Abstract
Background
This study aimed to examine radiologic microarchitectural changes in the mandibles of ovariectomized (OVX) rats through a systematic review and meta-analysis and to identify factors of the OVX rat model that influence on the bone microstructure.
Methods
Eligible articles were identified by searching electronic databases, including Embase, Medline, Web of Science, and KoreaMed, for articles published from January 1966 to November 2017. Two reviewers independently performed study selection, data extraction, and quality assessment. The pooled standardized mean difference (SMD) with 95% confidence intervals was calculated using a random-effects model. Subgroup analysis and meta-regression were performed to explore the effect of potential sources on the outcomes. The reliability of the results was assessed by sensitivity analysis and publication bias.
Results
Of 1160 studies, 16 studies (120 OVX and 120 control rats) were included in the meta-analysis. Compared to the control group, the OVX rats’ trabecular bone volume fraction (SMD = − 2.41, P < 0.01, I2 = 81%), trabecular thickness (SMD = − 1.73, P < 0.01, I2 = 73%) and bone mineral density (SMD = − 0.95, P = 0.01, I2 = 71%) displayed the bone loss consistent with osteoporosis. The trabecular separation (SMD = 1.66, P < 0.01, I2 = 51%) has widen in the OVX mandibular bone in comparison to the control group. However, the trabecular number showed no indication to detect the osteoporosis (SMD = − 0.45, P = 0.38, I2 = 76%). The meta-regression indicated that longer post-OVX periods led to greater changes in bone mineral density (β = − 0.104, P = 0.017). However, the rats’ age at OVX was not linked to bone microstructure change.
Conclusions
Using meta-regression and sensitivity analysis techniques, heterogeneity across the micro CT studies of OVX-induced osteoporosis was found. The major factors of heterogeneity were the region of interest and post-OVX period. Our assessment can assist in designing experiments to maximize the usefulness of OVX rat model.
Similar content being viewed by others
Background
Osteoporosis is a condition defined by reduced bone mass and microarchitectural deterioration of bone tissue, leading to a severe risk of deformity, pain, or skeletal fracture [1, 2]. Osteoporosis has become a major public health concern [3] which commonly occurs in postmenopausal women as a result of ovarian atrophy and the related decrease in circulating estrogens [4]. Osteoporosis is known to affect all skeletal sites, but the degree of bone loss and microarchitectural changes are not uniform [5]. Mandibular bony changes in systemic osteoporosis are a critical concern in the dental field as the success of implant osseointegration and another dental surgery is associated with the bone quality and quantity of the mandible [6, 7].
Ovariectomized (OVX) rats have been used as a preclinical model of postmenopausal humans to investigate mandibular changes [8, 9]. In OVX rats, bone mineral density (BMD) changes are similar to those in humans; therefore, BMD can be used as a common indicator of bone mass [10]. Some studies using OVX rats reported a relationship between systemic osteoporosis and mandibular bone loss [6, 11,12,13]; however, others have suggested a weak relationship, or no relationship at all [14,15,16,17]. The divergent results in the previous literature make it challenging to draw firm conclusions.
To find a reliable result in OVX rat studies, it is important to identify the factors that influence on bone structural changes. These animal studies can detect quite a heterogeneity in terms of species, designs, and intervention protocols compared to clinical trials [18]. In various studies of OVX rats, there are inconsistencies in the rat strain, the age of the rats, and post-OVX period. In addition, the irregular shape of the mandible hinders the establishment of criteria for observation site selection, which led to inconsistencies in the region of interest (ROI) among previous studies [19]. These factors underscore the need to establish a standard model by further investigating the characteristics of studies of OVX-induced osteoporosis. In such a context, meta-analysis can be a constructive tool to shed light on, and perhaps resolve, the lack of existing consensus.
Recently, researchers have examined trabecular morphology by using micro-computed tomography (micro-CT) to characterize bone deterioration in OVX rats [14]. Micro-CT has advantages, such as being non-destructive, fast, and easy compared to histological sections [20]. We aimed to examine radiologic microarchitectural changes in the mandibles of OVX rats through a systematic review and meta-analysis and to identify factors of the OVX rat model that influence on the bone microstructure.
Methods
Literature search and study selection
The systematic review and meta-analysis were conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement [21]. Studies of radiologic microarchitecture in OVX rats were searched in Embase, Medline, Web of Science, and KoreaMed (published from January 1966 to November 2017) using the following keywords: rat, rats, mandible, jaw bone, alveolar bone, osteoporotic, osteoporosis, ovariectomy, ovariectomized, and postmenopausal. Two of the authors (JHL and YHK) reviewed the title and abstract of the publications found in the literature search and made a preliminary selection. The final selection of studies was independently made by the same 2 individuals, according to the inclusion and exclusion criteria presented below (Table 1). Disagreements about the selection of studies and interpretation of data were settled by a third author (CL), and consensus was reached after discussion with all authors.
Data extraction
Data extraction was done independently by 2 authors (JHL and YHK). For each eligible study, we extracted data relating to study characteristics and outcomes using a pre-defined form including the name of the first author, the year of publication, the strain and number of the OVX and control rats, the rats’ age at OVX, the period after OVX surgery, the ROI of the mandible (mandibular body, mandibular condyle, M1 interradicular septum) and microarchitectural outcome (BV/TV, Tb.Th, Tb.Sp, Tb.N, BMD). Studies with more than one mandibular ROI were included independently in the meta-analysis. The mean value and standard deviation of the above data, as well as the number of OVX and control rats, were extracted for the meta-analysis. The data reported in graphs were estimated with GetData Graph Digitizer version 2.26 (Fedorov. S, 2013, Getdata-graph-digitizer.com, Russia).
Quality assessment
The methodological quality of the individual studies was assessed by 2 authors (JHL and YHK) independently based on the Collaborative Approach to Meta-analysis and the Review of Animal Data from Experimental Studies checklists [22] and the Instruments for Assessing Risk of Bias and Other Methodological Criteria of Published Animal Studies [23]. These guidelines assess quality using the following criteria: (1) sample size calculation; (2) random allocation to treatment; (3) husbandry conditions (e.g., breeding program, light/dark cycle, temperature, type of food, access to water, and environmental enrichment); (4) blinded assessment of outcomes; (5) compliance with animal welfare regulations; (6) disclosure of conflicts of interest; and (7) peer-reviewed publication. The quality scale ranged from 0 to 7 points. A third reviewer (CL) settled any disagreements between the 2 reviewers.
Statistical analysis
Microarchitectural changes in the mandibles of OVX rats were identified using the standardized mean difference (SMD) with 95% confidence intervals (CIs), using a random-effects model with the DerSimonian-Laird method. To explore heterogeneity among the studies, the Cochran Q statistic and Higgins’s I2 statistic were used for each outcome. P-values less than 0.1 or an I2 statistic greater than 50% was defined as statistically significant heterogeneity [24].
The subgroup analysis and meta-regression analysis were performed to identify factors potentially responsible for heterogeneity among the studies included in the meta-analysis. For potential sources that were categorical variables, subgroup analysis was used to compare their effect sizes. Subgroup analysis based on analysis of variance assumes between-study variance (τ2) to be the same in all subgroups. For potential sources that were continuous variables, meta-regression was used as an indicator of the possible influence on the effect size. The potential sources of interest were the strain of the rats, the age of the rats, the ROI of the mandible, the period after OVX, and the quality score of literature. Additionally, we performed a sensitivity analysis to assess the robustness of our findings.
The contour-enhanced funnel plot, Egger’s regression test, and Begg’s rank correlation test were applied to assess the presence of publication bias [25, 26]. When there was evidence of publication bias, potentially missing studies were imputed using the trim-and-fill method [27]. The number of missing studies was estimated using fail-safe numbers, as an additional method to assess publication bias. It has been suggested that a fail-safe number exceeding 5 K + 10 (K = N studies in the meta-analysis) should be considered acceptable [28]. Statistical analysis was performed using Review Manager (version 5.3.5 for Windows; the Cochrane Collaboration, Oxford, UK) and R software version 3.4.3 (The R Foundation for Statistical Computing; Vienna, Austria). All statistical tests were 2-sided, and P values < 0.05 were considered to indicate statistical significance.
Results
Selection of literature
The literature selection strategy is shown in Fig. 1. A total of 1160 publications from electronic databases were identified through electronic and manual searches. After screening the titles and abstracts, 45 studies remained. The full text of the 45 remaining studies was thoroughly reviewed. Of those, 29 studies were excluded due to the use of medication or treatment as an intervention for the OVX rats; the assessment of sites other than the mandible; the absence of bone morphometric parameters using micro-CT or BMD measurements; being reviews, letters, or expert opinion publications; or the absence of available full-text versions. Subsequently, 16 articles were finally selected for the qualitative synthesis.
Characteristics of the included studies
The characteristics of the eligible studies are shown in Table 2. The meta-analysis included 16 comparative assessments of post-OVX microarchitectural changes (120 rats in the OVX group and 120 rats in the control group). In each study, one particular ROI such as mandibular body or condyle was selected from multiple comparisons into the Meta-analysis (Kim KH [29] and Kuroda S [30]). One of the 16 studies was written in Korean [29], and the rest were in English.
Different rat strains were used in each study, including Sprague-Dawley, Wistar, Fischer, and Lewis-Brown-Norway rats. The age of the rats when the OVX was performed ranged from 4 weeks to 25 weeks. Two studies did not report the age of the rats [19, 40]. The sample size of the OVX and control groups in all 16 studies ranged from 3 to 10. The post-OVX period for radiologic microarchitecture assessments was performed varied from 4 weeks to 48 weeks. Thirteen studies used 1 post-OVX period, while 3 studies had 4 or more post-OVX periods. In such cases, only the final measurements were included. Moreover, the bone morphometric parameters of trabecular microarchitecture were diverse. Data on BMD were found in 10 studies [12, 15, 19, 29, 30, 32,33,34, 36], trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) were available in 9 studies [6, 12, 31,32,33, 35, 38,39,40], and trabecular number (Tb.N) was reported in 7 studies [6, 12, 32, 33, 35, 38, 40]. The most commonly selected ROIs in the mandible was the mandibular body and the interradicular septum of the first molar (M1). The rest of the studies used the entire mandible and mandibular condyles.
Quality assessment
The risk of bias for all 16 studies is shown in Table 3. Eight studies received a quality score of 4 or higher [6, 12, 15, 31, 34, 36, 38, 40]. The lowest quality score was 1 [19, 29], and the highest quality score was 6 [31]. No study described the sample size calculation, and 2 studies used a blinding method in outcome assessment [31, 36]. Twelve studies mentioned the random allocation of rats to groups [6, 12, 15, 19, 31,32,33,34,35,36,37,38, 40]; however, none stated the precise method of randomization. Ten studies mentioned the husbandry conditions of the rats [6, 12, 15, 29, 31, 34, 36,37,38], and all studies except for 4 explained their compliance with animal welfare regulations [19, 29, 37, 39]. A statement of potential conflicts of interest was only presented in 4 studies [12, 31, 35, 40].
Meta-analysis
BV/TV changes in the mandibles of OVX rats
Nine studies included BV/TV as an outcome. Meta-analysis using a random-effects model indicated that the mandibles of the OVX rats exhibited significantly lower BV/TV values (SMD = − 2.41, 95% CI: − 3.51 to − 1.31, P < 0.01, Fig. 2). High heterogeneity among the included studies was detected (Cochrane Q test: P < 0.01, I2 = 81%, Fig. 2).
Tb.Th changes in the mandibles of OVX rats
Tb.Th was measured in 9 studies. The meta-analysis using a random-effects model revealed that the OVX group had significantly lower Tb.Th values than the control group (SMD = − 1.73, 95% CI: − 2.56 to − 0.91, P < 0.01, Fig. 3). High heterogeneity among the included studies was detected (Cochrane Q test: P < 0.01, I2 = 73%, Fig. 3).
Tb.Sp changes in the mandibles of OVX rats
Nine studies assessed Tb.Sp. The meta-analysis using a random-effects model revealed that the OVX group has significantly higher Tb.Sp values than the control group (SMD = 1.66, 95% CI: 1.05 to 2.26, P < 0.01, Fig. 4). High heterogeneity among the included studies was detected (Cochrane Q test: P = 0.04, I2 = 51%, Fig. 4).
Tb.N changes in the mandibles of OVX rats
Only 7 studies included measures of Tb.N, with a very small effect size (SMD = − 0.45, 95% CI: − 1.47 to 0.56); non-significant effects were found (P = 0.38, Fig. 5).
BMD changes in the mandibles of OVX rats
An analysis of BMD data comprising 12 intergroup comparisons, generated from 16 original studies, was performed. The meta-analysis using a random-effects model revealed that the OVX group had significantly lower BMD values than the control group (SMD = − 0.95, 95% CI: − 1.71 to − 0.20, P = 0.01, Fig. 6). High heterogeneity among the included studies was detected (Cochrane Q test: P < 0.001, I2 = 71%, Fig. 6).
Subgroup analysis
To further explore the potential sources leading to heterogeneity, we performed a subgroup analysis based on strain, ROI and quality score. After stratification by strain, no subgroup differences were displayed. The comparison of the OVX and control group in Sprague-Dawley rats showed significant differences in BV/TV, Tb.Th, Tb.Sp, and BMD outcome. Sprague-Dawley rat group has sustained the high heterogeneity between the studies. In comparison, the subgroup of Wistar rats showed significant differences between the OVX and control groups in BV/TV, Tb.Th, and Tb.Sp and revealed a reduction in heterogeneity in parameters except for BMD.
Stratification by ROI led to a statistically significant result in subgroup differences among the mandibular body, mandibular condyle, and interradicular septum of the M1. The interradicular septum of the M1 subgroup has shown significant differences between the OVX and control groups in BV/TV, Tb.Th, Tb.Sp and BMD outcomes. There was a reduction of heterogeneity in interradicular septum of the M1 except for Tb.N. Moreover, the mandibular body subgroup showed significant differences between the OVX and control groups in BV/TV, Tb.Sp and Tb.N, with no heterogeneity among the studies.
The Quality score has been divided by high quality and low-quality subgroup, the high-quality has 4 scores out of 7, low-quality has 3 scores out of 7. The high quality and low quality have not shown subgroup difference. In a high-quality group, there was a significant difference between OVX and control groups in Tb.Th, Tb.Sp. The low-quality group, there was a significant difference between OVX and control groups in BT/TV, Tb.Sp. Except for low-quality studies, there is no effect on heterogeneity. The detailed results of the subgroup analysis are shown in Table 4.
Meta-regression analysis
The potential influence of the characteristics of continuous variables such as the rats’ age at OVX and the post-OVX period was assessed with meta-regression. We discovered that the duration after OVX was the factor with a significant influence on the heterogeneity of BMD in the meta-analysis (β = − 0.106, 95% CI: − 0.20 to − 0.02, P = 0.017). In the meta-regression plot, the duration after OVX showed an inverse relationship with SMD (Fig. 7). The rats’ age showed no statistically significant effects on bone microstructure outcomes. Detailed results of the meta-regression analysis are presented in Table 5.
Sensitivity analysis
We conducted a sensitivity analysis to assess the robustness of our results. For studies of OVX rat on BMD, exclusion of the shorter post-OVX period sample [15, 19, 34, 36, 37] considerably enhanced the effect size (SMD = − 1.77, 95% CI: − 2.57 to − 0.96, P < 0.001). The heterogeneity (I2) among the studies was reduced from 71 to 21%.
In studies using the mandibular body with ROI, exclusion of the shorter post-OVX period sample considerably increased the effect size of BMD (SMD = − 1.09, 95% CI: − 1.95 to − 0.24, P = 0.012) and there was no heterogeneity among the studies (I2 = 0%, P = 0.873). For OVX rat using M1 interradicular septum, except for the shorter post-OVX period studies, increased the effect size of BMD (SMD = − 2.13, 95% CI: − 3.52 to − 0.73, P = 0.003) with slightly increased heterogeneity (I2 = 51%, P = 0.129).
Publication bias
The Begg’s funnel plot and the Egger’s test were used to assessing the publication bias of the studies. Contour-enhanced funnel plots revealed no evidence of publication bias for Tb. N or BMD (Fig. 8d and e), as confirmed by the Begg’s test and Egger test (Tb.N: PBegg = 1.00, PEgger = 0.386; BMD: PBegg = 0.484, PEgger = 0.214, Table 6). However, asymmetries were found in the contour-enhanced funnel plots for BV/TV, Tb.Th, and Tb.Sp indicating the presence of publication bias (Fig. 8a, b, and c). Moreover, the results of the Begg and Egger’s tests also provided clear evidence of publication bias (BV/TV: PBegg = 0.012, PEgger = 0.005; Tb.Th: PBegg = 0.037, PEgger = 0.034; Tb.Sp: PBegg = 0.022, PEgger = 0.022; Table 6).
Contour-enhanced funnel plot for the analysis of publication bias, combined with a trim-and-fill analysis. a Funnel plot of publication bias in BV/TV changes in the OVX rats. b Funnel plot of publication bias in Tb.Th changes in the OVX rats. c Funnel plot of publication bias in Tb.Sp changes in the OVX rats. d Funnel plot of publication bias in Tb. N changes in the OVX rats. e Funnel plot of publication bias in BMD changes in the OVX rats. OVX, ovariectomy; BV/TV, trabecular bone volume fraction; Tb.Th, trabecular number; Tb. Sp, trabecular thickness; Tb.N, trabecular separation; BMD, bone mineral density
The trim-and-fill method was implemented to estimate the influence of publication bias by imputing potentially missing studies. After adjusting for publication bias, the effect size of these 3 outcomes was reduced, yet they remained statistically significant. (BV/TV: P = 0.032, Tb.Th: P = 0.020, Tb.Sp: P < 0.001). Additionally, a high fail-safe number was found for each outcome (300 for BV/TV, 215 for Tb.Th, and 239 for Tb.Sp). This number represents the minimum number of unpublished studies required to make the meta-analysis non-significant. The full details of the publication bias analysis are shown in Table 6.
Discussion
Heterogeneity in animal studies is caused by variation in research methods; biological characteristics based on species, sex, and age; interventions; and measurements of the main effect [18, 41]. This meta-analysis of systemic osteoporosis and mandibular bone changes showed that the outcomes were heterogeneous across studies. Investigating the causes and effects of this heterogeneity can assist in designing experiments to maximize the usefulness of animal models.
BMD is commonly used in clinical analysis as a valuable tool for osteoporosis risk assessment [42]. Bone morphometric parameters such as BV/TV, Tb.Th, Tb.Sp and Tb. N are also commonly measured on micro-CT images to assess the quality of the bone [43]. This meta-analysis revealed radiologic microarchitectural change consistent with osteoporosis in the mandibles of OVX rats. BV/TV, Tb.Th, Tb.Sp and BMD displayed consistent with bone loss, in terms of the effect size, in the OVX group compared to control groups, while Tb. N did not show a significant bone loss. This result implies that Tb. N is an ambiguous parameter for detecting bone microstructure changes in the mandible. The Tb. N implicates average of the trabecular number which requires a long-term observation until the number of trabecular changes. Nonetheless, the thickness and separation denote the change of microstructure in a predictable time period to produce the matured outcome.
The meta-analysis performed in this study found heterogeneity across studies for all outcomes (BV/TV, Tb.Th, Tb.Sp, Tb.N, and BMD). The impact of various characteristics was investigated through a subgroup analysis, meta-regression, and sensitivity analysis.
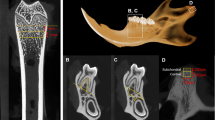
The trabecular bone in the femur and lumbar vertebrae has generally been analyzed with an ROI located 1 mm above the epiphyseal growth plate [44]. However, it is difficult to establish a unified ROI for the mandible due to the presence of teeth and the complex morphology of the mandible, which consists of the alveolar process, basal bone, and condyle [45]. In this meta-analysis, the mandibular body, condyle, M1 interradicular septum, and/or the entire mandible were selected as the ROI. In the subgroup analysis, the mandibular ROI was found to be an influential moderator of microarchitectural changes in response to estrogen deficiency. As Johnston and Ward [46] suggested, the M1 interradicular septum should be used as the ROI in the mandible due to its well-characterized site and distinctive response to estrogen depletion [46]. Thus, the molar region of the mandible is a definable model that has been widely used [47]. Similarly, the results of our subgroup analysis reflect significant differences in the M1 interradicular septum, showing robust bone microstructural changes between the OVX and control groups.
OVX-induced alterations in the proximal tibia, the femoral neck, and lumbar spine are known to reduce trabecular bone volume in fewer than 12 weeks after OVX [48,49,50,51]. However, the post-OVX period required for bone structural changes to occur in the mandible is not conclusively known. The majority of studies had cost and time restraints which restricts a single time period was observed for bone deterioration in the mandible based on the effects of osteoporosis. In our quantitative meta-analysis, meta-regression indicated an association between the post-OVX period and BMD changes in the rats’ mandible. This result reported that with longer post-OVX periods, a more notable decrease in BMD was exhibited. In addition, the sensitivity analysis revealed that the heterogeneity among the studies of BMD in OVX rats was reduced by excluding samples with a shorter post-OVX period. As the osteoporosis review article by Dervis [52] argued, insufficient duration of the experimental investigation leads to a failure to detect OVX-induced changes in the mandible. Johnston and Ward’s [46] review states that longer post-OVX periods are associated with greater effects on bone structural changes in the mandible. Therefore, a sufficient OVX duration should be considered as part of the design of studies using OVX rats.
The most common types of osteoporosis rat models are sexually mature models (3 months old) and skeletally mature models (12 months old) [8]. The notion of using skeletally mature animals is based on their similarity to human cases of postmenopausal bone loss [53]. In contrast, sexually mature models are used to eliminate the possibility of bone loss and disease caused by aging [8]. Most of the studies included chose 12-week-old rats as sexually mature rats, and rats aged 24 weeks as skeletally immature. However, 3 of the studies used rats under 8 weeks, which is known to be inappropriate from Kalu’s [8] review, which found that young rats may have lower bone mass due to repressed growth in contrast to control groups with rapid growth. Thus, bone loss in rats under 8 weeks may result from impaired growth, rather than accelerated bone loss, as observed following menopause. The exploration of heterogeneity among studies showed that the age of the rats did not affect heterogeneity. Our meta-regression results suggest that differences in the rats’ age at OVX had no effect on OVX-induced changes in the mandible. Therefore, our results concur with those of a previous study that reported that the confounding effect on bone loss was greatly reduced as rat growth slowed after 12 weeks of age [53]. Although skeletal growth is not complete, 12-week-old OVX rat models are most commonly used for osteoporosis modeling because they have similar characteristics to skeletally mature rats [54]. In addition, sexually mature rats respond much more rapidly to OVX than aged rats, reducing the time and cost of the study [53].
Publication bias is a considerable problem for the validity of meta-analyses, as studies with significant or positive results have a better chance to be published than studies with non-significant results [55]. Systematic reviews of animal studies are more vulnerable to publication bias than those of clinical trials [56]. Non-significant or unfavorable studies may not be published for commercial reasons [38]. This meta-analysis identified clear publication bias for the outcomes of BV/TV, Tb.Th, and Tb.Sp. However, the trim-and-fill method indicated that the publication bias led to minor changes in the effect size and demonstrated a high fail-safe number. Based on this result, our meta-analysis can be considered reliable and not meaningfully influenced by publication bias.
The results of the meta-analysis must be interpreted cautiously in accordance with the limitations of this study. As only full-text articles in English and Korean were included in the meta-analysis, eligible studies in other languages may have been overlooked. The absence of clarification about the random allocation method and lack of blind assessments reflect low methodological quality in the included studies. The statistical power may not have been sufficient, as a limited number of studies were included in the subgroup analysis and meta-regression. However, the findings of this study can fully assist in the design and interpretation of further studies and in the selection of an OVX animal model.
Conclusions
In conclusion, our study indicates that the mandibular microarchitectural changes and OVX-induced osteoporosis in the rat model show a significant relationship. However, heterogeneity showed that the differences in the ROI and post-OVX period had an effect on bone microstructural changes in OVX rats. Based on our heterogeneity assessment, the following experimental design for micro CT studies of mandibular osteoporosis in a rat model can be proposed: a 12 week-old rat model, use of the M1 interradicular septum as the ROI, and a sufficient observation period after ovariectomy.
Availability of data and materials
All data generated or analyzed during this study are included within the article.
Abbreviations
- BMD:
-
Bone mineral density
- BV/TV:
-
Trabecular bone volume fraction
- CIs:
-
Confidence intervals
- M1:
-
First molar
- Micro-CT:
-
Micro-computed tomography
- OVX:
-
Ovariectomized
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-analysis
- ROI:
-
Region of interest
- SMD:
-
Standardized mean difference
- Tb.N:
-
Trabecular number
- Tb.Sp:
-
Trabecular separation
- Tb.Th:
-
Trabecular thickness
References
Jiang G, Matsumoto H, Fujii A. Mandible bone loss in osteoporosis rats. J Bone Miner Metab. 2003;21(6):388–95.
Anon. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporosis Int. 2005;16(2):S129–38.
Zaffe D, Paganelli C, Cocchi D. Induction and pharmacological treatment of oral osteopenia in rats. Minerva Stomatol. 1999;48(3):45–62.
Gotfredsen A, Hassager C, Christiansen C. Total and regional bone mass in healthy and osteoporotic women. Basic Life Sci. 1990;55:101–6.
Hsu PY, Tsai MT, Wang SP, Chen YJ, Wu J, Hsu JT. Cortical bone morphological and trabecular bone microarchitectural changes in the mandible and femoral neck of ovariectomized rats. PLoS One. 2016;11(4):e0154367.
Li Y, He S, Hua Y, Hu J. Effect of osteoporosis on fixation of osseointegrated implants in rats. J Biomed Mater Res B Appl Biomater. 2017;105(8):2426–32.
Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15(3):175–91.
Francisco JI, Yu Y, Oliver RA, Walsh WR. Relationship between age, skeletal site, and time post-ovariectomy on bone mineral and trabecular microarchitecture in rats. J Orthop Res. 2011;29(2):189–96.
Turner RT, Maran A, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. Animal models for osteoporosis. Rev Endocr and Metab Disord. 2001;2(1):117–27.
Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodontal Res. 2002;37(2):161–5.
Li CL, Liu XL, Cai WX, Lu WW, Zwahlen RA, Zheng LW. Effect of ovariectomy on stimulating intracortical remodeling in rats. Biomed Res Int. 2014;2014:421431.
Liu H, Li W, Liu YS, Zhou YS. Bone micro-architectural analysis of mandible and tibia in ovariectomised rats: a quantitative structural comparison between undecalcified histological sections and micro-CT. Bone Joint Res. 2016;5(6):253–62.
Esteves CM, Moraes RM, Gomes FC, Marcondes MS, Lima GMG, Anbinder AL. Ovariectomy-associated changes in interradicular septum and in tibia metaphysis in different observation periods in rats. Pathol Res Pract. 2015;211(2):125–9.
Moriya Y, Ito K, Murai S. Effects of experimental osteoporosis on alveolar bone loss in rats. J Oral Sci. 1998;40(4):171–5.
Hara T, Sato T, Oka M, Mori S, Shirai H. Effects of ovariectomy and/or dietary calcium deficiency on bone dynamics in the rat hard palate, mandible and proximal tibia. Arch Oral Biol. 2001;46(5):443–51.
Anbinder AL, Prado Mde A, Spalding M, Balducci I, Carvalho YR, da Rocha RF. Estrogen deficiency and periodontal condition in rats: a radiographic and macroscopic study. Braz Dent J. 2006;17(3):201–7.
Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 2014;55(3):418–26.
Liu H, Gao K, Lin H, Zhang Y, Li BB. Relative skeletal effects in different sites of the mandible with the proximal tibia during ovariectomy and the subsequent estrogen treatment. J Oral Implantol. 2015;41:386–90.
Chappard D, Retailleau-Gaborit N, Legrand E, Basle MF, Audran M. Comparison insight bone measurements by histomorphometry and microCT. J Bone Miner Res. 2005;20(7):1177–84.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9 w264.
Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–8.
Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environ Health Perspect. 2013;121(9):985–92.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, vol. 4. New Jersey: Wiley; 2011.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638.
Kim KH, Shin SH, Kim UK, Pack BS, Chung IK. Effect on bone mineral density (BMD) of mandible in estrogen - deficiency rat. Maxillofac Plast Reconstr Surg. 2002;24(2):99–107.
Kuroda S, Mukohyama H, Kondo H, Aoki K, Ohya K, Ohyama T, Kasugai S. Bone mineral density of the mandible in ovariectomized rats: analyses using dual energy X-ray absorptiometry and peripheral quantitative computed tomography. Oral Dis. 2003;9(1):24–8.
Jiang L, Shen X, Wei L, Zhou Q, Gao Y. Effects of bisphosphonates on mandibular condyle of ovariectomized osteoporotic rats using micro-ct and histomorphometric analysis. J Oral Pathol Med. 2017;46(5):398–404.
Liu XL, Li CL, Lu WW, Cai WX, Zheng LW. Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin Oral Implants Res. 2015;26(4):392–8.
Liu Z, Yan C, Kang C, Zhang B, Li Y. Distributional variations in trabecular architecture of the mandibular bone: an in vivo micro-CT analysis in rats. PLoS One. 2015;10(1):e0116194.
Liu ZS, Liu LS, Kang C, Xie Q, Zhang B, Li Y. Effects of estrogen deficiency on microstructural changes in rat alveolar bone proper and periodontal ligament. Mol Med Rep. 2015;12(3):3508–14.
Mavropoulos A, Kiliaridis S, Rizzoli R, Ammann P. Normal masticatory function partially protects the rat mandibular bone from estrogen-deficiency induced osteoporosis. J Biomech. 2014;47(11):2666–71.
Patullo IM, Takayama L, Patullo RF, Jorgetti V, Pereira RM. Influence of ovariectomy and masticatory hypofunction on mandibular bone remodeling. Oral Dis. 2009;15(8):580–6.
Tanaka M, Ejiri S, Kohno S, Ozawa H. The effect of aging and ovariectomy on mandibular condyle in rats. J Prosthet Dent. 1998;79(6):685–90.
Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(4):495–502.
Yang J, Pham SM, Crabbe DL. Effects of oestrogen deficiency on rat mandibular and tibial microarchitecture. Dentomaxillofac Radiol. 2003;32(4):247–51.
Sun W, Wang YQ, Yan Q, Lu R, Shi B. Effects of Er-Zhi-Wan on microarchitecture and regulation of Wnt/beta-catenin signaling pathway in alveolar bone of ovariectomized rats. J Huazhong Univ Sci Technolog Med Sci. 2014;34(1):114–9.
Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, Lu L, Xiao DQ. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res. 2014;34(6):467–77.
Butz S, Wüster C, Scheidt-Nave C, Götz M, Ziegler R. Forearm BMD as measured by peripheral quantitative computed tomography (pQCT) in a German reference population. Osteoporosis Int. 1994;4(4):179–84.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86.
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–30.
Elovic RP, Hipp JA, Hayes WC. Ovariectomy decreases the bone area fraction of the rat mandible. Calcif Tissue Int. 1995;56(4):305–10.
Johnston BD, Ward WE. The ovariectomized rat as a model for studying alveolar bone loss in postmenopausal women. Biomed Res Int. 2015;2015:635023.
Faot F, Chatterjee M, de Camargos GV, Duyck J, Vandamme K. Micro-CT analysis of the rodent jaw bone micro-architecture: a systematic review. Bone Rep. 2015;2:14–24.
Li M, Shen Y, Wronski TJ. Time course of femoral neck osteopenia in ovariectomized rats. Bone. 1997;20(1):55–61.
Wronski TJ, Walsh CC, Ignaszewski LA. Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone. 1986;7(2):119–23.
Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43(3):179–83.
Wronski TJ, Dann LM, Horner SL. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1989;10(4):295–301.
Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):349–56.
Grynpas MD, Chachra D, Lundon K. Bone quality in animal models of osteoporosis. Drug Dev Res. 2000;49(3):146–58.
Leitner MM, Tami AE, Montavon PM, Ito K. Longitudinal as well as age-matched assessments of bone changes in the mature ovariectomized rat model. Lab Anim. 2009;43(3):266–71.
Yazdi MM, Jamalaldini MH, Sobhan MR, Jafari M, Mazaheri M, Zare-Shehneh M, Neamatzadeh H. Association of ESRalpha gene Pvu II T>C, XbaI a>G and BtgI G>a polymorphisms with knee osteoarthritis susceptibility: a systematic review and meta-analysis based on 22 case-control studies. Arch Bone Jt Surg. 2017;5(6):351–62.
ter Riet G, Korevaar DA, Leenaars M, Sterk PJ, Van Noorden CJF, Bouter LM, Lutter R, Elferink RPO, Hooft L. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS One. 2012;7(9):e43404.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2017R1D1A1B03033218). The funding body had no role in the design of the study or in the collection analysis and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SSH is the corresponding author and designed this study. JHL, YHK, CL, and BB conducted the literature search, study selection, data extraction and quality assessment. JHL performed the statistical analysis and wrote the draft of the manuscript. SSH, JHL, and CL revised the final version of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, JH., Han, SS., Lee, C. et al. Microarchitectural changes in the mandibles of ovariectomized rats: a systematic review and meta-analysis. BMC Oral Health 19, 128 (2019). https://doi.org/10.1186/s12903-019-0799-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-019-0799-0