Abstract
Background
High dose radiation therapy is commonly used in maxillofacial surgeries to treat a number of head and neck tumors. Despite its widespread use, little information is available regarding the effects of irradiation on bone cell viability and release of growth factors following dose-dependent irradiation.
Methods
Bone samples were collected from porcine mandibular cortical bone and irradiated at doses of 0, 7.5, 15, 30, 60 and 120 Grays. Thereafter, cell viability was quantified, and the release of growth factors including TGFβ1, BMP2, VEGF, IL1β and RANKL were investigated over time.
Results
It was observed that at only 7.5Gy of irradiation, over 85 % of cells were non-vital and by 60 Gy, all cells underwent apoptosis. Furthermore, over a 7-fold decrease in VEGF and a 2-fold decrease in TGFβ1 were observed following irradiation at all tested doses. Little change was observed for BMP2 and IL1β whereas RANKL was significantly increased for all irradiated samples.
Conclusions
These results demonstrate the pronounced effects of irradiation on bone-cell vitality and subsequent release of growth factors. Interestingly, the largest observed change in gene expression was the 7-fold decrease in VEGF protein following irradiation. Future research aimed at improving our understanding of bone following irradiation is necessary to further improve future clinical treatments.
Similar content being viewed by others
Background
High doses of irradiation therapy are routinely administered to patients for a large number of cancers affecting many organs [1, 2]. Furthermore, the use of bone allografts necessitates high dose irradiation for sample sterilization [1, 2]. Under normal circumstances, irradiation passes through a number of tissues where the minimization of doses is vital to the future survival of tissues. One tissue that commonly receives high doses of irradiation therapy in maxillofacial surgery is that of bone. Due to the high volume of bones found in the maxilla, it is common in head and neck procedures to pass high doses of irradiation through bony tissues [1, 2]. Common doses can range from 60 Gray to 70 Grays and such procedures have been highly successful for the treatment of many head and neck tumors [3, 4]. Despite successfully treating and managing these tumors, irradiation of bone has reported some drawbacks with a certain percentage of bone losing complete vitality and becoming necrotic [3, 4]. A group of experts have since recommended guidelines established for preventing the possibility of developing osteoradionecrosis of the jaw at doses exceeding 60 Grays [3, 4].
Furthermore, irradiation of bone is also commonly found in bone allograft sterilization procedures for bone tissue banking [5]. Although the full set of doses are commonly kept proprietary information [5], the release of subsequent growth factors from its content is a trademark commonly observed in demineralized bone allografts which may demonstrate signs of osteoinduction by showing signs of ectopic bone formation in various animal models. Irradiation of bone is also used in extracorporeal irradiation and has been implicates in not only oral and maxillofacial surgery, but also other disciplines such as otolaryngology [6] and orthopedics [7].
Marx et al. have been key oral maxillo-facial surgeons responsible for treating and developing treatment guidelines for patients presenting necrotic bone following irradiation [8–10]. While a number of attempts have been investigated to maintain optimal bone viability [11–13], a limited understanding of the cellular events that take place within bone following irradiation would benefit from further investigation.
Due to the widespread use of irradiation for various bone procedures including irradiation for tumors, sterilization of autologous bone transplants and bone tissue banking, it became of interest to our group to further investigate in a bench model the effects of dose-dependent irradiation on bone cell viability and release of growth factors. While it is known that irradiation is unfavorable for bone remodeling [14] and that an accumulation of evidence has been accumulating demonstrating that irradiation consequently affects microvasculature of bone-tissues [15], a detailed investigation of the in vitro mechanisms was studied to further increase our understanding of bone changes following irradiation. Therefore, the purpose of the present investigation was to determine the effects of irradiation on bone cell viability following irradiation at various single-doses including 0, 7.5, 15, 30, 60 and 120 Grays. Thereafter, bone graft morphology and surface proteins were analyzed via scanning electron microscopy and release of growth factors from the bone samples was quantified for TGFβ1, BMP2, VEGF, IL1β and RANKL at 15 min and 4 h following irradiation.
Methods
Bone collection
Bone was obtained from adult pigs (Metzgerei Balsiger, Wattenwil, Switzerland) and harvested from the buccal-sided mandibular cortical bone with a “bone scraper” (Hu-Friedy, Rotterdam, Netherlands) and placed into sterile plastic dishes as previously described [16]. Thereafter, bone was irradiated (single-dose) at the following doses: 0 (control), 7.5, 15, 30, 60 and 120 Grays. Briefly, bone samples were collected and exposed to 137Cs γ-rays at the dose rate of 0.83 Gy/min using a Gammacell®40 Exactor (Best Theratronics, Ottawa, Canada) for 0, 9, 18, 36, 72 and 144 min until the final dose of irradiation was reached. Thereafter, bone samples were immediately placed in a cell culture hood and experiments performed. For each experiment, four independent preparations of bone samples were available and all samples were performed in triplicate. Thereafter bone samples were either 1) fixed and assigned to scanning electron microscopy, 2) assigned for MTS analysis for cell viability or 3) left in PBS solution and samples collected after 15 min and 4 h for protein quantification using ELISA.
Scanning electron microscopy
Bone samples were fixed in 1 % glutaraldehyde and 1 % formaldehyde for 2 days for scanning electron microscopy (SEM). Following serial dehydration with ethanol, samples were critical point dried (Type M.9202 Critical Point Dryer, Roth & Co. Hatfield, PA, USA) and allowed to dry overnight as previously described [17, 18]. The following day, samples were sputter coated using a Balzers Union Sputtering Device (DCM-010, Balzers, Liechtenstein) with 10 nm of gold and analyzed microscopically using a Philips XL30 FEG scanning electron microscope to determine surface variations between samples.
Quantification of viable cells in bone samples
The cell viability in each of the bone samples was determined using the CellTiter 96® One Solution Cell Assay (MTS) (Promega, Madison, WI, USA) as previously described [19]. Briefly, 100 mg of harvested bone was treated with 80 μL of CellTiter96 aqueous solution dissolved in 400 μL of PBS. After 4 h of incubation, the cell viability was determined by measuring the absorbance at 490 nm on a 96-well plate-reader. Experiments were performed in triplicate with three independent experiments for each condition. Data was normalized to samples at 120 Gray with no signs of cell viability and analyzed for statistical significance using one-way analysis of variance with Tukey’s test.
ELISA protein quantification
Specific protein contents were determined for cell culture media incubated with 250 mg of bone samples. At time points 15 min and 4 h BMP2, TGFβ1, VEGF, IL1β and RANKL were quantified using an ELISA assays (RND Systems, Minneapolis, MN, USA) according to manufacturer’s protocol as previously described [17, 20]. Briefly, 100 μl of assay diluents and 50 μl of sample were incubated for 2 h at room temperature in antibody-precoated 96-well plates. Wells were washed 4 times with washing buffer, incubated for 2 h with peroxidase-conjugated antibody solution, washed again, followed by addition of 200 μl of substrate solution for 30 min and 50 μl of stopping solution for 30 min. Absorbance was measured at 450 nm on an Infinite 200 microplate reader (Tecan Group LTD, Männedorf, Switzerland). All samples were measured in triplicate and 3 independent experiments were performed. Statistical analysis was performed by two-way ANOVA with Bonferroni test.
Results
Scanning electron microscopy
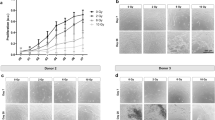
Bone samples were first visualized by SEM for morphologic differences before and after irradiation (Figs. 1, 2). Control samples were first utilized to determine surface characteristics prior to irradiation (Fig. 1). It was observed at 25 times magnification that bone samples presented many roughened topographies (Fig. 1). At higher resolution (400x magnification), the surface of control bone samples demonstrated many visible proteins on the surface of bone samples with a homogeneous surface coating of proteins typically found in native bone (Fig. 1). Following irradiation, bone samples visualized by SEM demonstrated a very homogeneous layer of surface proteins remaining on the bone surface (Fig. 2). Under the present conditions, very little change could be observed for bone samples at all doses with a common protein layer found across all surfaces independent of irradiation doses up to 120 Gy (Fig. 2).
Scanning electron micrograph (SEM) of irradiated bone samples at a magnification of 400 times for (a) 7.5, (b) 15, (c) 30, (d) 60 and (e) 120 Grays. Following increasing concentrations of irradiation, little to no changes in the surface homogeneity of proteins could be observed across all irradiated samples
Cell viability within bone samples
Bone samples were quantified by MTS assay in order to determine the amount of living bone cells found within samples following irradiation at doses ranging from 0 to 120 Gy. After only 7.5 Gy of irradiation, it was observed that over 85 % of all cells found within bone samples were non-vital (Fig. 3). After 60 Gy of irradiation, it was found that 0 % of cells were still viable (Fig. 3). The results from this experiment confirm the extremely damaging and significant effect of even small doses of irradiation on vitality of cells found within the bone matrix (Fig. 3).
Relative cell viability of bone cells following irradiation at varying concentrations of Grays relative to control samples. Over 85 % of all cells were non-vital in samples receiving as little as 7.5 Gy and all cells were non-vital following 60 Gy. (** denotes significantly higher than all other treatment modalities p < 0.01)
Release of growth factors from within bone samples
All bone samples were then quantified for release of growth factors including VEGF, TGFβ1, BMP2, RANKL and IL1β following 15 min and 4 h (Fig. 4, 5). It was first found that a high concentration of VEGF of 1517 +/- 142 pg/mL could be observed for control samples at 15 min and this was relatively maintained following 4 h of sample preparation (Fig. 4a). Interestingly, a marked and pronounced significant decrease of VEGF was observed in all irradiated bone following as little as 7.5 Gy of irradiation (Fig. 4a). No significant differences could be observed between all samples irradiated with bone at either 15 min or 4 h (Fig. 4a). Then TGFβ1 was quantified and although no significant differences could be observed at 15 min post irradiation, approximately a 2 fold significant increase was observed for control samples at 4 h (4621 +/- 1058 pg/mL). Interestingly, no differences in BMP2 protein concentration could be observed at both time points with very little expression observed in all samples (control samples = 127 +/- 15 pg/mL at 15 min and 146 +/- 30 pg/mL at 4 h).
Elisa quantification for growth factors including (a) vascular endothelial growth factor (VEGF), (b) transforming growth factor beta 1 (TGFB1) and (c) bone morphogenetic protein 2 (BMP2). a Following irradiation at all doses, a marked and significant decrease in VEGF was noted at both 15 min and 4 h. b Similarly, TGFB1 had a significant 2 fold decrease at 4 h for irradiated samples. c No changes in BMP2 could be observed at all time points following irradiation. (** denotes significantly higher than all other treatment modalities p < 0.01)
Elisa quantification for growth factors including (a) Receptor activator of nuclear factor kappa-B ligand (RANKL) and (b) interleukin 1-beta (IL1B). a Following irradiation at 4 h, a 2 fold decrease in RANKL could be observed for control samples when compared to all irradiated doses. b No changes in IL1B could be observed at all time points following irradiation. (# denotes significantly lower than all other treatment modalities p < 0.01)
Following growth factor concentration analysis, IL1β and RANKL were then quantified for the effects of bone irradiation on inflammatory cytokines and osteoclast differentiation marker (Fig. 5). First it was found that at 15 min post irradiation, no differences in RANKL expression could be observed between control samples and all irradiated bone samples (Fig. 5a). By 4 h, a significantly lower expression of RANKL could be observed when compared to all irradiated bone samples although all results were less than a 2 fold significant increase (Fig. 5a). No differences could be observed between all samples at either time points for IL1β expression (Fig. 5b).
Discussion
The purpose of the present manuscript was to determine in detail the cellular events that occur following irradiation of bone samples at increasing doses. Although clinically doses can range in intensity and duration, it has been suggested in the literature that doses exceeding 60 Gy are more commonly associated with osteoradionecrosis of the jaw [3, 4]. Furthermore, a number radiation therapy complications have been reported in the literature to date [21]. Therefore, the aim of this bench top study was to perform an in vitro investigation to further understand the cellular events taking place within bone samples following irradiation.
Interestingly, in the present study very little change in surface proteins was found between all bone samples following irradiation (Figs. 1, 2). Furthermore, it was observed that in all samples treated with irradiation, over an 85 % cell mortality was seen even following as little as 7.5 Gy (Fig. 3). This result was extremely surprising as it was initially thought that such small doses would have very little effect on cellular viability. It must be noted that this reported finding applies solely to an in vitro model and its extrapolation to the clinical reality is limited given that immune cells and regenerative cells would minimize free radicals and cells death when compared to the present in vitro model. Nevertheless, it remains striking that cell death occurred in such high numbers following such low levels of irradiation doses.
Thereafter, samples were investigated for growth factor and cytokine release following irradiation at both 15 min and 4 h post irradiation. The reason for selecting these time points was specifically to investigate the changes in cytokine release after a short time interval following initial irradiation (15 min) and also to determine how release of cytokines was affected after a later time point (4 h) following cell death from irradiation. The bone samples were first assessed for VEGF protein release. There was a marked and significant decrease in release of VEGF as early as 15 min post-irradiation (Fig. 4a). VEGF is one of the key growth factors responsible for angiogenesis and the effects of irradiation demonstrate the harsh effects on this potent growth factor. The results from this study further demonstrate and support the groups of clinical experts working with oxygen delivered in hyperbaric pressures for improved angiogenesis before and/or after irradiation therapy [11–13]. Although the clinical efficacy of using such treatment has come under speculation in recent years [22], the rational behind improving the angiogenic properties of bone for irradiated patients is logical and can further be explained by the present study as VEGF was the growth factor most notably down-regulated following irradiation (Fig. 4a). Future research aimed at addressing the in vivo release of VEGF from bone following irradiation may further add valuable data supporting the findings from the present study that irradiation has a significantly pronounced effect on VEGF protein release following irradiation at various doses.
Interestingly, it has been shown in a recent report that osteocytes are major contributors to the release of VEGF in vivo [23]. Furthermore, a subsequent in vitro report has demonstrated that proton irradiation with as little as 2 Gy is enough to suppress angiogenic genes in certain cell types [24]. Taken together with the results from the present study, it becomes extremely clinically relevant to further design strategies to limit the down-regulation of pro-angiogenic genes.
A second growth factor that has been extensively investigated by our group with respect to growth factor released from bone samples is TGFβ1 [17, 25–27]. In several studies analyzing the released protein content from bone (termed bone conditioned medium (BCM)), it was found that one of the likely paracrine factors displayed in bone remodeling is that of TGFβ1 [25]. It was found that by inhibiting TGFβ1 pathway, a 5-fold decrease in oral fibroblast activity was observed, thus confirming that much of the preliminary remodeling process caused by bone is likely governed by TGFβ1 signaling [27]. Thus, in the present study, a 2 fold significant decrease in TGFβ1 protein expression released from the bone samples following irradiation is likely to have a significant effect on future bone remodeling following irradiation. Furthermore, the combinatory reduction of both TGFβ1 and VEGF is hypothesized to pose major bone remodeling challenges further illustrating the necessary regenerative procedure to counteract these major drawbacks.
It was surprisingly observed in the present study that irradiation had virtually no effect on BMP2 protein expression (Fig. 4c). It may therefore be concluded that dying cells that are found within the bone matrix are not target cells for release of osteoinductive growth factors such as BMP2. Interestingly, it is commonly reported in the literature that certain forms of demineralized freeze-dried bone allografts (DFDBA) are osteoinductive whereas most if not all non-demineralized samples are non-osteoinductive [28]. Therefore, it may be concluded that within the present investigation, BMP2 is not a key player in bone remodeling of irradiated bone and likely BMP2 expression is only upregulated once the bone samples are resorbed by osteoclasts and BMP2 is thereafter released from content coming from within the bone matrix.
The results obtained with RANKL protein quantification also generated statistically significant differences at 4 h between control and irradiated bone. RANKL protein expression was up to 2 fold lower than certain irradiated bone samples. It must once again be highlighted that the largest percentage of cells found within bone samples are osteocytes, which account for approximately 90 % of all bone cells. It has previously been demonstrated that damaged or lack of osteocytes is routinely associated with reduced remodeling [29, 30] and dying osteocytes are able to signal for bone resorption by attracting osteoclasts through the release of RANKL [31, 32]. The in vitro findings in the present experiment further demonstrate and confirm the ability for bone samples undergoing high and fast rates of cell death are able to release osteoclast differentiation marker RANKL to the surrounding environment following cell death.
It must also be considered that one of the study limitation of the present study were that all bone samples received irradiation directly to bone in an in vitro model which might not simulate a clinical situation. An in vivo model would necessitate that all irradiation to bone would ultimately pass through overlying tissue which include epithelial, connective tissues, glands, muscles and a combination of them therefore absorbing some of the irradiation prior to bone. Furthermore, immune cells would counteract some of the free radicals produced by irradiation likely contributing to apoptosis of various cell types. Future investigation characterizing this interplay between these various cell types would further contribute to our understanding of bone remodeling following irradiation. Furthermore, in the present model, the bone periosteum was removed which might have a significant influence of the final outcome. Many of the progenitor cells found within bone are located within the periosteum and this complex interaction between periosteum and bone requires better understanding to better implement future regenerative procedures.
In context with some of the known literature, it has been debated for some years the influence of osteoradionecrosis on bone cell interactions. The original proposed and well-accepted ‘three-H concept’ of hypoxia, hypocellularity and hypovascularity as defined by Marx brings into question all the key elements of bone viability [33]. In light of the present findings, it becomes apparent that one of the key components downregulated after irradiation is that of VEGF thus giving evidence for a hypovascular and hypoxic environment. We also demonstrate the drastic changes in cell viability following only 7.5 Gy of irradiated bone. Although these doses would be significantly different in a human model and that the present in vitro model can only be vaguely extrapolated to a clinical situation, it remains highly pertinent information that 2 of the most affected genes, VEGF and TGFβ1, are prominent growth factors for bone regeneration.
Furthermore, most of the accumulated evidence from this manuscript seems to suggest that it is the osteocytes that are playing a key role in this process following irradiation. As most of the cells are apoptotic following irradiation, it becomes evident that they are major key players in maintaining tissue vascularity as they are key players in VEGF production. In previous histologic studies, it was found in human specimen samples from osteoradionecrotic bone after 36 Gy, a loss of osteocytes could be observed [34, 35]. Thus, it remains essential to further study the relationship between irradiated bone and most specifically osteocytes. Future research aimed at investigating protein release of growth factors such as TGFβ1 and VEGF using an animal model would be extremely advantageous. A further understanding of this relationship could provide more pertinent information to clinicians to better gear regenerative procedures for the treatment of osteoradionecrosis of the jaw and possibly provide better preventative measures for these patients prior to complications.
Conclusions
The analysis of bone samples following irradiation demonstrated quite profound effects on cell viability following irradiation and the release of growth factors responsible for bone regrowth and/or bone remodeling were also significantly affected. Over 85 % of cell death occurred following only 7.5 Gy of irradiation to bone samples and a 7-fold decrease of VEGF and a 2-fold decrease in TGFβ1 protein quantification were observed. Furthermore, RANKL was upregulated approximately 2 fold in samples receiving irradiation. As the effects of irradiation on bone viability and release of proteins was quite pronounced in the present study, it may thus becomes vital to better understanding the cell mechanisms taking place following irradiation. Future animal study investigating growth factor release from bone following irradiation would be beneficial.
Abbreviations
TGFβ1, transforming growth factor Beta 1; BMP2, bone morphogenetic protein 2; VEGF, vascular endothelial growth factor; IL1β, interleukin 1 beta; RANKL, receptor activator of nuclear factor kappa-B ligand; PBS, phosphate buffered solution; ELISA, enzyme-Linked Immunosorbent assay; SEM, scanning electron microscopy; BCM, bone conditioned medium
References
Fideler BM, Vangsness CT, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23(5):643–6.
Fideler BM, Vangsness CT, Moore T, Li Z, Rasheed S. Effects of gamma irradiation on the human immunodeficiency virus. A study in frozen human bone-patellar ligament-bone grafts obtained from infected cadavera. J Bone Joint Surgery. 1994;76(7):1032–5.
Curi MM, Dib LL. Osteoradionecrosis of the jaws: a retrospective study of the background factors and treatment in 104 cases. J Oral Maxillofacial Surgery. 1997;55(6):540–4. discussion 545-546.
Chopra S, Kamdar D, Ugur T, Ozlem E, Chen G, Peshek B, Marunick M, Kim H, Lin HS, Jacobs J. Factors predictive of severity of osteoradionecrosis of the mandible. Head Neck. 2011;33(11):1600–5.
Wei L, Miron RJ, Shi B, Zhang Y. Osteoinductive and Osteopromotive Variability among Different Demineralized Bone Allografts. Clin Implant Dent Relat Res. 2015;17(3):533–42.
Sugimoto H, Hatano M, Yoshida S, Sakumoto M, Kato H, Ito M, Yoshizaki T: Efficacy of concurrent superselective intra-arterial chemotherapy and radiotherapy for late-stage squamous cell carcinoma of the temporal bone. Clinical otolaryngology : official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. Clin Otolaryngol. 2015;40(5):500–4. doi:10.1111/coa.12431.
Ma Y, Xu W, Yin H, Huang Q, Liu T, Yang X, Wei H, Xiao J: Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. Eur Spine J. 2015;24(8):1754–60. doi:10.1007/s00586-015-3834-0. Epub 2015 May 6.
Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J American Dental Assoc. 1985;111(1):49–54.
Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral surgery Oral Medicine Oral pathology. 1987;64(4):379–90.
Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41(6):351–7.
Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. American J Surgery. 1990;160(5):519–24.
Van Merkesteyn JPR, Bakker DJ, Borgmeijer-Hoelen A. Hyperbaric oxygen treatment of osteoradionecrosis of the mandible experience in 29 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(1):12–6.
Jacobsson M, Kälebo P, Tjellström A, Turesson I. Bone cell viability after irradiation. An enzyme histochemical study. Acta Oncol. 1987;26(6):463-5. http://www.ncbi.nlm.nih.gov/pubmed/?term=Bone+cell+viability+after+irradiation%3A+An+enzyme+histochemical+study.
Koga DH, Salvajoli JV, Alves FA. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14(1):40–4.
Seemann I, Te Poele JA, Hoving S, Stewart FA. Mouse bone marrow-derived endothelial progenitor cells do not restore radiation-induced microvascular damage. ISRN Cardiology. 2014;2014:506348.
Sawada K, Caballe-Serrano J, Schuldt Filho G, Bosshardt DD, Schaller B, Buser D, Gruber R: Thermal processing of bone: in vitro response of mesenchymal cells to bone-conditioned medium. International journal of oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 2015;44(8):1060-6. doi:10.1016/j.ijom.2015.03.012. Epub 2015 Apr 11.
Miron RJ, Gruber R, Hedbom E, Saulacic N, Zhang Y, Sculean A, Bosshardt DD, Buser D. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin Implant Dent Relat Res. 2013;15(4):481–9.
Miron RJ, Hedbom E, Saulacic N, Zhang Y, Sculean A, Bosshardt DD, Buser D. Osteogenic potential of autogenous bone grafts harvested with four different surgical techniques. J Dent Res. 2011;90(12):1428–33.
Miron RJ, Caluseru OM, Guillemette V, Zhang Y, Gemperli AC, Chandad F, Sculean A. Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS One. 2013;8(8):e71008.
Sawada K, Fujioka-Kobayashi M, Kobayashi E, Schaller B, Miron RJ: Effects of Antiseptic Solutions Commonly Used in Dentistry on Bone Viability, Bone Morphology, and Release of Growth Factors. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. J Oral Maxillofac Surg. 2016;74(2):247-54. doi:10.1016/j.joms.2015.09.029. Epub 2015 Oct 3.
Jereczek-Fossa BA, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28(1):65–74.
Rice N, Polyzois I, Ekanayake K, Omer O, Stassen LF. The management of osteoradionecrosis of the jaws--a review. Surgeon. 2015;13(2):101–9.
Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–7.
Girdhani S, Lamont C, Hahnfeldt P, Abdollahi A, Hlatky L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat Res. 2012;178(1):33–45.
Caballe-Serrano J, Bosshardt DD, Buser D, Gruber R. Proteomic analysis of porcine bone-conditioned medium. Int J Oral Maxillofac Implants. 2014;29(5):1208–1215d.
Filho GS, Caballe-Serrano J, Sawada K, Bosshardt DD, Bianchini MA, Buser D, Gruber R. Conditioned medium of demineralized freeze-dried bone activates gene expression in periodontal fibroblasts in vitro. J Periodontol. 2015;86(6):827–34.
Peng J, Nemec M, Brolese E, Bosshardt DD, Schaller B, Buser D, Gruber R: Bone-Conditioned Medium Inhibits Osteogenic and Adipogenic Differentiation of Mesenchymal Cells In Vitro. Clinical implant dentistry and related research. Clin Implant Dent Relat Res. 2015;17(5):938-49. doi:10.1111/cid.12200. Epub 2014 Jan 27.
Wei L, Miron RJ, Shi B, Zhang Y: Osteoinductive and Osteopromotive Variability among Different Demineralized Bone Allografts. Clinical implant dentistry and related research. Clin Implant Dent Relat Res. 2015;17(3):533-42. doi:10.1111/cid.12118. Epub 2013 Jul 24.
Noble BS, Stevens H, Loveridge N, Reeve J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20(3):273–82.
Henriksen K, Leeming DJ, Byrjalsen I, Nielsen RH, Sorensen MG, Dziegiel MH, Martin TJ, Christiansen C, Qvist P, Karsdal MA. Osteoclasts prefer aged bone. Osteoporos Int. 2007;18(6):751–9.
Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17(11):2068–79.
Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23(6):915–27.
Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofacial Surgery. 1983;41(5):283–8.
Al-Nawas B, Duschner H, Grotz KA. Early cellular alterations in bone after radiation therapy and its relation to osteoradionecrosis. J Oral Maxillofacial Surgery. 2004;62(8):1045.
Grötz KA, Al-Nawas B, Piepkorn B, Reichert TE, Duschner H, Wagner W. Mikromorphologische Kieferveränderungen nach Bestrahlung. Mund Kiefer GesichtsChir. 1999;3(3):140–5.
Acknowledgements
This study was funded entirely by the department of Oral and Maxillofacial Surgery, University of Bern, Switzerland. We also thank Catherine Solioz for her skillful technical assistance. All authors declare no conflict of interest and have viewed and agreed to submission.
Funding
All data was funded by the Department of Cranio Maxillofacial Surgery, Inselspital, University of Bern, Switzerland.
Availability of data and materials
All data was presented in the main paper.
Authors’ contributions
KS, MFK and EK carried out the molecular analysis, SEM and ELISAs. KS, BS and RJM drafted the manuscript. JOB, BS and RJM participated in the design of the study. KS, MFK and EK performed the statistical analysis. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
No human subjects were used in this study. Animals utilized were utilized according to the guidelines by the University of Bern ethical standards and guidelines. This study utilized bone chips from adult pigs from a regular butcher undergoing daily euthanasia of animals (Metzgerei Balsiger, Wattenwil, Switzerland). All bone was obtained from the mandible. An ethical request from the local Canton of Bern (Switzerland) was waived by the IRB for this study since bone chips was only obtained from discarded material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sawada, K., Fujioka-Kobayashi, M., Kobayashi, E. et al. In vitro effects of 0 to 120 Grays of irradiation on bone viability and release of growth factors. BMC Oral Health 17, 4 (2017). https://doi.org/10.1186/s12903-016-0241-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-016-0241-9