Abstract
Background
The oral microbiota is a significant risk indicator for oral diseases, such as dental caries and periodontal inflammation. Much attention is presently paid to the development of functional foods (e.g. beverages containing cranberry constituents, or foods containing probiotics) that may serve as adjuncts for oral disease treatments (e.g. periodontitis and caries). Cranberry fruit, due to its unique chemical composition and antimicrobial potential, is a possible ingredient of such foods. The study aimed to investigate the effects of cranberry juice (CJ) and a cranberry functional beverage (mixture of 80% v/v apple juice, 20% v/v cranberry juice, and 0.25 g/100 mL ground cinnamon; CFB) on the growth and metabolic activity of selected oral bacteria.
Methods
Serial dilution pour plate method (SDPP) was used to examine the effect of CJ and CFB on the growth of Actinomyces naeslundii, Streptococcus mutans, and Lactobacillus paracasei subsp. paracasei. 48-h electrical impedance measurements (EIM) during the cultivation of A. naeslundii were applied to evaluate the utility of the method as a rapid alternative for the assessment of the antimicrobial potential of cranberry beverages.
Results
The tested bacteria differed in their susceptibility to the antimicrobial action of CJ and CFB, with L. paracasei subsp. paracasei being least vulnerable to CFB (according to SDPP). Although CJ at a concentration of 0.5 mL/mL, showed a bactericidal effect on the growth of S. mutans, A. naeslundii was more sensitive to CJ (SDPP). Its inhibitory effect on A. naeslundii was seen even at concentrations as small as 0.03125–0.125 mL/mL (SDPP and EIM). On the other hand, S. mutans seemed to be more vulnerable to CFB than A. naeslundii (SDPP).
Conclusions
CFB may be considered an adjunct in the treatment of oral diseases due to its action against selected oral pathogens, and not against the presumably beneficial L. paracasei subsp. paracasei. Bioelectrical impedance measurements appear to be a quick alternative to evaluating the antimicrobial activity of fruit beverages, but their utility should be confirmed with tests on other bacteria.
Similar content being viewed by others
Background
The pathogenic oral microbiota is a significant risk indicator for oral diseases, such as dental caries and periodontal inflammation [1]. Prevention and treatment of these diseases can include removing as many pathological microbiotas as possible using physical methods (tooth brushing, scaling, and root planning) or chemical methods (mouth rinses and antibiotic therapy). Some methods, such as antibiotic therapy, are questionable due to their possible side effects, such as the acquisition of resistance by oral pathogens or their negative effect on gastrointestinal tract microbiota. In recent years, the issue of mouthwash safety has also been raised. In particular, it has been suggested that regular long-term use of such items may lead to an overgrowth of pathogenic or resistant bacteria, eventually reducing the clinical efficacy of antibiotics [2,3,4]. Furthermore, the regular use of mouthwashes containing chlorhexidine or cetylpyridinium chloride is thought to contribute to an increased risk of hypertension (as a result of the destruction of oral microbes responsible for catalyzing the reduction of nitrate to nitrite) [2, 5,6,7,8,9,10,11] and to the development of prediabetes or diabetes [2]. Thus, much attention is presently being paid to the search for safe adjuncts for oral disease treatment. One solution may be found in so-called functional foods—items consumed as part of a regular diet and possessing health-related benefits that may contribute to diminishing the risk of specific chronic diseases [12]. In recent decades, cranberry fruit (Vaccinium macrocarpon) has received much attention regarding its possible use in the treatment of metabolic and oral diseases, including periodontal diseases [13,14,15]. The unique chemical composition of this fruit, including the presence of A-type procyanidins, makes it a potential ingredient of functional foods. It has previously been observed that cranberry compounds can inhibit the activity of Streptococcus sorbinus and Streptococcus mutans [15,16,17,18,19,20] and also possess antiadhesive potential against those bacteria, as well as against some periopathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum [15, 21,22,23,24,25]; it also seems that they can restrain bacterial biofilm formation [14, 26,27,28]. It has been suggested that cranberry constituents can modulate the host's immune response in the course of periodontitis [27, 29,30,31]. Apart from oral health, cranberry beverages have also been studied concerning their possible benefits in modulating inflammation and oxidative status in overweight adults and patients with metabolic syndrome [32,33,34]. However, the use of cranberry in functional foods production has to date been limited due to its tart and astringent taste, which affect the taste and flavor of food items it enriches. Attempts have been made to overcome this disadvantage by adding artificial sweeteners, such as sucralose, aspartame, and acesulfame K. However, undesirable descriptors—such as sweet and bitter aftertaste, bitterness, and metallic flavor—are frequently reported for such beverages [35, 36]. In our previous study, we developed a functional beverage containing cranberry juice without any added sugars or artificial sweeteners, intended to possess a highly acceptable taste and other organoleptic features while being an effective and risk-free agent for supporting standard nonsurgical periodontal treatment. Apple juice—a highly acceptable, widely available, and relatively economical resource—was used as a base of the beverage; 100% cranberry juice was used as the main bioactive compound and the proportion used made up the highest sensory acceptable percentage of the cranberry juice in the beverage, based on our unpublished consumer sensory evaluation of four different variants of the beverage with cranberry juice; ground cinnamon was also used to enrich the taste. These considerations led to the following formula: 80% v/v apple juice (from the variety Antonówka Zwykła), 20% v/v of cranberry juice (Vaccinium macrocarpon), and 0.25 g/100 mL of ground cinnamon. This beverage, which we refer to as the cranberry functional beverage (CFB), was successfully introduced as an adjunct to standard periodontal therapy in patients with gingivitis. Full details can be found in our previous work [37].
For the present study, we intended to characterize the antimicrobial potential of CFB in vitro; we hypothesized that CFB possesses antimicrobial activity against selected oral pathogens (Streptococcus mutans and Actinomyces naeslundii) and does not affect the growth of the presumably beneficial Lactobacillus paracasei subsp. paracasei. We assessed the effects of CFB on the growth of these oral bacteria using the serial dilutions pour plate (SDPP) method, and we compared the activity of CFB with the activity of 100% cranberry juice (CJ). Furthermore, we attempted to verify the results obtained using SDPP via the quick alternative method of electrical impedance measurement (EIM) on one selected pathogen, A. naeslundii.
Material and methods
Antimicrobial agents
Cranberry juice (CJ) was obtained from Vaccinium macrocarpon fruits. The fresh fruits were washed, heated to 80 °C, and pressed in a Bucher press (Niederweningen, Switzerland). Immediately after cooling, the CJ was stored at − 20 °C until the microbiological tests were performed. The CFB was produced according to the above formula by the firm Polska Róża Ernest Michalski. The chemical composition and nutritional value of CJ and CFB are shown in Table 1.
Phenolic compounds analysis
Reversed-phase (C18 column) ultra-high-performance liquid chromatography-electrospray ionization-mass spectrometry (RP-UHPLC-ESI–MS) analysis was performed using a Dionex UltiMate 3000 UHPLC (Thermo Fisher Scientific, Sunnyvale, CA, USA) coupled to a Bruker maXis impact ultrahigh resolution orthogonal quadrupole-time-of-flight accelerator (qTOF) equipped with an ESI source and operated in the positive- and negative-ion mode (Bruker Daltonik, Bremen, Germany). The RP chromatographic separation was achieved with a Kinetex™ 1.7 µm C18 100 Å, LC column 100 × 2.1 mm (Phenomenex, Torrance, CA, USA) according to Biesaga and Pyrzyńska [38]. The ESI–MS settings were previously described by Mildner-Szkudlarz et al. [39]. Molecular ions: [M + H]+ and [M-H]− for phenolic compounds were extracted from full scan chromatograms (± 0.003 m/z) and peak areas were integrated with TASQ 2.1 (Bruker Daltonik, Bremen, Germany). The compounds present in each sample were identified based on the retention time of standard and/or molecular mass and structural information from the MS detector during MS/MS experiments. Additionally, the hydrolysis of samples was performed to confirm the presence of phenolic glycosides identified using MSMS spectra. The occurring of phenolic aglycones or increase in their concentration after hydrolysis proves the presence of glycosides. The samples were hydrolyzed with 1.2 M HCl for 2 h at 90 °C using the method described previously by Nuutilla et al. [40]. The tandem mass spectrometric data were used for searching molecular structure using CSI:FingerID (Friedrich Schiller University, Jena Germany), which combines fragmentation tree computation and machine learning [41, 42]. Limit of quantification (LOQ where S/N > 15) was determined for caffeic acid, chlorogenic acid, p-coumaric acid, sinapic acid, quercetin and it was not lower than 0.01 µg/mL.
The content of selected phenolic compounds of CJ and CFB is given in Table 2. The presented compounds were selected based on their antibacterial potential known from previous studies or their abundance in the tested beverages. In general, CJ was richer in phenolic compounds compared to CFB.
Tested microorganisms
Strains of Actinomyces naeslundii (DSMZ 17,233), Streptococcus mutans (DSMZ 20,523), and Lactobacillus paracasei subsp. paracasei (DSMZ 4905) were used as test microorganisms. All strains were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Germany. These bacteria were cultured using the following media: Actinomyces Broth Vegitone for A. naeslundii; Casein-soy broth or agar with yeast extract for S. mutans (P-0236 or P-0237, BTL, Łódź, Poland), and MRS broth or agar for L. paracasei subsp. paracasei (CM0359 or CM0361, Oxoid, Hampshire, UK).
Preparation of test cultures
For both experiments, liquid monocultures of the tested bacterial strains were prepared. The cultures for SDPP were prepared in standard glass tubes to a volume of 5 mL. The cultures for electrical impedance measurement were prepared in special 10-mL measuring tubes that were incubated in the automated microbiological growth analyzer (BacTrac 4100, Sy-Lab, Austria). A series of two-fold dilutions of CJ (0.50 mL/mL CJ1, 0.25 mL/mL CJ2, 0.125 mL/mL CJ3, 0.0625 mL/mL CJ4, 0.03125 mL/mL CJ5) or CFB (0.50 mL/mL CFB1, 0.25 mL/mL CFB2, 0.125 mL/mL CFB3) with sterilized liquid growth media were prepared and inoculated with the test microorganism. The applied inoculum constituted 5% for SDPP and 10% for impedance measurement of the volume of the entire culture. Aside from the test cultures enriched with CJ or CFB and inoculated with the test microorganisms, reference cultures (RC) inoculated with the test microorganisms, but not enriched with CJ or CFB, were also prepared.
Serial dilutions pour plate (SDPP) method
To evaluate the inhibitory effects of CJ and CFB, the number of bacteria was determined at the 0 h time point and after 48 h of incubation at 37 °C. To that end, a volume of 0.1 mL of each liquid culture (after a series of decimal dilutions) was placed on sterile Petri dishes, covered with sterile agar medium; and incubated at 37 °C for 48 h. Colony forming units (cfu) were then counted, and dishes with cfu counts ranging from 30 to 300 were considered. The pour plate cultures were prepared in triplicate. The results of the experiment are presented as the mean number of cfu/mL of cultures after 48 h of incubation (Table 3). Further, the numbers of cfu/mL of each culture were log-transformed, and the differences (changes) between the log of the cfu/mL number at 48 h and 0 h in each culture were calculated.
Electrical impedance change measurements
The inhibitory effect of CJ and CFB against A. naeslundii was additionally verified by analyzing the electrical impedance changes in the growth medium during incubation. Such changes, caused by the metabolic activity of A. naeslundii during its growth in the medium containing CJ or CFB, were measured using an automated microbiological growth analyzer (BacTrac 4100, Sy-Lab, Austria). Special 10-mL measuring test tubes (Sy-Lab), equipped with four electrodes, were filled with 9 mL of growth medium containing CJ (0.50–0.03125 mL/mL) or CFB (0.50–0.125 mL/mL), and then inoculated with 1 mL inoculum of the tested bacteria. The measuring tubes were incubated at 37 °C in the analyzer’s thermostat. Changes in the electrical impedance were calculated using the formula:
where y—is the change (expressed in %) in the electrical impedance of the growth medium, y0—is the value of electrical impedance at the beginning of culturing, and yi—is the value of electrical impedance at a common point of measurement (measured every 10 min).
The changes in electrical impedance caused by the bacterial metabolic processes can be presented as a curve that parallels the classic microbial growth curve with lag, logarithmic, and stationary phases [41,42,43,44,45]. To facilitate comparative analysis, we employed an impedance threshold of 2% of the changes and determined the parameter of impedance detection time (IDT).
Statistical analysis
Data were analyzed using the T-test for independent variables, except for the comparison of IDT between CJ2 and RC, in which case the one-sample T-test was used. Statistical significance was set at p < 0.05. All analysis was performed using the StatSoft Statistica data analysis software system (version 13.1, 2016; www.statsoft.com).
Results
Actinomyces naeslundii
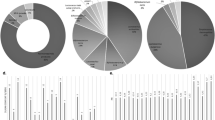
The number of cfu/mL of A. naeslundii at 48 h was significantly lower in CJ1, CJ3, CJ4, and CJ5 than in the reference culture (RC; Table 3). No significant difference was found between CJ2 and RC. In the CFB2 and CFB3 cultures, the number of cfu/mL at 48 h was substantially higher than in RC. No difference was seen between CFB1 and RC. Compared to changes in the number of cfu/mL in RC—which illustrates the growth of the tested microorganism under optimal growing conditions—an inhibitory effect of CJ was observed at concentrations of 0.50 mL/mL (CJ1) and 0.125–0.03125 mL/mL (CJ 3–5); surprisingly, a slight stimulation of A. naeslundii growth was seen at 0.25 mL/mL (CJ2, Fig. 1a). At the concentration of 0.50 mL/mL CFB, the intensity of A. naeslundii growth was comparable to that in RC, while at 0.125–0.25 mL/mL CFB, an intensification was seen (Fig. 1b).
Changes in the number of cfu/mL between 0 and 48 h time points. a, b A. naeslundii, c, d S. mutans, e, f L. paracasei subsp. paracasei. Results are expressed as means ± SDs. *, **, ***: Significantly lower than reference culture (*p < 0.05, **p < 0.01, ***p < 0.001). #, ##, ###: Significantly higher than reference culture (#p < 0.05, ##p < 0.01, ###p < 0.001). NS: not significantly different from reference culture. CJ, cranberry juice; CFB, cranberry functional beverage
Analyzing the changes in electrical impedance during incubation of the tested microorganisms an inhibiting effect in both cases (CJ as well as CFB) was observed. The significant differences in the value of impedance detection time (IDT) for all the tested juices concentrations were registered. In general, the higher was the concentration of CJ or CFB, the higher was the growth inhibition of the tested A. naeslundii (the longer was the IDT). In the highest concentration (0.50 mL/mL) a bactericidal effect of CJ was noted (Fig. 2a). The inhibition of A. naeslundii metabolic activity was noted in CJ2, CJ3, and CJ4 (as determined by the longer IDT in those cultures in comparison to RC). Similarly, at a concentration of 0.50 mL/mL of CFB, a bactericidal effect was seen (Fig. 2b). In CFB2 and CFB3, inhibition of A. naeslundii metabolic activity was also observed (IDT was significantly longer than in RC).
Streptococcus mutans
No growth of S. mutans was observed at 48 h in CJ1 (0.50 mL/mL; bactericidal effect, Table 3). The number of cfu/mL was significantly lower in CJ2 than in RC. In subsequent cultures enriched with CJ, the number of cfu/mL was higher than in RC. Regarding the cultures enriched with CFB, lower numbers of cfu/mL than in RC were observed solely in CFB1, while these values for CFB2 and CFB3 were higher than in the RC. Compared to the changes in the number of cfu/mL in RC, an inhibitory effect of CJ was observed at a concentration of 0.5 and 0.25 mL/mL (CJ1 and CJ2), and of CFB at a concentration of 0.50 mL/mL (CFB1, Fig. 1c, d). In all succeeding cultures, an intensification of S. mutans growth was observed.
Lactobacillus paracasei subsp. paracasei
At 48 h, the numbers of cfu/mL in CJ1 and CJ2 were significantly lower than in RC, and in CJ3 higher than in RC (Table 3). In the cultures enriched with CFB, significantly lower and significantly higher numbers of cfu/mL than in RC were observed in CFB1 and CFB2, respectively. Compared to the changes in the number of cfu/mL in RC, an inhibitory effect of CJ was observed only at a concentration of 0.50 mL/mL (CJ1), while an intensification in the growth of L. paracasei subsp. paracasei was observed at 0.125 mL/mL (CJ3; Fig. 1e). At no tested concentration did CFB inhibit the growth of L. paracasei subsp. paracasei, determined as changes in cfu/mL; at 0.25 mL/mL (CFB2), an intensification was observed in bacterial growth.
Discussion
The results of our study support the hypothesis that cranberry functional beverage (CFB) possesses a certain antimicrobial activity against the oral pathogens Actinomyces naeslundii and Streptococcus mutans, but not against Lactobacillus paracasei subsp. paracasei. The bacteria S. mutans and A. naeslundii differ in their vulnerability to the inhibitory action of CJ and CFB. CJ possesses greater antimicrobial activity than CFB against the tested oral bacteria, including both oral pathogens and L. paracasei subsp. paracasei.
The evaluation of the antimicrobial effect of the tested beverages was performed using two methods: serial dilutions pour plate (SDPP) method and electrical impedance measurement. Similar studies on inhibitory and bactericidal activity evaluation [46,47,48,49] have indicated the usefulness of the impedimetric technique as a fast and precise test. Our results confirmed that bioelectrical impedance measurement can be an adequate and rapid method for determining the antimicrobial potential of fruit beverages against oral bacteria, at least in the case of A. naeslundii.
Each of the tested microorganisms has its own specific optimal growing conditions and specific effect on dental plaque biofilm development, as well as possessing characteristic adaptation mechanisms to changes in growth conditions, which under in vivo conditions concern the dental plaque microenvironment [50,51,52]. All these aspects affect the vulnerability of particular microorganisms to antimicrobial agents. Among the bacteria, we tested here, Lactobacillus paracasei subsp. paracasei proved to be relatively tolerant to the presence of CJ or CFB in the growth media. The role of the Lactobacillus genus in oral health is yet not clear. Studies of the involvement of Lactobacillus in the initiation and progression of dental caries have been carried out for decades, though without resolving the issue. The Lactobacillus genus contains about 80 species of bacteria. Although the total number of Lactobacillus spp. present grows with the progression of dental caries, it needs to be emphasized that some Lactobacillus species—such as L. paracasei, L. plantarum, L. rhamnosus, and L. salivarius—possess inhibitory potential against certain cariopathogens, like S. mutans. The inhibitory action seems to be greater in strains obtained from chronic periodontitis patients than in those from healthy volunteers [50, 53,54,55]. In view of this, the lack of strong antimicrobial potential of CJ or CFB against L. paracasei subsp. paracasei should not be perceived negatively. There is no data on the effect of cranberry components on oral lactobacilli species. The results of the studies on food microflora indicate nonetheless, that probiotic Lactobacillus species (L. rhamnosus), are less susceptible to the antimicrobial action of cranberry or blueberry phenolic compounds compared to foodborne pathogens (E. coli, Listeria monocytogenes or Salmonella typhinurium) [56, 57]. Phenolic compounds are generally known for their inhibitory action on bacteria growth. In recent years, however, it has been suggested that polyphenols can have even stimulatory effects on the growth of some bacteria, including species of lactic acid bacteria. The mechanism that gives Lactobacillus species greater resistance for phenolic compounds compared to the sensitivity of some oral pathogens (as shown in the current study) or food pathogens is unclear. Concerning lactic acid bacteria, they rely heavily on energy-transducing systems to survive in incessantly changing and often-hostile environments. Most of these metabolic energy-generating systems offer the prevention of a lethal decrease of the internal pH [58, 59]. The interaction between phenolic compounds and lactic acid bacteria is bidirectional, e.g. lactic acid bacteria can determine the bioavailability of polyphenols, and polyphenols can affect the growth of bacteria. The impact of phenolic compounds on lactic acid bacteria is determined by many factors, such as the structure of polyphenol, its concentration, bacterial species, and its growth phase, metabolic abilities, and adaptation response [60]. It is worth noting that apple juice, which constitutes 80 v/v% of CFB, has recently been proposed as a suitable medium for delivering certain strains of Lactobacillus (e.g. L. paracasei, L. plantarum) and producing potentially probiotic fruit juices [61]. It would be reasonable to consider enriching CFB with probiotic bacteria, to develop a product with even greater pro-health properties.
Actinomyces naeslundii is a gram-positive early colonizer of the dental plaque biofilm. It has two types of fimbriae: type 1 fimbriae facilitate its adhesion to acidic, proline-rich salivary proteins and statherin (which is present in the salivary pellicle). Type 2 fimbriae are associated with the attachment of A. naeslundii to the glycosidic receptors on epithelial cells, polymorphonuclear leukocytes, and oral streptococci. A. naeslundii associates and forms biofilms with Streptococcus and periodontal pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum, which have been known to induce alveolar bone resorption (in the course of periodontitis). Studies on animal models have reported alveolar bone destruction induced by A. naeslundii [62]. Some data are indicating the potential of a high molecular weight nondialysable material (NDM) from cranberry to inhibit the coaggregation of A. naeslundii with other periodontopathogenic microorganisms [21, 63, 64]. We have determined that A. naeslundii seems to be more sensitive than S. mutans to the presence of CJ in growth media, as the inhibitory effect was observed even at low concentrations of CJ. This was demonstrated by both the SDPP and electrical impedance measurement methods. As expected, CJ was a more potent inhibitor of A. naeslundii growth and metabolic activity than was CFB. The electric impedance measurements demonstrated a dose-dependent relation between the concentration of CJ or CFB in the growth medium and the degree of inhibition of the metabolic activity of A. naeslundii, as determined by IDT. Both tested agents exhibited a bactericidal effect at the highest concentrations, as measured by IDT; this is consistent with the results of the SDPP method, but only for the CJ-enriched culture.
Streptococcus mutans is a primary colonizer of dental plaque and is considered one of the primary causative agents of dental caries. This bacterium possesses a wide range of virulence factors, the most important of which include sucrose-independent and sucrose-dependent adhesion within the dental plaque (which plays a prominent role in initiating the changes in plaque ecology that lead to dental caries), acidogenicity, acid tolerance, and the production of a wide range of mutacins. All these features give S. mutans an ecological advantage over other microorganisms during the processes of dental biofilm formation and maturation [51, 65,66,67,68,69,70,71]. It has previously been observed in in vitro models that certain phenolic fractions of cranberry fruits (e.g. NDM and A-type cranberry proanthocyanidins) have the potential to inhibit the activity of glucosyltransferases and fructosyltransferases from S. mutans, and may interfere in sucrose-dependent and sucrose-independent adhesion of the bacteria. However, from the viewpoint of our experiment, the most likely mechanism of antimicrobial action of CJ and CFB against S. mutans would seem to be the interruption of adaptive processes that occurs at low pH; this is referred to as the acid tolerance response. This process involves the ability of S. mutans to maintain a transmembrane pH gradient, with the interior of the cell being more alkaline; this is achieved by upregulation of a proton-translocating F1F0-ATPase that extrudes H+ as the external environment becomes more acidic. Acid-tolerant growth is also associated with changes in metabolic pathways, such as the downregulation or upregulation of certain proteins [69]. Previous in vitro studies have indicated the potential of cranberry phenolics to affect the acid tolerance response of S. mutans, decreasing its acidogenic capabilities [17, 72]. The inhibitory effect of CJ on the growth of S. mutans was evident at high concentrations (0.25–0.50 mL/mL), whereas the effect was noted even at lower concentrations of CJ in the case of A. naeslundii (0.03125–0.1250 mL/mL). A concentration of 0.50 mL/mL of CFB was effective in inhibiting the growth of S. mutans, though not of A. naeslundii, as measured by the SDPP method alone.
The antimicrobial potential of cranberry and other fruit and vegetable juices against oral pathogens has been studied before. However, most of these studies focused on juice extracts or biochemical compounds derived from fruits and vegetables [73,74,75]. Studies of juices in the form they are habitually consumed are sparse [76]. In our study, we tested the beverages in a form that can be directly incorporated into the diet, and this should be seen as a strength of our approach. One limitation of our study is the use of planktonic monocultures of bacteria; the dental biofilm is generally less sensitive to antimicrobial agents than the planktonic form, and shows greater pathogenic synergism, and is harder to remove via physical or chemical agents [51, 77, 78]. For this reason, the results of this study cannot be taken to demonstrate the effects of CJ or CFB on dental plaque in vivo. In fact, the anticipation of the actual action of any antimicrobial agent in in vitro models is nearly impossible. Molecular techniques have identified over 700 bacterial species in the subgingival microbiota, but about 50–60% of these are uncultivable [79, 80]. Nonetheless, in our previous study, where gingivitis patients drank 750 mL of the tested CFB daily for eight weeks, we did note a reduction in the number of S. mutans in dental plaque, compared to patients drinking water. In that study, having in mind the relatively high content of sugars of CFB, and thus possible risk of dental caries development or progression, we evaluated the number of S. mutans as a CFB safety control. We demonstrated then, that the consumption of CFB improves gingival and plaque indices, without posing a risk of caries development [37]. This is in agreement with the results of the current study. It should be also mentioned that the low pH of CFB and high content of acids might pose a risk of dental erosion. This pathological process is defined as the partial demineralization of the tooth surface caused by repeated exposure to acid. The microorganisms are not involved [81, 82]. However, taking into account the high phenolic content of CFB and its antioxidant capacity, this beverage should be recommended as part of a varied and well-balanced diet in which the erosive potential of fruit and fruit drinks is balanced by the consumption of milk and yoghurt (or other dietary calcium sources) that can serve as a protective factor.
Another methodological issue is that the numbers of cfu in the cultures at 0 h were not standardized in the pour plate experiment. However, the calculations allowed us to compare the changes in the number of cfu between the cultures enriched with CJ or CFB and the reference cultures.
Conclusions
In conclusion, the tested bacteria differed in their susceptibility to the antimicrobial action of CJ and CFB, with L. paracasei subsp. paracasei being, as expected, the least vulnerable to CFB. Although CJ at a concentration of 0.5 mL/mL showed a bactericidal effect on the growth of S. mutans, A. naeslundii proved more sensitive to the presence of CJ in the growth medium, as demonstrated by the inhibitory effect of CJ on A. naeslundii, which was seen even at concentrations as low as 0.03125–0.125 mL/mL. S. mutans seemed to be more vulnerable to CFB than A. naeslundii. The results allow us to state that CFB may be further investigated as a possible safe adjunct in oral disease treatment on account of its action against selected oral pathogens, and not against the presumably beneficial Lactobacillus paracasei subsp. paracasei. In addition, bioelectrical impedance measurement appears to be a rapid alternative method for evaluating the antimicrobial activity of fruit beverages, but its utility should be confirmed with tests on other bacteria.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CFB:
-
Cranberry functional beverage
- SDPP:
-
Serial dilutions pour plate
- EIM:
-
Electrical impedance measurements
- CJ:
-
Cranberry juice
- LOD:
-
Level of detection
- RC:
-
Reference culture
- cfu:
-
Colony forming units
- IDT:
-
Impedance detection time
References
Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7.
Joshipura KJ, Muñoz-Torres FJ, Morou-Bermudez E, Patel RP. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide Biol Chem. 2017;71:14–20.
Saleem HGM, Seers CA, Sabri AN, Reynolds EC. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016;16:214.
Yazdankhah SP, Scheie AA, Høiby EA, Lunestad B-T, Heir E, Fotland TØ, et al. Triclosan and antimicrobial resistance in bacteria: an overview. Microb Drug Resist Larchmt N. 2006;12:83–90.
Long J, Cai Q, Steinwandel M, Hargreaves MK, Bordenstein SR, Blot WJ, et al. Association of oral microbiome with type 2 diabetes risk. J Periodontal Res. 2017;52:636–43.
Sampaio-Maia B, Caldas IM, Pereira ML, Pérez-Mongiovi D, Araujo R. The oral microbiome in health and its implication in oral and systemic diseases. Adv Appl Microbiol. 2016;97:171–210.
Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593–615.
Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide Biol Chem. 2008;19:333–7.
Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2013;55:93–100.
Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens. 2015;28:572–5.
McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med. 2015;36:1177–85.
Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2:611–25.
Zare Javid A, Maghsoumi-Norouzabad L, Ashrafzadeh E, Yousefimanesh HA, Zakerkish M, Ahmadi Angali K, et al. Impact of cranberry juice enriched with omega-3 fatty acids adjunct with nonsurgical periodontal treatment on metabolic control and periodontal status in type 2 patients with diabetes with periodontal disease. J Am Coll Nutr. 2018;37:71–9.
La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010;54:1778–84.
Steinberg D, Feldman M, Ofek I, Weiss EI. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother. 2004;54:86–9.
Babu J, Blair C, Jacob S, Itzhak O. Inhibition of Streptococcus gordonii metabolic activity in biofilm by cranberry juice high-molecular-weight component. J Biomed Biotechnol. 2012;2012:590384.
Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, et al. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol Lett. 2006;257:50–6.
Koo H, Duarte S, Murata RM, Scott-Anne K, Gregoire S, Watson GE, et al. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010;44:116–26.
Feng G, Klein MI, Gregoire S, Singh AP, Vorsa N, Koo H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutans glucan-mediated adhesion and transcriptome responses within biofilms. Biofouling. 2013;29:629–40.
Philip N, Bandara HMHN, Leishman SJ, Walsh LJ. Effect of polyphenol-rich cranberry extracts on cariogenic biofilm properties and microbial composition of polymicrobial biofilms. Arch Oral Biol. 2019;102:1–6.
Weiss EL, Lev-Dor R, Sharon N, Ofek I. Inhibitory effect of a high-molecular-weight constituent of cranberry on adhesion of oral bacteria. Crit Rev Food Sci Nutr. 2002;42(3 Suppl):285–92.
Yamanaka A, Kimizuka R, Kato T, Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol Immunol. 2004;19:150–4.
Labrecque J, Bodet C, Chandad F, Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J Antimicrob Chemother. 2006;58:439–43.
Polak D, Naddaf R, Shapira L, Weiss EI, Houri-Haddad Y. Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. gingivalis and F. nucleatum mixed infection. J Periodontol. 2013;84:1019–25.
Feldman M, Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113:438–47.
Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. 2015;145:1185–93.
Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42:589–92.
Philip N, Bandara HMHN, Leishman SJ, Walsh LJ. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur J Oral Sci. 2019;127:122–9.
Bodet C, Chandad F, Grenier D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. J Periodontal Res. 2007;42:159–68.
La VD, Howell AB, Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J Dent Res. 2009;88:627–32.
Tipton DA, Babu JP, Dabbous MK. Effects of cranberry components on human aggressive periodontitis gingival fibroblasts. J Periodontal Res. 2013;48:433–42.
Chew B, Mathison B, Kimble L, McKay D, Kaspar K, Khoo C, et al. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial. Eur J Nutr. 2018. https://doi.org/10.1007/s00394-018-1643-z.
Simão TNC, Lozovoy MAB, Simão ANC, Oliveira SR, Venturini D, Morimoto HK, et al. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br J Nutr. 2013;110:1885–94.
Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res N Y N. 2011;31:190–6.
de Rocha IFO, Bolini HMA. Passion fruit juice with different sweeteners: sensory profile by descriptive analysis and acceptance. Food Sci Nutr. 2015;3:129–39.
Freitas MLF, de Dutra MBL, Bolini HMA. Sensory profile and acceptability for pitanga (Eugenia uniflora L.) nectar with different sweeteners. Food Sci Technol Int Cienc Tecnol Los Aliment Int. 2016;22:720–31.
Woźniewicz M, Nowaczyk PM, Kurhańska-Flisykowska A, Wyganowska-Świątkowska M, Lasik-Kurdyś M, Walkowiak J, et al. Consumption of cranberry functional beverage reduces gingival index and plaque index in patients with gingivitis. Nutr Res. 2018;58:36–45.
Biesaga M, Pyrzyńska K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013;136:46–54.
Mildner-Szkudlarz S, Siger A, Szwengiel A, Bajerska J. Natural compounds from grape by-products enhance nutritive value and reduce formation of CML in model muffins. Food Chem. 2015;172:78–85.
Nuutila AM, Kammiovirta K, Oksman-Caldentey K-M. Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem. 2002;76:519–25.
Noble PA. Hypothetical model for monitoring microbial growth by using capacitance measurements—a minireview. J Microbiol Methods. 1999;37:45–9.
Paquet J, Lacroix C, Audet P, Thibault J. Electrical conductivity as a tool for analysing fermentation processes for production of cheese starters. Int Dairy J. 2000;10:391–9.
Wawerla M, Stolle A, Schalch B, Eisgruber H. Impedance microbiology: applications in food hygiene. J Food Prot. 1999;62:1488–96.
Ben-Yoav H, Freeman A, Sternheim M, Shacham-Diamand Y. An electrochemical impedance model for integrated bacterial biofilms. Electrochim Acta. 2011;56:7780–6.
Dweik M, Stringer RC, Dastider SG, Wu Y, Almasri M, Barizuddin S. Specific and targeted detection of viable Escherichia coli O157:H7 using a sensitive and reusable impedance biosensor with dose and time response studies. Talanta. 2012;94:84–9.
Zhang X, Wang X, Cheng H, Zheng Y, Zhao J, Qu K. A universal automated method for determining the bacteriostatic activity of nanomaterials. J Hazard Mater. 2021;413:125320.
Sibirtsev VS, Naumov IA, Kuprina EÉ, Olekhnovich RO. Use of impedance biotesting to assess the actions of pharmaceutical compounds on the growth of microorganisms. Pharm Chem J. 2016;50:481–5.
Lasik M, Dobrucka R, Konieczny P. Impedimetric test for rapid determination of performic acid (PFA) biocidal activity toward Escherichia Coli. Acta Sci Pol Technol Aliment. 2013;12:385–94.
Chorianopoulos NG, Giaouris ED, Skandamis PN, Haroutounian SA, Nychas G-JE. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J Appl Microbiol. 2008;104:1586–96.
Badet C, Thebaud NB. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. 2008;2:38–48.
Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2:435–44.
Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. Actinomyces naeslundii in initial dental biofilm formation. Microbiology. 2009;155:2116–26.
Kõll-Klais P, Mändar R, Leibur E, Marcotte H, Hammarström L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20:354–61.
Teanpaisan R, Piwat S, Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. 2011;53:452–9.
Wannun P, Piwat S, Teanpaisan R. Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe. 2014;27:17–21.
Lacombe A, McGivney C, Tadepalli S, Sun X, Wu VCH. The effect of American cranberry (Vaccinium macrocarpon) constituents on the growth inhibition, membrane integrity, and injury of Escherichia coli O157:H7 and Listeria monocytogenes in comparison to Lactobacillus rhamnosus. Food Microbiol. 2013;34:352–9.
Lacombe A, Wu VCH, White J, Tadepalli S, Andre EE. The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiol. 2012;30:124–31.
Torres AF, Mesquita PAR, Campos FM, Couto JA, Tóth IV, Rangel AOSS, et al. Development of a flow injection method for monitoring cell membrane damage of wine lactic acid bacteria. Microchim Acta. 2007;159:87–93.
Lacombe A, Wu VCH. The potential of berries to serve as selective inhibitors of pathogens and promoters of beneficial microorganisms. Food Qual Saf. 2017;1:3–12.
Jasnowska-Małecka J. Wzrost Lactobacillus plantarum w obecności wybranych związków polifenolowych. Acta Sci Pol Biotechnol. 2016;15:17–26.
Garcia EF, de Oliveira AA, Luciano WA, de Albuquerque TMR, de Oliveira Arcanjo NM, Madruga MS, et al. The performance of five fruit-derived and freeze-dried potentially probiotic Lactobacillus strains in apple, orange, and grape juices. J Sci Food Agric. 2018;98:5000–10.
Sato T, Watanabe K, Toyama T, Kumada H, Sasaki H, Hamada N. The role of Actinomyces naeslundii peptidoglycan in alveolar bone resorption. J Oral Biosci. 2014;56:54–7.
de Medeiros AKB, de Melo LA, Alves RAH, Barbosa GAS, de Lima KC, da Porto Carreiro AF. Inhibitory effect of cranberry extract on periodontopathogenic biofilm: an integrative review. J Indian Soc Periodontol. 2016;20:503–8.
Weiss EI, Lev-Dor R, Kashamn Y, Goldhar J, Sharon N, Ofek I. Inhibiting interspecies coaggregation of plaque bacteria with a cranberry juice constituent [published erratum appear in J Am Dent Assoc 1999 Jan; 130(1):36 and 1999 Mar; 130(3):332]. J Am Dent Assoc. 1998;129:1719–23.
Banas JA, Vickerman MM. Glucan-binding proteins of the oral streptococci. Crit Rev Oral Biol Med. 2003;14:89–99.
Banas JA. Virulence properties of Streptococcus mutans. Front Biosci J Virtual Libr. 2004;9:1267–77.
Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86.
Hung W-C, Tsai J-C, Hsueh P-R, Chia J-S, Teng L-J. Species identification of mutans streptococci by groESL gene sequence. J Med Microbiol. 2005;54(Pt 9):857–62.
Len ACL, Harty DWS, Jacques NA. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiol Read Engl. 2004;150(Pt 5):1339–51.
Napimoga MH, Höfling JF, Klein MI, Kamiya RU, Gonçalves RB. Transmission, diversity and virulence factors of Streptococcus mutans genotypes. J Oral Sci. 2005;47:59–64.
Welin-Neilands J, Svensäter G. Acid tolerance of biofilm cells of Streptococcus mutans. Appl Environ Microbiol. 2007;73:5633–8.
Gregoire S, Singh AP, Vorsa N, Koo H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. J Appl Microbiol. 2007;103:1960–8.
Alberto MR, Rinsdahl Canavosio MA, Manca de Nadra MC. Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Electron J Biotechnol. 2006;9:205–9.
Gulube Z, Patel M. Effect of Punica granatum on the virulence factors of cariogenic bacteria Streptococcus mutans. Microb Pathog. 2016;98:45–9.
Ferrazzano GF, Scioscia E, Sateriale D, Pastore G, Colicchio R, Pagliuca C, et al. In vitro antibacterial activity of pomegranate juice and peel extracts on cariogenic bacteria. BioMed Res Int. 2017;2017:2152749.
Behera S, Khetrapal P, Punia SK, Agrawal D, Khandelwal M, Lohar J. Evaluation of antibacterial activity of three selected fruit juices on clinical endodontic bacterial strains. J Pharm Bioallied Sci. 2017;9(Suppl 1):S217–21.
Jakubovics NS. Talk of the town: interspecies communication in oral biofilms. Mol Oral Microbiol. 2010;25:4–14.
Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16:729–39.
Al-hebshi NN, Shuga-Aldin HM, Al-Sharabi AK, Ghandour I. Subgingival periodontal pathogens associated with chronic periodontitis in Yemenis. BMC Oral Health. 2014. https://doi.org/10.1186/1472-6831-14-13.
Díaz PI, Kolenbrander PE. Subgingival biofilm communities in health and disease. Rev Clínica Periodoncia Implantol Rehabil Oral. 2009;2:187–92.
Saads Carvalho T, Lussi A. Chapter 9: acidic beverages and foods associated with dental erosion and erosive tooth wear. In: Zohoori FV, Duckworth RM, editors. Monographs in oral science. S Karger AG; 2020. p. 91–8.
Enam F, Mursalat M, Guha U, Aich N, Anik MI, Nisha NS, et al. Dental erosion potential of beverages and bottled drinking water in Bangladesh. Int J Food Prop. 2017;20:2499–510.
Acknowledgements
Not applicable.
Funding
This work was financed by the National Science Center, Poland (Project Number N N 312 124139).
Author information
Authors and Affiliations
Contributions
MW, JB, and MLK conceived and designed the experiments. ERK prepared the beverages. ASz analysed phenolic compounds of beverages. PMN and MLK performed the experiments and analysed the data. PMN, MW, and JB wrote the manuscript with input from all the other authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nowaczyk, P.M., Bajerska, J., Lasik-Kurdyś, M. et al. The effect of cranberry juice and a cranberry functional beverage on the growth and metabolic activity of selected oral bacteria. BMC Oral Health 21, 660 (2021). https://doi.org/10.1186/s12903-021-02025-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-021-02025-w