (2008) The AGE/RAGE axis in diabetes-accelerated atherosclerosis. Clin Exp Pharmacol Physiol 35:329–334
Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM (2005) Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54:3103–3111
Nożyński J, Zakliczyński M, Konecka-Mrowka D, Zielinska T, Zakliczynska H, Nikiel B, Mlynarczyk-Liszka J, Mrowka A, Zembala-Nozynska E, Pijet M, Rdzanowska K, Lange D, Przybylski R, Zembala M (2012) Advanced glycation end product accumulation in the cardiomyocytes of heart failure patients with and without diabetes. Ann Transplant 17:53–61
Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M (2005) Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 54:3274–3281
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM (2009) RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20:742–752
Fukami K, Yamagishi S, Coughlan MT, Harcourt BE, Kantharidis P, Thallas-Bonke V, Okuda S, Cooper ME, Forbes JM (2014) Ramipril inhibits AGE-RAGE-induced matrix metalloproteinase-2 activation in experimental diabetic nephropathy. Diabetol Metab Syndr 6:86
Fukami K, Yamagishi S, Ueda S, Okuda S (2008) Role of AGEs in diabetic nephropathy. Curr Pharm Des 14:946–952
Yamagishi S, Matsui T, Nakamura K, Inoue H, Takeuchi M, Ueda S, Okuda S, Imaizumi T (2008) Olmesartan blocks inflammatory reactions in endothelial cells evoked by advanced glycation end products by suppressing generation of reactive oxygen species. Ophthalmic Res 40:10–15
Thomas MC, Forbes JM, Cooper ME (2005) Advanced glycation end products and diabetic nephropathy. Am J Ther 12:562–572
Nakashima S, Matsui T, Takeuchi M, Yamagishi SI (2014) Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis. Horm Metab Res 46:717–721
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (81473383) and the National Science and Technology Major Projects (2012ZX09103101-078; 2013ZX09103-001-008; 2013ZX09102106).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WYH, LY and DGH designed the experiments; WYH, LYH, and HGR performed the experiments; WYH and HGR analyzed the data; WYH and DGH wrote the paper. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, YH., Liu, YH., He, GR. et al. Esculin improves dyslipidemia, inflammation and renal damage in streptozotocin-induced diabetic rats. BMC Complement Altern Med 15, 402 (2015). https://doi.org/10.1186/s12906-015-0817-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-015-0817-y