Abstract
Background
The aim of this study was to evaluate the effects of traditional and whitening toothpastes on the color and surface roughness of different composite resin materials.
Methods
Eighty disc-shaped samples were prepared for each of the following composite resins: nano-hybrid (Filtek Ultimate Universal; 3 M/ESPE, Saint Paul, USA), micro-hybrid (Charisma Smart; Kulzer, Hanau, Germany) and supra-nano-filled (Omnichroma; Tokuyama, Tokyo, Japan). Each composite-resin sample was randomly divided into the following four subgroups (n = 20 per group): Group 1, control; Group 2, traditional toothpaste (Colgate Total 12; Colgate Palmolive, New York, USA); Group 3, peroxide-based toothpaste (Colgate Optic White; Colgate-Palmolive, New York, USA); and Group 4, blue covarine-based toothpaste (Meridol Gentle White; CP-GABA, Hamburg, Germany). The samples for the toothpaste subgroups were immersed in a coffee solution for 10 min and washed twice a day before each brushing cycle. The specimens were brushed for 30 days. Color analyses were performed using a spectrophotometer (SpectroShade Micro, MHT, Italy). Surface roughness analyses were conducted using a profilometer (Surftest SJ-210 Mitutoyo, Tokyo, Japon). The color and surface roughness analyses were performed at baseline and 1, 7 and 30 days after each treatment. Furthermore, surface topography analysis was performed using Scanning Electron Microscopy (FEG 250-FeiQuanta, the Netherlands). The data were analysed with a three-way robust ANOVA and Bonferroni post-hoc correction (p < 0.05).
Results
The smallest color change was observed for the micro-hybrid composite resin, and the greatest color change was observed for the nano-hybrid composite resin. Based on the tested composite resin samples, the greatest color change was obtained after using blue covarine–based toothpaste, while the smallest color change was observed after using peroxide-based toothpaste. Moreover, the supra-nano-filled composite resin samples exhibited the lowest roughness values (robust ANOVA test, p < 0.001). There was no statistically significant difference between the mean values of roughness for the composite, group and time interaction (p = 0.937).
Conclusion
Charisma Smart composite resin exhibited significantly lower staining than all the other composite resins tested after using all toothpastes included in the study. Further laboratory and clinical studies are needed to fully understand the long-term effectiveness of whitening toothpaste on composite resin materials.
Similar content being viewed by others
Introduction
The desire for aesthetic improvement has led to the introduction of numerous materials and methods for teeth whitening and restoration in dentistry [1, 2]. Some people choose home-whitening techniques, including the use of whitening toothpaste, due to favourable bleaching results. Whitening toothpaste provides satisfactory results over a short period of time [3]. This kind of toothpaste usually contains whitening agents and abrasives that can remove extrinsic stains from teeth in a fast, suitable and cheap manner [3,4,5]. Hydrogen peroxide and carbamide peroxide are frequently used as whitening agents in different concentrations. Teeth whitening occurs due to oxy-reduction reactions, as pigments are reduced into smaller molecules with peroxide-containing toothpaste [3, 6]. Furthermore, extrinsic stains can be removed with abrasives. During brushing, abrasive particles become lodged between the bristles of the toothbrush and the surfaces of the teeth. The stains are removed due to the hardness of the abrasive, thus cleaning the surface of the tooth. However, only the extrinsic stains of the tooth are affected by this mechanism rather than the natural tooth color or internal discoloration [3, 7,8,9]. At the same time, optical modifying toothpastes contain pigments, such as blue covarine, which can change the apparent color of teeth by depositing a thin, semi-transparent film of bluish pigment on the dental surface. This film modifies the interaction of incident light, making teeth appear whiter and brighter [7, 10,11,12,13].
The color change of composite resin may be affected by factors such as matrix type, filler type and coloring agents. Discoloration of composite resin can intensify due to contact with staining agents or alcoholic and acidic media, which are present in the diets of most people, further degrading the organic matrix. Roughness is another factor that may cause composite resin discoloration. The surface characteristics of composite resins can be improved using high-quality finishing and polishing methods, as rough surfaces may cause discolorations, plaque accumulation, recurrent caries and gingival irritations, in addition to producing inconvenience and cleaning difficulties [14].
The literature has reported that whitening toothpastes with abrasive particles may produce roughness on the surfaces of composite resin materials [15]. It has been noted that increasing porosity on the surface can change the composite resin by causing volumetric loss or high water withdrawal [16]. During brushing, the polymer matrix of the composite resin can degrade and change the surface hardness of the composite resin, subsequently enhancing pigmentation [8, 17, 18]. Moreover, the composite resin surface remains rough because the bristles of the toothbrush cannot abrade and smoothen fillers in the way that the rubber core or disc used for polishing the material can [8]. Abrasion can lead to changes in the surface of materials, thus affecting the contours and colors of teeth due to surface roughness [18].
In the literature, several clinical and laboratory studies have evaluated the effects of whitening toothpastes on the discoloration and surface roughness of composite resin [9, 16]. However, the recent development of new agents and materials means that our knowledge of this topic is limited [16]. Therefore, the aim of this in vitro study was to evaluate the effects of traditional, peroxide-based and blue covarine-based toothpastes on the color change and surface roughness of nano-hybrid, micro-hybrid and supra-nano-filled composite resin materials. In addition, we analysed the morphological changes in the composite resin surfaces by using scanning electron microscopy (SEM) after the application of the toothpastes. The null hypothesis was that there would be no differences in color change and surface roughness of the nano-hybrid, micro-hybrid and supra-nano-filled composite resin samples after brushing with the selected whitening toothpastes.
Materials and methods
We used three direct restorative materials. Details of the composite resin materials used in the study are provided in Table 1.
Sample size calculation
The software G*Power (G*Power Ver. 3.0.10, Germany) was used to determine the minimum sample size, with 95% statistical significance and 0.80 test power in 1.0 effect size. For this study, the calculated minimum sample size was 17. To prevent possible data loss, three samples were added to each group, as we decided that the study would be performed with 20 samples for each group.
Sample preparation
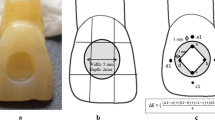
Eighty disc-shaped samples were created for each composite resin using a Teflon matrix (2 mm in height and 10 mm in diameter). Filtek Ultimate Universal and Charisma Smart composite resin materials were used in the A2 shade for standardisation. Omnichroma composite resin was a single-shade material. The specimens were cured with a halogen curing unit at a light intensity of 400–550 mW/cm2 and using a standard curing mode (Demetron LC, Kerr, Collins Ave., USA) for each side for 20 s according to the instructions of the manufacturer. The intensity of the light-curing unit was verified with a calibrated radiometer for every five specimens.
A mylar strip was placed over the composite resin and pressed using a glass plate removed after curing to provide a flat surface. The samples were polished using an electric handpiece at 15,000 rpm and a series of polishing discs (Super-Snap, Rainbow Technique Kit, Shofu Inc, Kyoto, Japan) for 10 s (coarse, medium, fine and superfine). The thickness of each sample was measured with a digital caliper (Mitutoyo, Kawasaki, Japan) for standardisation. After polishing, the specimens were kept in deionized water at 37 °C for 24 h [19].
Then, the samples were kept in artificial saliva (0.33 g of KH2PO4, 0.34 g of Na2HPO4, 1.27 g of KCl, 0.16 g of NaSCN, 0.58 g of NaCl, 0.17 g of CaCl2, 0.16 g of NH4Cl, 0.03 g of glucose, 0.2 g of urea, 0.002 g of ascorbic acid and 2.7 g of mucin in 1000 mL of distilled water) in an incubator (FN055-Nuve, Ankara, Turkey) at 37 °C for 24 h.
The sample for each composite resin (n = 80) was randomly divided into the following four subgroups (n = 20 per group) based on the type of toothpaste used in the study:
Group 1. For the control group, the samples were stored in artificial saliva at 37 °C throughout the study period. The artificial saliva was changed every day.
Group 2. The samples were brushed with Colgate Total 12 (Colgate Total 12, Colgate-Palmolive, New York, USA) toothpaste after immersion in a coffee solution twice a day for 10 min for 30 days.
Group 3. The samples were brushed with Colgate Optik White toothpaste (Colgate Optic White, Colgate-Palmolive, New York, USA) after immersion in a coffee solution twice a day for 10 min for 30 days.
Group 4. The samples were brushed with Meridol Gentle White toothpaste (Meridol Gentle White, CP-GABA, Hamburg, Germany) after immersion in a coffee solution twice a day for 10 min for 30 days.
During resting periods, the samples were stored in artificial saliva at 37 °C, and the artificial saliva was changed every day during the study period.
Calibration
All of the color and surface roughness analyses of the samples were performed by one investigator (GC). She was trained by using a spectrophotometer and profilometer [20]. To validate, the investigator measured the color and surface roughness of the ten samples from each composite resin material that was not included in the study. The analysis was performed two times with an interval of one week between two measurements. This resulted in an intra-agreement rate of 98%, considered high reproducibility.
Blinding
According to a list constituted by RANDOM.ORG, all specimens of each composite resin group were numbered and randomly assigned into four subgroups. The sequence of toothpaste application was randomized with the same technique [20].
Staining in coffee solution and brushing simulation
For each daily brushing cycle, the samples were first immersed in 2 ml of coffee solution at 37 ºC for 10 min and then washed with distilled water. The coffee solution was constructed using 1 tsp of soluble coffee (Nescafe Original, Nestle, Araras, Sao Paulo, Brazil) dissolved in 50 ml of boiling water [18].
The specimens in the brushing subgroups were also brushed with toothpaste twice a day using an electric toothbrush (Oral B Vitality Plus 2D Cross Action, Germany) for five seconds on each surface for 30 days. We chose five seconds per sample because that is the typical amount of time that a person brushes a stained surface [21, 22]. The horizontal brushing technique was used by one investigator (GC) to eliminate potential differences among investigators and standardize the brushing force. The toothpaste was diluted in distilled water every brushing cycle (1:3) (in weight) [20].
After filtering the liquids of the specimens, gently rinsing them with distilled water, and drying them with paper tissue, we performed color measurements. The toothpastes used in this study are presented in Table 2.
Color measurement
The color analysis of the samples was performed by a blind-trained investigator at baseline (before the staining) and 1 day, 7 days and 30 days after the brushing to determine the color change rate. To avoid the influence of light in the environment, the measurements were performed in a windowless dark laboratory.
The color analyses of the composite resin samples were conducted using a spectrophotometer (SpectroShade Micro, MHT, Italy) according to the CIE L*a*b* coordinates that help identify the color of an object in a three-dimensional color space. The chromaticity coordinates are the axes of a* and b*. The L* axis, perpendicular to a* and b*, shows the perceived color lightness.
Before all measurements, the spectrophotometer was calibrated according to manufacturer recommendations. The measuring tip of the instrument was placed at a right angle to the sample surface and at the same distance each time. The color of each sample was measured three times. The images of the samples were then opened and saved using the device’s computer software (Spectroshade Micro-Software-Version 3.01, MHT, Italy). Measurements were taken based on the images, and the samples’ L*, a*, and b* values were recorded. The L* parameter (white-black range) demonstrated the brightness of the samples, the a* parameter (red-green range) demonstrated the redness and the b* parameter demonstrated the yellowness (yellow-blue range).
The color changes (ΔE00) of the samples were calculated using the CIEDE2000 formula: ΔE00 = [(ΔL´/KLSL)2 + (ΔC´/KCSC)2 + (ΔH´/KHSH)2 + RT(ΔC´/KCSC) X (ΔH´/KHSH)2]1/2, where ΔL´, ΔC´, and ΔH´ are the distinctions in terms of lightness, chroma and hue, respectively, between the two samples in CIEDE2000. RT is the rotation function that accounts for the interaction between chroma and hue differences in the blue zone. SL, SC and SH are the weighting functions that adjust the total color difference for variation according to the location of the color difference pair at L*, a*, and b* coordinates. The parametric factors (KL, KC and KH) are correction terms for experimental conditions, as described by Sharma et al. [1, 23].
The color changes were calculated between baseline and 1 day [ΔE00 (1 day−baseline)], 7 days [ΔE00 (7 days−baseline)] and 30 days [ΔE00 (30 days−baseline)] after brushing.
Surface roughness measurement
Surface roughness analyses of the composite resin samples were conducted using a profilometer (Surftest SJ-210 Mitutoyo, Tokyo, Japon). The values were recorded at the baseline and 1 day, 7 days and 30 days after brushing with the toothpaste to evaluate the rate of surface roughness. The measurements were performed at a speed of 0.25 mm/sn and with a cut-off of 0.80 mm. Three readings were taken for each sample, and the averages of these values (Ra, µm) were recorded across the four evaluation periods.
Surface topography measurement
After 30 days of brushing with different kinds of toothpaste, two samples were randomly selected from each composite resin subgroup to analyse their surface topography. The analysis was performed using SEM (FEG 250-FeiQuanta, the Netherlands). The most representative images were archived for illustration.
Statistical analysis
The data were analysed using the R programme. The normality distribution of the data was analysed using the Shapiro-Wilks test. The WRS2 package was used to compare non-normal distributed values of color and roughness according to the composite resin, group and time. The data were examined using a three-way robust analysis of ANOVA with a pruned mean. Multiple comparisons were performed using Bonferroni post hoc correction. The significance level was p < 0.05 for all tests.
Results
Color analysis
The mean values and standard deviations for L*, a*, b* and ΔE00 are presented in Table 3a, 3b, 3c and 3d. We found a statistically significant difference between the L*, a* and b* values according to composite resin, group and time (p < 0 0.01, p < 0.01 and p = 0.001) (Table 4).
Charisma had the highest L* (67.0) and a* (1.49) values, while Omnichroma had the lowest L* (63.6) and a* (-4.00) values (Tables 3a and 3b). The b* value for Filtek (17.2) was higher than the b* values for Charisma (16.3) and Omnichroma (8.2) (Table 3c). Whereas the smallest color change (ΔE00) was observed for Charisma (1.81), the greatest color change was exhibited by Filtek (5.18) (Table 3d). Moreover, we found that the highest L* values and the lowest a* and b* values belonged to the control group for all the tested composite resin materials. The L* values decreased and the a* and b* values increased over time for all the tested composite resin materials.
The greatest color change (ΔE00) occurred in Group 4 (4.73) and after four weeks (6.47) for all the tested composite resin materials (Table 3d). Furthermore, we observed a statistically significant difference between L*, a*, b* and ΔE00 values in terms of the composite resin, group and time interaction (p = 0.01) (Table 4). The highest mean L* value was obtained by Group 4 of Filtek at baseline (68.6), while the lowest mean L* value was obtained by Group 2 of Filtek (54.3) after four weeks (Table 3a). The greatest color change (ΔE00) was observed for Group 2 of the Filtek after 30 days (Table 3d).
Surface roughness analysis
The mean values and standard deviations of the results for the surface roughness values are presented in Table 5. We found statistically significant differences between the surface roughness values according to composite resin, group and time (Table 6). Omnichroma (0.167) exhibited lower roughness values than Charisma (0.230) and Filtek (0.235). The highest surface roughness values were obtained by Group 4 (0.225) and after four weeks (0.226) by all test groups (Table 5). Furthermore, there was no statistically significant difference between the mean values of roughness according to the composite resin, group and time interaction (p = 0.937) (Table 6).
Scanning electron microscopy (SEM) analysis
There were no cracks, fractures or ruptures of inorganic particles in the control and toothpaste groups of the tested composite resin materials (see Figs. 1, 2 and 3).
Discussion
The results showed that the null hypothesis (i.e. that there would be no differences in color change and surface roughness of nano-hybrid, micro-hybrid and supra-nano-filled composite resin samples after brushing with whitening toothpastes) was unsupported.
This study examined the in vitro effects of brushing for 30 days with whitening toothpaste on the color and surface roughness of composite resins. The abrasiveness of the toothpaste can be measured using relative dentine abrasivity (RDA) [16]. Although the abrasives in toothpastes can prevent extrinsic staining of teeth, the abrasiveness of the toothpaste should be safe and at tolerable levels. It is recommended that the RDA of toothpaste not exceed 250 and that whitening toothpaste have an average RDA ranging between 60 and 100 or higher than 100, as detailed by the International Organization for Standardization (ISO) [16, 24, 25]. Therefore, for our study, we selected toothpastes with similar relative RDA rates and moderate abrasiveness.
We used a coffee solution for the staining because coffee (one of the most commonly used beverages) causes the greatest discoloration of restorative materials [19, 26, 27]. Moreover, the high temperature and acidity of coffee can cause composite resin discoloration. In addition to surface staining, coffee can cause subsurface staining due to its polar and delayed release colorants being absorbed by the composite resin surface [28]. Furthermore, scholars have reported that a greater temperature in the environment can hasten the discoloration of restorative materials. Therefore, composite resin samples were stored in an incubator at 37 ºC to stimulate the oral environment. The coffee solution was prepared by the investigator for every brushing cycle to minimise bacterial growth [19]. To deal with the problem of the visual assessment of color, devices such as colorimeters and spectrophotometers have been used in previous studies [19, 29]. According to the literature, color can be detected with a spectrophotometer device 33% more accurately and 93.3% more objectively than using the visual method [30]. Therefore, we performed color assessments with a digital spectrophotometer device.
The CIE L*a*b color system is generally used to measure color in dentistry [31]. The CIE L*a*b system can provide a standardised technique for measuring ΔE* values in an accurate manner. The small color changes identified by this system offer the advantages of improved objectivity, repeatability and sensitivity [29]. However, we selected the CIEDE 2000 color difference formula (ΔE00) to calculate single-number shade pass and avoid failures in evaluating minor to medium color disparities, rather than the previous CIE L*a*b system [19]. Scholars have found that color variation can be determined using perceptibility and acceptability thresholds. In our study, the perceptibility (ΔE00) and 50:50% acceptability thresholds were set at 0.8 and 1.8, respectively [19, 31]. Finally, in the present study, the perceptibility and acceptability values were higher after 30 days for all composite resin materials, which correlated with the previous study performed by Rohym et al. [19].
The organic components and filler particle properties of composite resin materials cause discoloration [32]. Aggregated filler and glass particles are vulnerable to porosities, with water absorption leading to staining and color changes in the nano-filled composite resin [33, 34]. Therefore, triethylene glycol dimethacrylate (TEGDMA) leads to further deterioration of the matrix/fill particle bond and brushing this composite resin may lead to further removal of these particles from the surface and thus to reduced color stability in the nano-filled composite resin material [1, 35]. In a study examining color change in nano-hybrid and micro-hybrid composite resins, the authors reported that the greatest color change occurred in the nano-hybrid composite resin containing TEGDMA [36]. In another study, the color stability and surface roughness of nano-filled, nano-hybrid, and micro-hybrid composite resins were reduced after brushing with whitening toothpaste, and it was reported that the greatest color change occurred in the nano-hybrid composite resin material; these results correlate with our findings. Furthermore, the same study reported that the late penetration of food-simulating substances through the polymer matrix may cause discoloration in the nano-hybrid composite resin [37]. Similar to the literature, in our study, the greatest discoloration was found in the nano-hybrid composite resin (Filtek Ultimate Universal, 3 M/ESPE, Saint Paul, USA). This may be due to the type and amount of filler particles in the composite resin material. We believe that the TEGDMA ratio of the nano-hybrid composite resin may lead to increased water absorption and increased polymer solubility [38].
It is known that there is a significant difference between the nano-hybrid and micro-hybrid composite resins in terms of filler size. According to the manufacturer, the nano-filled composite resin contains nanoclusters consisting of zirconia (4–11 nm) and silica (20 nm) nanoparticles, while the micro-hybrid composite resin contains micro-glass particles. Nanoclusters have been noted to have micropores that facilitate fluid absorption and pigment retention. In addition, nanoparticles contain large amounts of atomic particles on their surfaces [36]. It has been said that the quantum effect changes with the size of the particle and that the shrinking of particle size leads to the effects becoming apparent. The quantum effect of nanoparticles exposes them to their simple agglomeration within dust particles, thereby making them susceptible to different surface interactions, including the adsorption of other substances [39]. In line with the literature, our study revealed that the color change of the nano-hybrid composite resin occurred at a higher rate than that of the micro-hybrid composite resin.
Furthermore, scholars have reported that the nano-hybrid composite resin is more unstable in terms of color than the supra-nano-filled composite resin (Omnichroma), which correlated with our results [40]. The lower filler content and presence of nanoclusters can explain the lower color resistance of the nano-hybrid composite resin compared to the supra-nano-filled resin composite (Omnichroma) [19, 41, 42]. In this study, we evaluated the effects of toothpaste on the color stability of composite resins rather than evaluating the coloration of composite resins. We found that traditional toothpaste, peroxide-based toothpaste and blue covarine-based toothpaste could not prevent the coloration of the coffee-colored supra-nano-filled composite resin samples (Omnichroma).
The literature states that filler size and surface roughness are not affected by tooth brushing, but the average surface roughness value generally increases in composite resins with larger filler sizes [9]. This is explained by the gradual removal of fillers after tooth brushing. The larger the filler size, the more filler will be removed and the more the surface roughness of the material will increase. However, the shape of the filler, the distance between the fillers, the composite resin matrix composition, the chemical bond between the filler particles and the degree of conversion after polymerisation are factors that must be considered [43]. Scholars have reported that there is a material-dependent interaction between toothpaste abrasiveness and the surface roughness of restorative materials [44]. Various studies have determined that the surface roughness values for micro-hybrid composite resins after the use of whitening paste are higher than for nanocomposite resin systems, as supported by our findings [43,44,45]. In our study, although there was no statistically significant difference between the surface roughness values of the micro-hybrid composite resin and the nano-hybrid composite resin after 30 days of brushing with whitening toothpaste, the numerical values for the micro-hybrid composite resin were higher than those of the nanohybrid composite resin. In addition, in the current study, the lowest surface roughness values belonged to the supra-nano-filled composite resin.
In addition, it has been mentioned in the literature that surface roughness can cause the external discoloration of composite resin materials [19, 41]. The wearing out of the resin composite can lead to the debonding of the inorganic fillers from the resin matrix, which can cause voids and increase surface roughness, thereby creating a surface that is susceptible to external stains [41, 46]. Moreover, the resin matrix is a key element in staining susceptibility [22, 29, 47]. The discoloration of the resin matrix depends on the hydrophilicity of the resin matrix and the water absorption of the material, as indicated in several studies [19, 46, 48]. Moreover, our finding that the Omnichroma composite resin containing a matrix composition based on TEGDMA and urethane dimethacrylate (UDMA) was more discolored than the Charisma composite resin correlated with the results of previous studies [19, 42, 49]. Although we evaluated the effects of whitening toothpaste on nano-hybrid, supra-nano-filled and micro-filled composite resin materials, our study had limitations. More specifically, we could not mimic the factors affecting the restorative materials in the oral cavity, such as microbiota, salivary circulation, temperature and pH changes, as done in other in vitro studies [19, 49]. Therefore, it was not possible to precisely simulate the conditions of the oral cavity.
Conclusion
Within the limitations of the present study, the findings showed after 30 sequential days, traditional and whitening toothpastes could not decrease discoloration on the micro-hybrid, nano-filled and supra-nano-filled composite resin samples caused by the coffee solution to the level below the perceptibility threshold. The smallest color change was observed in the micro-hybrid composite resin and the greatest color change was observed in the nano-hybrid composite resin. The greatest color change was obtained after using a toothpaste based on blue covarine, while the smallest color change was observed after using peroxide-based toothpaste. The supra-nano-filled composite resin samples had the lowest surface roughness values compared to the micro-hybrid and nano-filled composite resin samples. Additional laboratory and clinical studies are needed to fully understand the long-term effectiveness of whitening toothpaste on composite resin materials.
Data Availability
The datasets used and/or analysed in this study are available from the corresponding author upon reasonable request.
References
Roselino RML, Chinelatti AM, Alandia-Román CC, Pires-de-Souza PCF. Effect of brushing time and dentifrice abrasiveness on color change and surface roughness of resin composites. Braz Dent J. 2015;26:507–13.
Amaral CM, Rodrigues JA, Erhardt MC, Araujo MW, Marchi GM, Heymann HO, Pimenta LA. Effect of whitening dentifrices on the superficial roughness of esthetic restorative materials. J Esthet Restor Dent. 2006;18:102–8.
Tomás MBD, Pecci-Lloret PM, Guerrero-Gironés J. Effectiveness and abrasiveness of activated charcoal as a whitening agent: a systematic review of in vitro studies. Annals of Anatomy - Anatomischer Anzeiger. 2023;245:151998.
Ghajari MF, Shamsaei M, Basandeh K, Galouyak MS. Abrasiveness and whitening effect of charcoal-containing whitening toothpastes in permanent teeth. Dent Res J. 2021;18–51.
Viana ÍEL, Weiss GS, Sakae LO, Niemeyer SH, Borges AB, Scaramucci T. Activated charcoal toothpastes do not increase erosive tooth wear. J Dent. 2021;109:103677.
Casado BGS, Moraes SLD, Souza GFM, Guerra CMF, Souto-Maior JR, Lemos CAA, Vasconcelos BCE, Pellizzer EP. Efficacy of dental bleaching with whitening dentifrices: a systematic review. Int J Dent. 2018;7868531.
Joiner A. Whitening toothpastes: a review of the literature. J Dent. 2010;38:e17–e24.
Neme AL, Frazier KB, Roeder LB, Debner TL. Effect of prophylactic polishing protocols on the surface roughness of esthetic restorative materials. Oper Dent. 2002;27:50–8.
Heintze SD, Forjanic M. Roughness of different dental materials before and after simulated toothbrushing in vitro. Oper Dent. 2005;30:617–26.
Van Loveren C, Duckwort RM. Anti-calculus and whitening toothpastes. Monogr Oral Sci. 2013;23:61–74.
Collins LZ, Naeeni M, Platten SM. Instant tooth whitening from a silica toothpaste containing blue covarine. J Dent. 2008;36:21–5.
Joiner A, Philpotts CJ, Alonso C, Ashcroft AT, Sygrove NJ. A novel optical approach to achieving tooth whitening. J Dent. 2008;36:8–14.
Vaz PTV, Jubilato PD, Oliveira MRM, Bortolatto FJ, Floros CM, Dantas RAA, Oliveira Junior BO. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: which one is the most effective? J Appl Oral Sci. 2019;27:1–8.
Rodrigues SC, Dala Nora B, Mallmann A, May GL, Jacques BL. Repolishing resin composites after bleaching treatments: Effects on color stability and smoothness. Oper Dent. 2019;44(1):54–64.
Roopa KB, Basappa N, Prabhakar RA, Raju SO, Gagandeep L. Effect of whitening dentifrice on micro hardness, colour stability and surface roughness of aesthetic restorative materials. J Clin Diagn Res. 2016;10:ZC06–11.
Roselino L, Tonani R, Sbardelotto C, Alves A, Arruda C, Tirapelli C, Pires-de-Souza F. Color stability and surface roughness of composite resins submitted to brushing with bleaching toothpastes: an in situ study. J Esthet Restor Dent. 2019;31:486–92.
Schroder T, Silva BP, Basso RG, Franco CM, Maske TT, Cenci SM. Factors affecting the color stability and staining of esthetic restorations. Odontology. 2019;107:507–12.
Silva MT, Dantas BCD, Franco TT, Franco TL, Huhtala LRFM. Surface degradation of composite resins under staining and brushing challenges. J Dent Sci. 2019;14:87–92.
Rohym S, Tawfeek MH, Kamh R. Effect of coffee on color stability and surface roughness of newly introduced single shade resin composite materials. BMC Oral Health. 2023;23:1–13.
Koç Vural U, Bagdatli Z, Yilmaz EA, Yalçın Çakır F, Altundaşar E, Gurgan S. Effects of charcoal-based whitening toothpastes on human enamel in terms of color, surface roughness, and microhardness: an in vitro study. Clin Oral Investig. 2021;25(10):5977–85.
Bezgin T, Özer L, Tulga Öz F, Özkan P. Effect of toothbrushing on color changes of esthetic restorative materials. J Esthet Restor Dent. 2015;27(Suppl 1):65–S73.
Reinhardt WJ, Balbierz MM, Schultz MC, Simetich B, Beatty WM. Effect of tooth-whitening procedures on stained composite resins. Oper Dent. 2019;44(1):65–75.
Sharma G, Wu W, Dalal EN. The CIEDE2000 color-difference formula: implementation notes, supplementary test data, and mathematical observations. Color Res Appl. 2005;30:21–30.
Yap AU, Yap SH, Teo CK, Ng JJ. Finishing/polishing of composite and compomer restoratives: effectiveness of one-step systems. Oper Dent. 2004;29:275–9.
Jones C, Billington R, Pearson G. The in vivo perception of roughness of restorations. Br Dent J. 2004;196:42–5.
Paolone G, Formiga S, De Palma F, Abbruzzese L, Chirico L, Scolavino S, Goracci C, Cantatore G, Vichi A. Color stability of resin-based composites: staining procedures with liquids-A narrative review. J Esthet Restor Dent. 2022;34(6):865–87.
Ayad NM. Susceptibility of restorative materials to staining by common beverages: an in vitro study. Eur J Esthet Dent. 2007;2:236–47.
Mehrgan S, Kermanshah H, Omrani RL, Ahmadi E, Rafeie N. Comparison the effect of charcoal-containing, hydrogen peroxide-containing, and abrasive whitening toothpastes on color stability of a resin composite: an in vitro study. BMC Oral Health. 2021;19:594.
Hussain SK, Al-Abbasi SW, Refaat MM, Hussain AM. The effect of staining and bleaching on the color of two different types of composite restoration. J Clin Exp Dent. 2021;13:e1233.
Paul S, Peter A, Pietrobon N, Hämmerle CH. Visual and spectrophotometric shade analysis of human teeth. J Dent Res. 2002;81:578–82.
ElAziz RHA, Gadallah LK, Saleh RS. Evaluation of charcoal and sea salt lemon-based whitening toothpastes on color change and surface roughness of stained teeth. J Contemp Dent Pract. 2022;23(2):169–75.
Mozzaquatro LR, Rodrigues CS, Kaizer MR, Lago M, Mallmann A, Jacques LB. The effect of brushing and aging on the staining and smoothness of resin composites. J Esthet Restor Dent. 2006;22:211–22.
Nasim I, Neelakantan P, Sujeer R, Subbarao CV. Color stability of microfilled, microhybrid and nanocomposite resins – an in vitro study. J Dent. 2010;38(Suppl 2):137–42.
Choi MS, Lee YK, Lim BS, Yang HC. Changes in surface characteristics of dental resin composites after polishing. J Mater Sci. 2005;16:347–53.
Lee YK, Powers JM. Color changes of resin composites in the reflectance and transmittance modes. Dent Mater. 2007;23:259–64.
Ertaş E, Güler U, Yücel AC, Köprülü H, Güler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–6.
Roselino LM, Cruvinel DR, Chinelatti MA, Pires-de-Souza FC. Effect of brushing and accelerated ageing on color stability and surface roughness of composites. J Dent. 2013;41:54–61.
Silva MT, Sales SLLA, Pucci RC, Borges BA, Torres GRC. The combined effect of food-simulating solutions, brushing, and staining on color stability of composite resins. Acta Biomater Odontol Scand. 2017;3:1–7.
Scholl JA, Koh AL, Dionne JA. Quantum plasmon resonances of individual metallic nanoparticles. Nature. 2012;483:421–7.
Soliman YA, Mahmoud EM, Gepreel MH, Aff RR. The ability of coffee to stain nanohybrid composite resins. Alexa Dent J. 2021;46:91–5.
Reddy PS, Tejaswi SLK, Shetty S, Annapoorna MB, Pujari CS, Thippeswamy MH. Effects of commonly consumed beverages on surface roughness and color stability of the nano, micro hybrid and hybrid composite resins: an in vitro study. J Contemp Dent Pract. 2013;14(4):718–23.
Aydın N, Topçu FT, Karaoğlanoğlu S, Oktay EA, Erdemir U. Effect of finishing and polishing systems on the surface roughness and color change of composite resins. J Clin Exp Dent. 2021;13(5):e446–454.
Oliveira GU, Mondelli RFL, Rodrigues MC, Franco EB, Ishikiriama SK, Wang L. Impact of filler size and distribution on roughness and wear of composite resin after simulated toothbrushing. J Appl Oral Sci. 2012;20(5):510–6.
Pires-de-Souza FDP, Garcia LDR, Roselino LDR, Naves LZ. Color stability of silorane-based composites submitted to accelerated artificial ageing-an in situ study. J Dent. 2011;39(Suppl1):18–24.
Yılmaz C, Kanık Ö. Investigation of surface roughness values of various restorative materials after brushing with blue covarine containing whitening toothpaste by two different methods: AFM and profilometer. Microsc Res Tech. 2022;85(2):521–32.
Bagheri R, Burrow MF, Tyas M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J Dent. 2005;33(5):389–98.
Patil A, Muliya VS, Pentapati KC, Kamath S. Effect of green, tulsi, and areca teas on the color stability of two composite resin materials – an in vitro spectrophotometric analysis. Clin Cosmet Investig Dent. 2020;12:423–8.
Kangwankai K, Sani S, Panpisut P. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE. 2017;12(11):e0187757.
Bilmez YZ, Şeker O, Köse HD, Aslan GB. Evaluation of liquid sorption and color stability of dental composites after exposure to common lactation teas. Int Dent Res. 2021;11(Suppl 1):228–33.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
GK designed the study. GC contributed to the material preparation and data collection. GC and GK contributed to analyzing and interpreting data and drafting the manuscript.GK contributed to critically revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Colak, G., Katirci, G. In Vitro evaluation of the effects of whitening toothpastes on the color and surface roughness of different composite resin materials. BMC Oral Health 23, 580 (2023). https://doi.org/10.1186/s12903-023-03277-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03277-4