Abstract
Background
There is increasing recognition that prehabilitation is important as a means of preparing patients physically and psychologically for cancer treatment. However, little is understood about the role and optimal nature of prehabilitation for gynaecological cancer patients, who usually face extensive and life-changing surgery in addition to other treatments that impact significantly on physiological and psychosexual wellbeing.
Review question
This scoping review was conducted to collate the research evidence on multimodal prehabilitation in gynaecological cancers and the related barriers and facilitators to engagement and delivery that should be considered when designing a prehabilitation intervention for this group of women.
Methods
Seven medical databases and four grey literature repositories were searched from database inception to September 2021. All articles, reporting on multimodal prehabilitation in gynaecological cancers were included in the final review, whether qualitative, quantitative or mixed-methods. Qualitative studies on unimodal interventions were also included, as these were thought to be more likely to include information about barriers and facilitators which could also be relevant to multimodal interventions. A realist framework of context, mechanism and outcome was used to assist interpretation of findings.
Results
In total, 24 studies were included in the final review. The studies included the following tumour groups: ovarian only (n = 12), endometrial only (n = 1), mixed ovarian, endometrial, vulvar (n = 5) and non-specific gynaecological tumours (n = 6). There was considerable variation across studies in terms of screening for prehabilitation, delivery of prehabilitation and outcome measures. Key mechanisms and contexts influencing engagement with prehabilitation can be summarised as: (1) The role of healthcare professionals and organisations (2) Patients’ perceptions of acceptability (3) Factors influencing patient motivation (4) Prehabilitation as a priority (5) Access to prehabilitation.
Implications for practice
A standardised and well evidenced prehabilitation programme for women with gynaecological cancer does not yet exist. Healthcare organisations and researchers should take into account the enablers and barriers to effective engagement by healthcare professionals and by patients, when designing and evaluating prehabilitation for gynaecological cancer patients.
Similar content being viewed by others
Introduction
Prehabilitation offers the opportunity to improve patients’ physical and mental function, through buffering the deconditioning related to cancer treatments between the time of diagnosis and recovery [1]. Prehabilitation has been shown to reduce pulmonary and overall morbidity and improve post-operative gait, cardiovascular function and urinary continence in those undergoing major cancer surgeries [1, 2] It also has the potential to improve health related quality of life in the longer term [3]. Multimodal programmes generally consist of a combination of medical management, physical activity, nutrition and psychological wellbeing and are considered more effective than standard care approaches or unimodal interventions [4, 5]. Gynaecological cancers consist of vulvar, cervical, vaginal, endometrial, and ovarian tumours. The latter in particular are associated with increased mortality and morbidity, often due to late and advanced presentation [6, 7]. Women with endometrial cancer have better survival overall, but over 50% are obese and therefore at risk of cardiovascular disease and other co-morbidities [8]. Suboptimal conditioning prior to surgery is likely to exacerbate post-treatment side-effects already experienced by gynaecological cancer patients undergoing chemotherapy and radiotherapy, such as gastrointestinal and sexual dysfunction, urinary incontinence, menopause and lymphoedema [9, 10]. This in turn, costs healthcare services a significant amount of money in rehabilitation [11].
The potential for prehabilitation in gynaecological cancers has been recognised [12] but little is known about the specific prehabilitation needs of women facing gynaecological cancer treatment and the barriers and facilitators influencing engagement in and outcomes of prehabilitation. This is important to the targeting and personalisation of prehabilitation programmes to enhance uptake and effectiveness [13].
Methodology
The aim of this scoping review was to explore the empirical and theoretical evidence for multimodal prehabilitation amongst women with gynaecological cancers, with particular emphasis on the enablers and barriers to prehabilitation delivery, engagement, and adherence in this patient group. Scoping reviews are particularly relevant for examining the extent, range and nature of the evidence on a topic and for summarising findings from a heterogeneous body of knowledge [14]. This review used a realist lens to enable a detailed exploration of factors likely to influence the success of a complex intervention, such as prehabilitation [15]. Realist approaches focus on the contexts and mechanisms that lead to particular outcomes, thus helping to explain how and why interventions may or may not work [16]. Other reviewers have combined scoping and realist approaches to understand complex contexts [17].
This review follows the Joanna Briggs Institute (JBI) guidelines for scoping reviews [18], Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [14]. The review protocol has been published in an open access forum [19].
The review was conducted following the key steps outlined by JBI:
1. Define the review questions 2. Determine the inclusion criteria 3. Search strategy 4. Evidence screening and selection 5. Data extraction 6. Data analysis 7. Presentation of the results [18].
Step 1 Define the review questions Given the complexity of prehabilitation as an intervention, it is important not only to understand what has worked or is perceived to work based on measured or predicted outcomes, but also the mechanisms and the context which may operate as facilitators and barriers, and thus influence the success of a prehabilitation intervention [20]. Our research questions were:
-
1.
How does the gynaecological cancer literature define ‘prehabilitation’?
-
2.
What are the intended and unintended outcomes for gynaecological cancer patients participating in a prehabilitation programme?
-
3.
What are the key components, skills and contexts required by the healthcare team to implement a successful prehabilitation programme in this population?
-
4.
What are the facilitators and barriers to engaging in prehabilitation amongst patients with gynaecological cancers?
Step 2 Determine the inclusion criteria To be included in this review, all studies needed to investigate and or report on the role, impact and/or influencers surrounding prehabilitation, from the perspectives of either gynaecological cancer patients and/or health professionals. All study designs were included in this scoping review, on the basis that that they met the inclusion criteria outlined in Table 1. Study abstracts as well as protocols for ongoing trials of relevant multimodal prehabilitation interventions were included in the final review as the authors felt these provided key insights into the nature, delivery and intended outcomes of prehabilitation interventions. Quantitative studies and protocols were included only if they addressed multimodal prehabilitation programmes. Qualitative studies describing unimodal programmes were also included, as their results were likely to be beneficial in understanding facilitators and barriers which could also be relevant to taking part in multimodal programmes.
Any articles published in a language other than English, were excluded due to limited translation resources.
Step 3 Search Strategy All searches for relevant literature were carried out by the research librarian following discussion with the research team to predefine search terms (see Additional file 1). Articles were retrieved on the 6th October 2021 using the major search terms ‘gynaecology’ ‘cancer’ and ‘prehabilitation’ from the date of database inception to September 2021. A comprehensive set of seven databases were searched using the National Health Service’s Healthcare Database (HDAS) to encompass medical, nursing, allied health and psychological literature relevant to multimodal prehabilitation. These included Allied and Complementary Medicine Database (AMED), British Nursing Index (BNI), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Emcare, Medical Literature Analysis and Retrieval System Online (MEDLINE) and Psychological Information Database (PsycINFO). Since the search was conducted, HDAS has been discontinued, however, the underlying databases remain available via other platforms and the same search strategy can be replicated. The search was also conducted in the Cochrane Library platform, across the Cochrane Database of Systematic Reviews, Central Register of Clinical Trials and Cochrane Clinical Answers, using an identical set of keywords and subject headings to the MEDLINE version of the original search. Additionally a search for grey literature was conducted in National Institute for Health and Care Excellence (NICE) Evidence Search and Turning Research into Practice (TRIP) Database, and using the search engine Google. In depth search strategies can be found in Additional file 1: Tables S1 and S2. For completeness, the reference lists of all included papers were reviewed for possible inclusion.
Step 4 Evidence Screening and Selection Duplications were removed from all retrieved articles using HDAS’ deduplication function. All retrieved abstracts were uploaded to Covidence, for independent screening by the first and last author. Full texts of papers for possible inclusion were then reviewed by the same authors using Covidence [21]. Any disagreement in decision making was discussed and consensus was reached between the two reviewers at each stage of the screening, thus, a third reviewer was not required. We did not conduct critical appraisal as this is not generally recommended in scoping reviews [18].
Steps 5 and 6 Data extraction and analysis All data were extracted using the JBI Reviewers’ Manual as a guide [22]. A summary table was compiled to include details of title, year of publication, country, study design, sample, key findings related to the scoping review, strengths and limitations (Table 2). Additionally, data on all interventions, including those described within registered trial protocols, were categorised using the Template for Intervention Description and Replication (TIDieR) checklist [23] (Table 3). This allows a more detailed understanding of the components of interventions, how they are delivered and tailored and how they are evaluated. Findings related to barriers and facilitators to engagement with prehabilitation were considered in relation to the Context-Mechanism-Outcome framework [22].
Results
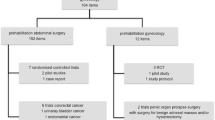
In total, 24 studies were included in this scoping review (Fig. 1) and the results of the review are presented in narrative form, Tables 2, 3 and Fig. 2.
Overview of studies
The 24 studies included nine registered protocols for randomised controlled trials of prehabilitation interventions [24,25,26,27,28,29,30,31,32], six observational studies (of which four were abstracts only) [33,34,35,36,37,38], three mixed-methods studies [39,40,41], two qualitative studies [42, 43], two cross-sectional surveys [44, 45], one cost-effectiveness analysis [46] and one systematic review [47] (Table 2).
The largest number of studies originated from the USA (n = 7). Other countries of origin were UK (n = 3), Denmark (n = 3), France (n = 3), India (n = 2), Spain (n = 2), Brazil (n = 1), Prague (n = 1), China (n = 1), The Netherlands (n = 1). UK data were limited to three abstracts describing pilot interventions [33,34,35], two of which related to ovarian cancer patients [33, 34], whilst the remaining abstract included only one gynaecological cancer patient in the study population [35]. The majority (n = 12) of studies included patients with ovarian cancer only. Six studies described their populations as ‘gynaecological cancers’ and five included a mixed group of ovarian, endometrial and vulvar cancers. One study included patients with endometrial cancer only. No relevant studies were found which included cervical or vaginal cancers. Sample sizes ranged from 1 to 194 gynaecological patients and the mean age range of participants was 58–88 years old, although some trials are open to patients from 16 years and above.
How gynaecological cancer studies define prehabilitation
Prehabilitation interventions included in this review varied in their nature and duration. Our eligibility criteria excluded unimodal intervention studies e.g., those focussed on physical activity or nutritional optimisation only, but in fact these were also labelled as prehabilitation. Other studies described enhanced recovery after surgery (ERAS) programmes as prehabilitation. The authors of this scoping review agreed that ERAS is a separate intervention, although it might complement prehabilitation to provide effective pre-operative work up. Therefore, studies which included multimodal prehabilitation as a component of ERAS or in addition to it, were included in the present review, but studies referring to ERAS alone were excluded.
Sixteen studies described multimodal prehabilitation interventions. The three mixed-methods studies were from the same research group and described the same prehabilitation intervention, so these were considered as one study (Table 3).
These interventions all varied in terms of programme setting, nature and delivery of prehabilitation, participant criteria, duration of prehabilitation and measured outcomes. The reported duration of prehabilitation ranged from 2 weeks to 12 months, but most studies were unclear about the duration of intervention or contact time with health professionals in the prehabilitation period. Few studies provided a comprehensive description of all aspects of their prehabilitation intervention. Only two studies reported using theory to underpin the design of their complex intervention.
In terms of programme setting, most interventions adopted entirely remote supervision (n = 8) of which some were reliant on wearable technology and smartphone applications (n = 3). Some interventions were supervised face to face (n = 1) whilst others provided flexibility between facility-based supervision and remote supervision (n = 2). The remaining studies were unclear about the programme setting (n = 6). Only a few studies stated explicit involvement of a multidisciplinary team to deliver the individual components of prehabilitation (n = 8).
All programmes featured a physical activity and nutrition component (n = 16) and the majority of these also included a psychological component (n = 13). Interventions also included pharmaceutical reconciliation (n = 1), smoking cessation (n = 1), alcohol and smoking cessation (n = 1), anaemia management (n = 1) and pre-operative anaesthetic review (n = 1).
Physical activity
Most of the interventions used screening tools to obtain baseline parameters for physical fitness, from which physical activity was prescribed. The 6- minute walk test (6MWT), a measure of mobility related function in older adults was commonly used. Other screening measures included grip strength, the maximum rate of oxygen the body is able to use during exercise (VO2 max), Five times Sit to Stand, The 5-item FRAIL scale (Fatigue, Resistance, Ambulation, Illnesses, & Loss of Weight), Short Questionnaire to Assess Health-Enhancing Physical Activity (SQUASH) and The International Physical Activity Questionnaires (IPAQ). Less than 50% of the interventions described their physical activity component in detail, beyond ‘physical therapy’ intervention or ‘exercise’. Of those which did, cardiovascular exercise to increase the heartrate, resistance training, circuit training and increasing daily step count were mentioned. Approved resources such as the Macmillan Cancer Support ‘Move More’ booklet were provided to all participants in one study.
Nutrition
Screening tools were utilised by studies to assess for the risk of malnutrition, however, this was not as common as screening for physical function. Taking baseline anthropometry was the most common method of nutritional screening. Some studies utilised validated tools such as the Patient Generated-Subjective Global Assessment (PG-SGA), whilst a few used the Malnutrition Universal Screening Tool (MUST) or an adapted version of it. Interventions were commonly described as ‘dietetic consultation’ or ‘nutritional input, education or activity’. Two interventions made specific recommendations around increasing dietary protein and one intervention focussed on using a soy-based probiotic.
Psychological wellbeing
Several validated tools were used to establish baseline psychological health and wellbeing amongst participants including the Short Form -36 questionnaire (SF-36) and Hospital Anxiety and Depression Score (HADS). However, the descriptions of psychological interventions ranged from being vague i.e., ‘psychological help’, ‘counselling’, ‘support’ and ‘coping strategies’ to more specific techniques like relaxation, mindfulness, cognitive behavioural therapy and motivational interviewing.
The intended and unintended outcomes of participation in prehabilitation for gynaecological cancer patients
Interventional studies and protocols reported a wide variety of intended outcomes. These include improvements in physical conditioning and function (n = 10), post-operative complications (n = 7), quality of life (n = 7), nutritional status (n = 6), adherence to advice (n = 3), length of stay (n = 3), qualitative outcomes (n = 3), readmissions (n = 2), delays in surgery (n = 1), patient volume (n = 1), innate immune response (n = 1), cost-effectiveness (n = 1), sleep (n = 1), general symptoms (n = 1), progression free survival (n = 1) and overall survival (n = 1).
Only a few published studies reported actual outcomes of their prehabilitation programme, the majority of which were positive. Even fewer papers discussed unintended outcomes of their interventions i.e. adverse or surprising outcomes. In a UK based tertiary centre, multimodal prehabilitation delivered remotely during neoadjuvant chemotherapy to twenty five ovarian cancer patients led to a significant reduction (24–4%) in delays to major debulking surgery [33]. A case study of an octogenarian undergoing a hysterectomy for endometrial cancer found that a three week, tailored, multidisciplinary led, home-based prehabilitation programme improved her functional and mental capacity post-operatively but did not improve her overall nutritional status [36].
A mixed-methods study, in which thirty ovarian cancer patients were given written multimodal recommendations including exercises to increase the heart rate found that activity increased overall, mainly through walking and cycling [39]. However, many participants reported preparedness in additional ways to those recommended by the leaflet. For example, practical activities such as gardening, household cleaning, bag packing etc. Whilst this was not an intended outcome, the authors commented on the importance of encouraging tasks which contribute to the recovery period as a future consideration for prehabilitation programmes. In the same study, all participants were provided with information and resources on smoking cessation, however, none of the six smokers stopped smoking during the pre-operative period. Miralpeix et al. suggest the use of a hospital pulmonary programme, consisting of behavioural support and nicotine replacement therapy to support smoking cessation [47], however, this recommendation formed part of a theoretical model generated by the authors, the outcomes of which are not yet known.
The key components, skills and contexts required by the healthcare team to implement a successful prehabilitation programme
Only four studies provided information about the components (e.g. guidelines, defined roles), skills (knowledge) and contexts (capacity and cost-effectiveness) required for healthcare teams. The only qualitative study to investigate the views of healthcare professionals found that clinicians value having a strong evidence base in order to advocate prehabilitation [43]. Defined roles for all members of the multidisciplinary team were also considered essential to streamline the process of prehabilitation. Oncologists in this study did not feel they had the capacity to oversee prehabilitation, therefore the authors presented a model in which the clinical nurse specialist was at the core of screening and triage, provided there were clear guidelines and screening tools available to support their role.
A cross-sectional survey of peri-operative practices amongst 100 surgical and gynaecological oncologists in India found that 98% of respondents advised prehabilitation, of which 71% recommended trimodal interventions (physical activity, nutrition and psychological input) [44]. In another survey of 136 Indian anaesthesiologists, gynaecological oncologists, and intensivists, 76% recommended preoperative exercise and even greater proportion recommended correction of anaemia, smoking cessation, and alcohol consumption. Immunonutrition was the least recommended intervention [45]. Interestingly, based on the survey responses, the psychological component of prehabilitation was considered non-essential. Only one study provided data from an organisational perspective. A cost-effectiveness study based on model inputs in the USA suggested that prehabilitation could potentially save over $9,000 per patient in a cohort of over 4,000 women [46].
The facilitators and barriers to participating in prehabilitation amongst patients with gynaecological cancers
The existing evidence provides useful insights into key mechanisms and contexts acting as facilitators or barriers to engagement with prehabilitation. These can be summarised in the following themes: (1) Factors affecting patients’ views of the acceptability of prehabilitation (2) Factors affecting the motivation of patients to engage in prehabilitation (3) Prehabilitation as a priority (4) Access to prehabilitation.
Factors affecting patients’ views of the acceptability of prehabilitation
Very few studies directly explored the acceptability of prehabilitation from the patient’s perspective. Of nine protocols, only two documented their intention to assess acceptability and or satisfaction with the programme through exit interviews/questionnaires [25, 28]. However, all qualitative studies [26, 28], 32,33,34 reported something about the acceptability of prehabilitation from the patients’ perspective, mostly suggesting that patients are positive about engaging with prehabilitation due to the perceived and actual health benefits.
For a cohort of women in Denmark, prehabilitation was considered acceptable if it fitted in with their everyday lives and allowed them to carry out other tasks which helped them ‘prepare’ for surgery, such as meal preparation, laundry, gardening [40, 41]. Following the recommendations provided, women were ready to accept prehabilitation as being beneficial for health and wellbeing, but spending time with loved ones, funeral planning and finances were considered equally as important by some.
Ovarian cancer patients undergoing neo-adjuvant chemotherapy in the USA, were willing to engage in exercise despite lack of participation in structured physical activity at the point of diagnosis [42]. In depth interviews with those who were prehabilitation-naïve revealed that patients were theoretically willing to undertake 15–30 min of exercise on 3–7 days of the week. Activities such as walking, strength training and yoga/stretching were considered most acceptable.
Only one study commented on the acceptability of nutritional recommendations, in which patients felt that nutritional optimisation extended beyond the recommendations of a ‘high protein’ diet and should be more inclusive to fruits and vegetables [40]. Studies reporting baseline characteristics of participants found high rates of sarcopenia and malnutrition [33, 37, 38] and it is therefore unsurprising that nutritional components of prehabilitation are focussed around increasing protein intake [39, 47]. No studies specifically reported on the acceptability of psychological components.
Factors affecting the motivation of patients to engage in prehabilitation
Motivation appears to be a key mechanism influencing the engagement of patients with prehabilitation. Qualitative studies have revealed that patients believe that prehabilitation is beneficial to their health and wellbeing, treatment-related outcomes and cancer-related outcomes [39, 41, 42], and as such, these beliefs are motivating. Patients who participated in a UK based multimodal prehabilitation programme reported being more motivated to make long term lifestyle changes, as did their families [35].
The need for a support system to motivate patients was also identified. In three qualitative studies with ovarian cancer patients, support systems were available through colleagues, friends, and/or healthcare professionals [35, 42, 43]. However, one study found that some patients preferred not to ‘burden’ family members by relying on them, and therefore, identified healthcare professionals as the most appropriate motivators [41].
In addition to having a human support system, participants taking part in remote prehabilitation interventions identified progress tracking in the form of pedometers and diaries as highly motivating [40, 41, 43]. This supports the use of wearable devices in several of the trial protocols [31] whereby patients will have real-time fitness measures and outcomes that can be reported to healthcare professionals.
Prehabilitation as a priority
Another key factor influencing engagement is the degree to which patients prioritise prehabilitation, specifically in the context of time to treatment. Qualitative studies in Denmark and The Netherlands highlighted patients’ concerns around the short duration between diagnosis and surgery [41, 43]. With as little as a 1–2 week pre-operative period, patients felt the need to prioritise preparing for ‘life and death’ such a socialising, financial tasks and life administration.
A concern raised by patients was the large amount of time already spent in the hospital for appointments, and the possibility that prehabilitation programmes would require further attendance [43]. To address these concerns, van der Zanden et al. asked patients and healthcare professionals for their opinions on delaying surgery to allow for more pre-operative optimisation. They concluded that patients are unlikely to delay due to anxiety and a lack of evidence base supporting the decision to postpone surgical intervention. [43]. Several studies took advantage of three to six cycles of neo-adjuvant chemotherapy as a period for prehabilitation prior to surgery [31, 33, 34].
Access to prehabilitation
Qualitative findings suggested that prehabilitation needed to be locally accessible due to the cost of transport, appointment burden and limited pre-operative time [42, 43]. Several interventions included remote/home based interventions in their design [31, 33, 35, 39]. In the ‘Marsden Mile’ programme, initial results had revealed poor attendance to facility-based exercise [33], which led to the development of an entirely remote programme [34]. Lack of attendance to facility-based sessions was mainly due to ill-health, a finding highlighted in several other qualitative studies [41,42,43]. In these studies, patients suggested that their physical and mental health can act as barriers to engaging with society as well as the activities expected of them as part of prehabilitation.
No studies discussed whether ethnicity or age affected the accessibility of their prehabilitation interventions, although Polen De et al. did comment on the potential limitations of their entirely Caucasian cohort [41]. Three of the trial protocols excluded patients on the basis of poor understanding or inability to speak/write the primary language [32].
Figure 2 synthesises the contexts and mechanisms influencing engagement with prehabilitation. It illustrates the factors contributing to healthcare professional and patient engagement.
Discussion
This scoping review aimed to summarise the quantitative and qualitative evidence for prehabilitation in women with gynaecological cancers, using a realist approach. To our knowledge, this is the first review to do this. We were already aware that there are no published trials for multimodal prehabilitation within gynaecological cancer [48], however our review provides a summary of several ongoing randomised controlled trials for which protocols have been published. Pilot observational studies suggest that prehabilitation is beneficial for this group, however, sample sizes of gynaecological cancer patients have been small and results are mostly limited to published abstracts [33,34,35, 38]. Our findings reveal several barriers and facilitators which need to be taken into account in future prehabilitation interventions for this group.
We acknowledge the limitations of this review. Firstly, it is possible that studies may have been missed by database searching as well those which were published after the search date. Secondly, this review only included studies with multimodal programmes involving more than one non-medical intervention, due to their perceived ability of meeting the complex needs of cancer patients. Therefore, studies reporting on unimodal prehabilitation programmes or those concentrating on medical management and optimisation, may have been missed.
Although descriptions of the interventions included in the scoping review are limited, our analysis of the contexts, mechanisms, and outcomes for prehabilitation provide useful insights into the factors that need to be considered in the design and implementation of prehabilitation for women with gynaecological cancer. It is now widely understood that the success of a complex intervention depends on the theory underpinning its design [44], which helps to explain the mechanisms underlying patient behaviour, based on what works for them and their circumstances [45]. Unfortunately, however, only two interventions found in the present study described the use of a logic change model [30] and framework [49] in their development. Moreover, few evaluated the acceptability of their interventions, despite this being an important consideration for complex interventions [50].
One study presented a working prehabilitation template for women undergoing surgery [47], and whilst it is detailed, flexible and plausible, it does not fully reflect the factors that might influence engagement with prehabilitation that we have identified. The qualitative literature in this field illustrates the complexity of delivering prehabilitation and sheds light on some of these factors. Our review suggests that both patients’ and healthcare professionals’ needs, views and respective roles must be considered in a successful prehabilitation programme. In order for healthcare professionals to engage with and deliver prehabilitation, they need a strong evidence base for prehabilitation within gynaecological cancer; defined roles for delivering prehabilitation within the multidisciplinary team and clear guidance around screening and triage of patients. Given that the existing literature does not yet provide strong evidence and clear guidance, engaging healthcare professionals may be challenging at this time.
The included studies suggest that patients value accessible prehabilitation services that are supported by a knowledgeable and motivated multidisciplinary team. Although it seems that surgical and gynaecological oncologists in some countries actively recommend prehabilitation as part of peri-operative management [29, 30], and that many believe it is important, there is a lack of awareness amongst professionals of the availability of prehabilitation services [49]. This suggests that there is still work to be done to educate the workforce around prehabilitation and to develop effective referral pathways between primary and secondary care.
Ease of access to prehabilitation emerged as an important factor. The coronavirus pandemic has accelerated the trend towards remotely delivered interventions, and several of the ongoing trials identified in this review utilise home-based prehabilitation models. Completed studies suggest that home-based multimodal prehabilitation is feasible and leads to improvements in a range of outcomes [51]. However, findings from qualitative studies reveal the importance of accessible support and supervision as a motivator, either through an opportunity to meet others face to face or to monitor and encourage patients to keep on track with their prehabilitation goals. The potential for digital interventions in this field is huge, but lack of access, confidence and competence in relation to technology can present obstacles [31]. Given that gynaecological cancers are more common in those aged 75–79 years old [52, 53], the confidence, skills and access to technology in an older population must be considered.
Whilst there will be emerging evidence from ongoing randomised controlled trials, the heterogeneity of study designs, programme settings, participant eligibility criteria and measured outcomes is significant. The majority of multimodal prehabilitation programmes do incorporate trimodal components encompassing physical activity, nutrition, and psychological interventions. Some also include smoking and alcohol cessation and medication reconciliation whilst others omit the psychological component of prehabilitation. The way in which the individual components of the programme are delivered and what is expected of patients also differ widely across the trials. Outcome measures for post-operative complications, cardiovascular health, functional activity, and health related quality of life are generally included in most studies, however, there are no two trials which have the same set of primary and secondary outcomes.
The lack of standardisation across interventions and outcome measures means that concluding benefit in future work through a meta-analyses may prove challenging. The inability to draw significant improvement benefit of prehabilitation due to the heterogeneity of studies was recently seen in a systematic review in hepatobiliary cancers [54] and has led to a call for standardisation amongst the colorectal community [55]. Greater consistency of outcome measures would also strengthen the evidence base in gynaecological cancer.
It is worth highlighting that the majority of ongoing studies focus on patients with ovarian cancer rather than other gynaecological cancers. This is unsurprising given the high incidence of comorbidity and sarcopenia in this group, as well as the need for pre-operative conditioning prior to major abdominal surgery [7]. However, some cancer centres work under guidance to perform primary debulking surgery for ovarian cancer within two weeks from diagnosis [56], which leaves a very short window of opportunity for prehabilitation. The findings of this review suggest that women may find it difficult to achieve prehabilitation goals as well as to come to terms with diagnosis and prepare for ‘life and death’ during this limited period. Prehabilitation programmes may also need to address issues that are at the forefront of patients’ minds, including socialising, domestic tasks, financial preparation and legal paperwork [39, 41].
Qualitative studies included in this review have focussed primarily on White cohorts [39,40,41,42,43]. Although the incidence of gynaecological cancers is greater amongst White women in the UK [57], there is evidence of increasing incidence and mortality related to endometrial cancer in Black women [57]. This points to a need to ensure that future studies reflect our diverse population and shed light on the factors which influence engagement with prehabilitation amongst different racial groups and ethnic minorities.
Conclusion
This scoping review illustrates that the evidence for prehabilitation in gynaecological cancer patients is limited, although there are several randomised controlled trials underway. Since a standardised and well accepted prehabilitation programme for this cohort does not yet exist, healthcare organisations and researchers should consider the factors affecting the delivery and engagement of health professionals and patients when designing one. This means taking in to account the needs, knowledge and capacity of healthcare professionals as well as the practical considerations around patient accessibility and acceptability of prehabilitation in the context of wider preparation following a cancer diagnosis. The findings of this review provide important insights into these issues.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files.
Abbreviations
- 5STS:
-
Five times sit to stand
- 6MWT:
-
Six-minute walk test
- BMI:
-
Body mass index
- CASP:
-
Critical appraisal skills programme
- ERAS:
-
Enhanced recovery after surgery
- FACT-G:
-
Functional assessment of cancer therapy-general
- FRAIL scale:
-
Fatigue, resistance, ambulation, illnesses, and loss of weight
- GLIM:
-
Global leadership initiative on malnutrition
- HADS:
-
Hospital anxiety and depression scale
- IPAQ:
-
International physical activity questionnaire
- JBI:
-
Joanna Briggs institute
- MUST:
-
Malnutrition universal screening tool
- NACT:
-
Neoadjuvant chemotherapy
- PG SGA-SF:
-
Patient generated subjective global assessment short form
- PRISMA:
-
Preferred reporting items for systematic reviews
- QLQ-C30:
-
Quality of life questionnaire for cancer patients
- RBANS:
-
Repeatable battery for the assessment of neuropsychological status
- RAMSES:
-
Realist and meta-narrative evidence syntheses-evolving standards
- SF-12:
-
12-item short form survey
- SF-36:
-
36-item short form survey
- SQUASH:
-
Short questionnaire to assess health enhancing physical activity
- TIDieR:
-
Template for intervention description and replication
- VO2 max:
-
Maximum oxygen consumption
- WAIS:
-
Wechsler adult intelligence scale
References
Faithfull S, Turner L, Poole K, Joy M, Manders R, Weprin J, et al. Prehabilitation for adults diagnosed with cancer: a systematic review of long-term physical function, nutrition and patient-reported outcomes. Eur J Cancer Care. 2019;28(4): e13023.
Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. 2019;43(7):1661–8.
Ngo-Huang A, Parker NH, Bruera E, Lee RE, Simpson R, O’Connor DP, et al. Home-based exercise prehabilitation during preoperative treatment for pancreatic cancer is associated with improvement in physical function and quality of life. Integr Cancer Ther. 2019;1(18):1534735419894061.
Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol. 2017;56(2):295–300.
Brahmbhatt P, Minnella EM, Randall IM, Santa MD. Multimodal prehabilitation: a mini review of contemporary research. Curr Anesthesiol Rep. 2021. https://doi.org/10.1007/s40140-021-00490-1.
Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Prev Biomark. 2017;26(4):632–41.
Fotopoulou C, Planchamp F, Aytulu T, Chiva L, Cina A, Ergönül Ö, et al. European society of gynaecological oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int J Gynecol Cancer. 2021;31(9):1199–206. https://doi.org/10.1136/ijgc-2021-002951.
Papatla K, Huang M, Slomovitz B. The obese endometrial cancer patient: how do we effectively improve morbidity and mortality in this patient population? Ann Oncol. 2016;27(11):1988–94.
Muls A, Taylor A, Lalondrelle S, Kabir M, Norton C, Hart A, et al. A proposed tailored investigational algorithm for women treated for gynaecological cancer with long-term gastrointestinal consequences. Support Care Cancer. 2020;28(10):4881–9.
Grover S, Hill-Kayser CE, Vachani C, Hampshire MK, DiLullo GA, Metz JM. Patient reported late effects of gynecological cancer treatment. Gynecol Oncol. 2012;124(3):399–403.
Muls AC, Lalji A, Marshall C, Butler L, Shaw C, Vyoral S, et al. The holistic management of consequences of cancer treatment by a gastrointestinal and nutrition team: a financially viable approach to an enormous problem? Clin Med Lond Engl. 2016;16(3):240–6.
Schneider S, Armbrust R, Spies C, du Bois A, Sehouli J. Prehabilitation programs and ERAS protocols in gynecological oncology: a comprehensive review. Arch Gynecol Obstet. 2020;301(2):315–26.
Mina DS, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, et al. Multiphasic prehabilitation across the cancer continuum: a narrative review and conceptual framework. Front Oncol. 2021. https://doi.org/10.3389/fonc.2020.598425.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11(1):21.
Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci. 2012;7(1):33.
Myette E, Riva M. Surveying the complex social-ecological pathways between resource extraction and Indigenous Peoples’ health in Canada: A scoping review with a realist perspective. Extr Ind Soc. 2021;8(2): 100901.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement. 2021;19(1):3–10.
Saggu RK (2022) Considerations for multimodal prehabilitation in women with gynaecological cancers: A scoping review using realist principles
Pawson R, Tilley N. An introduction to scientific realist evaluation. In: Chelimsky E, Shadish W, editors. Evaluation for the 21st century: a handbook. Oaks: SAGE Publications; 1997. p. 405–18. https://doi.org/10.4135/9781483348896.n29.
Covidence Better systematic review management [Internet]. Covidence. [cited 2021 Dec 13]. Available from: https://www.covidence.org/
Chapter 11: Scoping reviews - JBI Manual for Evidence Synthesis - JBI Global Wiki [Internet]. [cited 2021 Oct 5]. Available from: https://jbi-global-wiki.refined.site/space/MANUAL/3283910770
Hoffmann T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Die TIDieR Checkliste und Anleitung – ein Instrument für eine verbesserte Interventionsbeschreibung und Replikation. Gesundheitswesen. 2016;78(03):175–88.
NCT04862325. SOPHIE Trial: surgery in Ovarian Cancer With PreHabilitation In ERAS. https://clinicaltrials.gov/show/NCT04862325 [Internet]. 2021 May 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02254917/full
NCT04789694. Prehabilitation in Patients With Advanced Stage Ovarian Cancer Planned for Interval Debulking Surgery. https://clinicaltrials.gov/show/NCT04789694 [Internet]. 2021 Mar 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02249751/full
NCT04596384. Home-Based Telemonitoring Program for Functional Recovery and Symptoms in Gastrointestinal, Genitourinary, or Gynecologic Cancer Patients Undergoing Abdominal Surgery. https://clinicaltrials.gov/show/NCT04596384 [Internet]. 2020 Nov 30 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02196712/full
NCT04505111. Prehabilitation Plus ERAS vs ERAS in Gynecological Surgery. https://clinicaltrials.gov/show/NCT04505111 [Internet]. 2020 Aug 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02137857/full
NCT04451369. Connected Prehabilitation Program During Neo Adjuvant Chemotherapy. https://clinicaltrials.gov/show/NCT04451369 [Internet]. 2020 Jul 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02133995/full
NCT04298827. Gyn Onc Prehab Study. https://clinicaltrials.gov/show/NCT04298827 [Internet]. 2020 Mar 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02088837/full
NCT04284969. PROADAPT-ovary/EWOC-2. https://clinicaltrials.gov/show/NCT04284969 [Internet]. 2020 Mar 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02088523/full
Lambaudie E, Bannier C, Braticevic CV, Goetgheluck CZ, Boher J-M, Soussan PB, et al. TRAINING-Ovary 01 (connecTed pRehabiliAtIoN pelvIc caNcer surGery): multicenter randomized study comparing neoadjuvant chemotherapy for patients managed for ovarian cancer with or without a connected pre-habilitation program. Int J Gynecol Cancer. 2021;31(6):920–4. https://doi.org/10.1136/ijgc-2020-002128.
NL8699. Multimodal intensive prehabilitation in high impact surgery to reduce postoperative complications. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NL8699 [Internet]. 2020 Oct 31 [cited 2021 Oct 8]; Available from: https://www.cochranelibrary.com/central/doi/https://doi.org/10.1002/central/CN-02173254/full
Raman VV, Harvey E, Wills A, Shovel L, Codet-Boisse J, Kasivisvanathan R, et al. Impact of a remote prehabilitation programme in reducing delays to patients having surgery for advanced gynaecological cancer. Anesth Analg. 2021;133(3):1482.
Shovel L, Dunne J, Kasivisvanathan R, Whibley J, Fernandes A. A tertiary cancer centre experience of prehabilitation for surgical ovarian cancer patients receiving neoadjuvant chemotherapy: the royal Mile - Marsden integrated lifestyle and exercise programm. Clin Nutr ESPEN. 2019;31:138.
Knowles C, DunneJ DF, Ashcroft J, Byrne J, Rigby C, Byrne C, Jones L, Fenwick S. Prehab matters: a prehabilitation service for cancer patients undergoing major abdominal surgery. Physiotherapy. 2019;105:e132. https://doi.org/10.1016/j.physio.2018.11.122.
Carli F, Brown R, Kennepohl S. Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesth J Can Anesth. 2012;59(8):779–84.
Ngo-Huang A, Herbert A, Fontillas RC, Parker NH, Asumbrado R, Garg N, Dibaj S, et al. Frequency of sarcopenia, sarcopenic obesity, and changes in physical function in surgical oncology patients referred for prehabilitation. Integr Cancer Ther. 2021;20:153473542110001. https://doi.org/10.1177/15347354211000118.
Ngo-Huang A, Fontillas RC, Gupta E, Sahai SK, Popovich S, Andrabi T, et al. Implementing prehabilitation as part of enhanced recovery after surgery (ERAS) efforts at a comprehensive cancer center: a team-based approach. J Clin Oncol. 2018;36(30_suppl):137–137. https://doi.org/10.1200/JCO.2018.36.30_suppl.137.
Beck A, Vind Thaysen H, Hasselholt Soegaard C, Blaakaer J, Seibaek L. Prehabilitation in cancer care: patients’ ability to prepare for major abdominal surgery. Scand J Caring Sci. 2021;35(1):143–55.
Beck A, Vind Thaysen H, Hasselholt Soegaard C, Blaakaer J, Seibaek L. What matters to you? An investigation of patients’ perspectives on and acceptability of prehabilitation in major cancer surgery. Eur J Cancer Care. 2021;30(6):e13475.
Beck A, Thaysen HV, Soegaard CH, Blaakaer J, Seibaek L. Investigating the experiences, thoughts, and feelings underlying and influencing prehabilitation among cancer patients: a qualitative perspective on the what, when, where, who, and why. Dis Rehabilit. 2022;44(2):202–9.
Polen-De C, Langstraat C, Asiedu GB, Jatoi A, Kumar A. Advanced ovarian cancer patients identify opportunities for prehabilitation: a qualitative study. Gynecol Oncol Rep. 2021;1(36): 100731.
van der Zanden V, van der Zaag-Loonen HJ, Paarlberg KM, Meijer WJ, Mourits MJE, van Munster BC. PREsurgery thoughts – thoughts on prehabilitation in oncologic gynecologic surgery, a qualitative template analysis in older adults and their healthcare professionals. Dis Rehabilit. 2021. https://doi.org/10.1080/09638288.2021.1952319.
Dhiman A, Ray MD. enhanced recovery after gynecological/oncological surgeries: current status in India. Indian J Gynecol Oncol. 2020. https://doi.org/10.1007/s40944-020-00458-9.
Bhandoria G, Solanki SL, Bhavsar M, Balakrishnan K, Bapuji C, et al. Enhanced recovery after surgery (ERAS) in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a cross-sectional survey. Pleura Peritoneum. 2021;6(3):99–111.
Dholakia J, Montemorano L, Cohn DE, Straughn JM, Dilley SE. Prehabilitation is a cost-saving method with improved outcomes for medically frail patients undergoing surgery for epithelial ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2020;159:74.
Miralpeix E, Mancebo G, Gayete S, Corcoy M, Solé-Sedeño JM. Role and impact of multimodal prehabilitation for gynecologic oncology patients in an enhanced recovery after surgery (ERAS) program. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2019;29(8):1235–43.
Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;1(39):156–62.
Beck A, Vind Thaysen H, Hasselholt Soegaard C, Blaakaer J, Seibaek L. Prehabilitation in cancer care: patients’ ability to prepare for major abdominal surgery. Scand J Caring Sci. 2021;35(1):143–55.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17(1):88.
Wu F, Rotimi O, Laza-Cagigas R, Rampal T. The feasibility and effects of a telehealth-delivered home-based prehabilitation program for cancer patients during the pandemic. Curr Oncol. 2021;28(3):2248–59.
Uterine cancer statistics [Internet]. Cancer Research UK. 2015 [cited 2021 Dec 13]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer
Ovarian cancer statistics [Internet]. Cancer Research UK. 2015 [cited 2021 Dec 13]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer
Dagorno C, Sommacale D, Laurent A, Attias A, Mongardon N, Levesque E, et al. Prehabilitation in hepato-pancreato-biliary surgery: a systematic review and meta-analysis. J Visc Surg. 2021. https://doi.org/10.1016/j.jviscsurg.2021.07.003.
Pearson I, Blackwell S, Fish R, Daniels S, West M, Mutrie N, et al. Defining standards in colorectal optimisation: a Delphi study protocol to achieve international consensus on key standards for colorectal surgery prehabilitation. BMJ Open. 2021;11(3): e047235.
Fotopoulou C, Hall M, Cruickshank D, Gabra H, Ganesan R, Hughes C, et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2017;213:123–39.
Shirley MH, Barnes I, Sayeed S, Finlayson A, Ali R. Incidence of breast and gynaecological cancers by ethnic group in England, 2001–2007: a descriptive study. BMC Cancer. 2014;18(14):979.
Acknowledgements
This research project was funded by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC) through a Royal Marsden (RM) Partners Pan London Fellowship. MW is also supported by the NIHR Imperial BRC and CS by the NIHR RM and Institute of Cancer Research BRC. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding
This review has been funded by the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC) through a Royal Marsden Partners Pan London Fellowship. The funders were not involved in the data collection, analysis, interpretation and writing of the study but had input in to its design.
Author information
Authors and Affiliations
Contributions
RKS designed the protocol for the review; carried out data selection, extraction, analysis and drafted the manuscript. PB contributed to the conception of the review and carried out the literature search in all repositories listed in this paper. JB contributed to the conception of the review and drafting the manuscript. SGM contributed to the conception of the review and drafting the manuscript. CH contributed to the conception of the review and drafting the manuscript. PL contributed to the conception of the review and drafting the manuscript. AHM contributed to the conception of the review and drafting the manuscript. CS contributed to the conception and design of the review and substantively revised the manuscript. MW co-designed the review and substantively contributed to the data extraction and analysis. She also substantively revised the manuscript. All authors have approved the submitted version and have agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional File 1
. Extended Search Strategy. Tables highlighting the detailed search strategy for each repository and the removal of deduplications during screening.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saggu, R.K., Barlow, P., Butler, J. et al. Considerations for multimodal prehabilitation in women with gynaecological cancers: a scoping review using realist principles. BMC Women's Health 22, 300 (2022). https://doi.org/10.1186/s12905-022-01882-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01882-z