Abstract
Background
Oxidative stress is a disturbance in the natural balance between oxidative and anti-oxidative processes, which is the major effective factor in cardiovascular disorders and metabolic syndrome (MetS), due to the role of pro-oxidants in inducing oxidative stress, and as a result, the occurrence and exacerbation of components of metabolic syndrome and cardiovascular risk factors, this cross-sectional study was conducted with the aim of investigating the relationship between the status of dietary pro-oxidants score (POS) and metabolic parameters including serum lipids, glycemic markers and blood pressure among obese adults.
Methods
338 individuals with obesity (BMI ≥ 30 kg/m 2), aged between 20 and 50 years were recruited in the present cross-sectional study. A validated food frequency questionnaire (FFQ) was used to determine the dietary pro-oxidant score (POS). Analysis of variance (ANOVA) with Tukey’s post-hoc comparisons after adjustment for confounders and multivariable logistic regression analysis were performed to determine the association of cardiometabolic risk factors among the tertiles of POS.
Results
Participants with higher POS had lower levels of body mass index (BMI), weight and waist circumference (WC). There were no significant associations between metabolic parameters including glycemic markers and lipid profile in one-way ANOVA and multivariate multinomial logistic regression models.
Conclusions
The findings of this study revealed that greater dietary pro-oxidant intake might be associated with lower BMI, body weight, and WC in Iranian obese individuals. Further studies with interventional or longitudinal approaches will help to better elucidate the causality of the observed associations.
Similar content being viewed by others
Introduction
Oxidative stress (OS) is a multifaceted, complicated process that develops from an imbalance between reactive oxygen species generated by pro-oxidants and antioxidant defense [1]. Numerous studies have demonstrated that cellular pathways’ high oxidative stress is a major contributor to cardiovascular disorders and manifestations of the metabolic syndrome MetS [2,3,4]. Multiple metabolic abnormalities, including obesity, type 2 diabetes (T2D), and the consequences of T2D known as the MetS, are caused by imbalances in the energy and redox potential of cells, these imbalances are triggered by the disruption of important biological reactions [5]. MetS is characterized as a pathophysiological association and combination of cardiometabolic risk factors that are known to increase a person’s risk of CVD and T2DM [6]. According to studies, MetS affects over 30% of the world’s population, making it a serious worldwide public health problem [7], in Iran, MetS presently affects 30.4% of the population and is increasing significantly [8]. Pathophysiology of MetS is a highly complicated and unclear. The concept that disruption of the normal balance between oxidative and anti-oxidative processes, resulting in redox state in the cell and tissues, may play a significant role in its manifestations is supported by a number of studies [5, 9]. A cross-sectional study on 113 elderly subjects indicated a strong relationship between the accumulation of Mets components and the aggravation of oxidative stress [10]. According to the research conducted so far, there is absolutely no doubt that oxidative stress and MetS are related [11], so many studies are focused on preventing (OS) in MetS. Diet could provide more precursors for endogenous lipid peroxidation and induce oxidative stress biomarkers by dietary components such as polyunsaturated fatty acids particularly ω-3 fatty acids with a high double bond index and heme iron [12]. Through the Fenton reaction, iron may produce reactive oxygen species (ROS), which results in oxidative stress and greater rates of lipid peroxidation [13]. Syrovatka P et al. [14] shown a substantial association between ferritin and obesity parameters and metabolic syndrome in healthy males, suggesting that higher body iron storage may cause reduced insulin sensitivity through increased oxidative stress [14]. Another dietary pro-oxidant is PUFAs, which are more vulnerable to oxidation and the production of LDL-oxidized blood due to their high level of unsaturation [15], highly unsaturated n-3 PUFA supplements have been shown to enhance oxidative stress in both humans and animals according to research [16,17,18,19]. Despite the fact that it is well recognized that saturated fatty acids (SFAs) in general encourage abdominal obesity, dyslipidemia, insulin resistance, systemic inflammation and impaired glucose tolerance [20,21,22]. SFAs overexposure has been shown to enhance the production of pro-inflammatory cytokines, disrupt insulin signaling, and drive apoptosis marked by both endoplasmic reticulum (ER) deficits and oxidative stress in a variety of cell types in vitro [23,24,25,26]. Leptin, an adipocyte-derived hormone that is increased in obese people and may cause oxidative stress, could be the physiological factor behind the association between obesity and oxidative stress [27]. Considering the role of pro-oxidants in oxidative stress and as a result the increases in the number of MetS components and cardio-metabolic risk factors, as well as limited research focusing only on pro-oxidant factors, this present study aimed to evaluate the association between dietary triggers of oxidative stress and cardio metabolic risk factors including blood pressure, lipid profile, glycemic and insulin related factors among apparently metabolically healthy subjects with obesity.
Method & materials
Study population
In the present cross-sectional study, 338 randomly chosen volunteers who were both male and female with obesity (BMI ≥ 30 kg/m2) and between the ages of 20 and 50 were chosen from previous research [28, 29] which have been done in the last four years from 2019 to 2022. The study excludes women who are pregnant, breast feeding, or postmenopausal, individuals who have had gastric bypass surgery or other weight loss operations, cancer, liver or kidney problems, cardiovascular diseases, diabetes mellitus, or cancer, and subjects who take any medications or dietary supplements that could affect weight. Meanwhile, the study protocol was approved by the ethics committee of Iran’s Tabriz University of Medical Sciences after all participants read and signed an informed consent form (Registration number: IR.TBZME-D.REC.1400.454).
Sociodemographic data
We gathered demographic information through questionnaires and interviews. The socioeconomic status (SES) score was estimated using the data gathered on educational attainment, employment status, home ownership, and family size. Individuals reported their greatest degree of education, and we used education as a categorical variable. This variable was rated on a scale of 0 to 5 points as follows: illiterate: 0, less than diploma: 1, associate degree: 2, bachelor: 3, master: 4, and higher: 5. The employment status of female participants was divided into five groups (housewife, employee, student, self-employed, and others), while the occupational status of male participants was divided into the following categories: unemployed: 1, worker, farmer and rancher: 2, others: 3, employee: 4, and self-employed: 5. Individuals were categorized as belonging to families of 3 and under 3 (score 1), 4–5 (score 2), and over 6 (score3) persons. In addition, if they didn’t own a home, they got a score of 1, and if they did, they got a score of 2. Depending on the respondents’ total SES score, which varied from 1 to 15, the individuals were then categorized into three categories. Using the Persian version of the DASS-21[30], the frequency of depression, anxiety, and stress-related symptoms during the preceding weeks was evaluated. Age, marital status, educational level, nursing experience, work unit, shift work, and work hours per week are the seven items on this questionnaire that evaluate a subject’s sociodemographic features. A maximum score of three and a minimum score of zero are assigned to the questions on a Likert scale. On each scale, the score may be between 0 and 21. A visual analogue scale (VAS) was used to assess the state of the appetite in the morning following fasting state (VAS)[31]. To illustrate the two extremes of the VAS, the phrases “I’m not at all hungry” and “I have not been so hungry” were inserted at the opposite ends of a 100-mm line. This questionnaire asked about past and projected food intake as well as questions regarding hunger, satiety, fullness, cravings for sweet, salty, and fatty meals. A shortened version of the international physical activity questionnaire (IPAQ) was used to estimate the participants’ level of physical activity [32,33,34].
Dietary assessments and calculation of dietary pro-oxidant score (POS)
We used a validated, semi-quantitative food frequency questionnaire (FFQ) that was adapted for the Iranian population to gather data on dietary consumption. The Iranian household manual’s recommendations for portion sizes, cooking yields, and dietary food amounts were used to ask participants to keep records of all the foods and beverages they consumed on a daily, weekly, monthly, or yearly basis. Every food item’s reported frequency was modified to daily intake and converted to grams. In this study, three dietary components known to have a pro-oxidant effect were selected including iron and saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA) [35,36,37]. The total amount of consumption of all three factors was calculated and they were categorized into tertiles by reverse scoring. The higher POS denotes the lower dietary pro-oxidant intake and was more favorable.
Anthropometric assessments
A wall stadiometer with a sensitivity of 0.5 cm Seca scale (Seca Co., Hamburg, Germany) was used for measuring the participants’ height while individuals were asked to take off their shoes for height measurement. Weight of participants was measured, without extra clothes, by using Seca scale (Seca Co., Hamburg, Germany) with 0.1 kg precision. The bioelectrical impedance analysis (BIA) method was employed by Tanita, BC-418 MA (Tokyo, Japan) to estimate body composition which measures the body fat percentage, fat mass (FM), fat free mass (FFM), and predicted muscle mass. When exhaling, the distance between the iliac crest and the lowest rib was measured using a tape measure to the closest 0.1 cm.
Measurement of blood biomarkers and blood pressure assessments
All measurements were taken after at least 12 h of overnight fasting. A trained physician used a standard mercury sphygmomanometer to measure the subject’s systolic and diastolic blood pressure on the right arm after 10 to 15 min of rest and sitting state. The average of two measured blood pressure was recorded for each participant. All individuals provided 10 ml of venous blood for sampling, which was centrifuged at 4500 rpm for 10 min to separate the serum and plasma samples. A commercial kit was used to analyze the serum levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol, and fasting blood glucose (Pars Azmoon, Tehran, Iran). Additionally, the Friedewald equation was used to quantify the amount of low-density lipoprotein cholesterol [38]. Enzyme-linked immunosorbent assay (ELISA) kit was used to determine serum concentrations of insulin (Bioassay Technology Laboratory, Shanghai Korean Biotech, Shanghai City, China). Fasting insulin (IU/ml) + fasting glucose (mmol/l)/22.5 was used to calculate the homeostatic model assessment for insulin resistance (HOMA-IR), and the quantitative insulin sensitivity check index (QUICKI) was estimated as 1/[log fasting insulin (U/mL) + log glucose (mmol/L)].
Statistical analysis
The Statistical Package for Social Sciences (version 21.0; SPSS Inc, Chicago IL) was used to perform the statistical analysis, with a significance level of P < 0.05. For categorical variables, data were reported as frequency (%), and for continuous variables, as mean (standard deviation). The Chi-square test and one-way analysis of variance (ANOVA) were used, respectively, to assess the differences in discrete and continuous variables across different tertiles of POS. In addition, three multivariable-adjusted and unadjusted models of multinomial logistic regression were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for test the presence of possible association of cardiometabolic risk factors among the POS tertiles.
Results
The present cross-sectional study was conducted among 338 obese participants (mean BMI of 32.62 kg/ m2 and mean age of 40.78 years old) which includes 41% male. Table 1 shows the general characteristics and biochemical parameters of the participants across quartiles of POS. Older subjects scored higher on the prooxidant score, indicating a direct relationship between age and dietary prooxidant intake (P = 0.03). According to the results of this study, a higher POS that indicates lower intake of pro-oxidants was accompanied by lower weight (P = 0.004), lower BMI levels (P = 0.001). WC was also lower in higher tertiles of POS than in lower tertile (P = 0.02). The significant level of association between BMI, weight and WC and POS remained even after adjusting for confounding factors (P < 0.001). No significant difference in other demographic variables such as SES score, DASS, appetite, physical activity was found among POS tertiles (P > 0.05). Tables 2 and 3 show the comparison of dietary intake of POS components, energy, macronutrients, and food groups in different tertiles of POS. A statistically meaningful lower intake of POS components (including iron, SFA and PUFA)(Table 2), fruit, meat group, energy, fat and MUFA was observed in the highest tertiles of POS (P < 0.05),while percentage of carbohydrate intake was higher in higher tertiles of POS (P < 0.001)(Table 3). Table 4 represents the odds ratios (ORs) and 95% confidence intervals (CIs) of the cardiometabolic risk factors across different tertiles of POS after adjustment for potential confounders in three models of crude, adjusted for age and sex and adjusted for age, sex, BMI, SES and dietary energy intake. Subjects in the second tertile of POS had a reduced risk of higher SBP levels (OR = 0.968, P = 0.04), and a higher risk of increased DBP levels (OR = 1.042, P = 0.05) compared with those with the lower PCOS tertiles in the crude and age, sex adjusted models. However, after adjustment for age and gender, BMI, SES and dietary energy intake (3rd model), these associations lost their significance.
Discussion
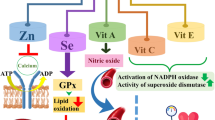
To the best of our knowledge, no prior research has been conducted to explore the association between dietary pro-oxidants and cardiometabolic risk factors. In this cross-sectional research of obese adults, we evaluated dietary pro-oxidant consumption, indicated by pro-oxidant score (POS), and its relationship with cardiometabolic risk factors. As the primary finding of the present study, a better status of dietary pro-oxidants (determined by higher POS tertiles with a reduction in the consumption of dietary factors including iron, PUFA, and SFA that can induce pro-oxidant state) was associated with improved weight, BMI, and waist circumference (WC). Consumption of a high SFA diet led to an increase in the expression of inflammatory genes in adipose tissue and a decrease in the expression of genes involved in fatty acid -oxidation and the synthesis of triglycerides, according to a parallel controlled-feeding trial carried out in 20 people who were centrally overweight who are at risk of metabolic syndrome [39], this finding can explain the association of SFA as one of the pro-oxidant factors and obesity that we indicated. Furthermore, significant interactions between genetic risk score and total fat, SFA, and MUFA intake were discovered in 497 Asian Indian individuals, revealing that high SFA intake is significantly associated with larger WC than low SFA intake in individuals with high genetic risk, also low SFA intake was shown to be associated with smaller WC in individuals with higher genetic risk compared to those with lower genetic risk [40]. A study involving 2163 participants from the US population hypothesized that SFA may play a major role in modulating the effects of fat mass and obesity associated (FTO) polymorphisms that are associated with BMI/obesity, results indicated that high-SFA intake instead of total fat intake could be more associated in increasing the effects of the FTO risk allele on BMI [41]. In addition, the intake of SFA, MUFA, and PUFA is positively correlated with the risk of obesity and higher BMI, according to epidemiological cross-sectional research [42,43,44]. While PUFA-rich diets, particularly n-3 PUFA, are thought to be useful in preventing several metabolic disorders [45,46,47], the other side of the coin is that PUFAs are vulnerable to free radical oxidation[48], which makes them turn into a pro-oxidant agent. Results of a cross-sectional study with 895 individuals demonstrate strong associations between PUFAs and ROS generation in young and middle-aged groups in multiple regression models, suggesting that PUFAs may enhance oxidative stress and have deleterious consequences for the body [49, 50]. However, the reduction of systolic and diastolic blood pressure by PUFA supplementation has been demonstrated in treated hypertensive subjects [51]. In addition, an increase in the content of these fatty acids in cell membranes is linked to a decrease in blood pressure caused by omega-3 PUFAs, which is likely to depend on the composition of the cell membrane at birth, which in turn may be influenced by dietary patterns and even genetic factors [52]. Results of a meta-analysis of 70 randomized controlled trials (RCTs) which investigates the effect of omega-3 PUFAs including eicosapentaenoic acid + docosahexaenoic acid (EPA + DHA) on BP showed that both hypertensive and normotensive patients had statistically significant BP reductions brought on by omega-3 PUFA [53]. This effect of PUFAs on blood pressure could justify the effect of increasing diastolic blood pressure in second tertile of POS that we observed in multivariate multinomial logistic regression (Table 4). Excess iron levels are harmful and can produce reactive oxygen species that cause lipid peroxidation and DNA damage [54,55,56,57,58] as well as increased prevalence of metabolic syndrome its components [59, 60]. A meta-analysis of fourteen observational studies indicated that the dietary iron level has significant positive association with MetS [61]. In a cross-sectional research of 1567 Japanese individuals with type-2 diabetes, Ferreira ED et al.[62] found that regardless of macronutrient and fiber intake, participants in the highest quartile of iron intake had a significantly higher risk of obesity in the 30- to 54-year-old age group. Meanwhile, studies have shown that serum ferritin level has a positive correlation with WC, BMI [63,64,65]. Hence, the mentioned findings could be consistent with our findings regarding the significant relationship between pro-oxidant factors and weight, BMI, and WC (Table 1). The summary of the mechanisms of these three pro-oxidant factors involved in the regulation of obesity is given in Fig. 1. According to results of our research, there is no correlation between tertiles of POS and SBP, DBP, FBS TC, TG, HDL-C, LDL-C, insulin, HOMA-IR, or QUICKI. Noruzi et al. also reported no significant relationship between components of the MetS and combined pro- and antioxidant exposure status, indicated by oxidative balance score, except for increased WC and DBP among 847 Iranian participants [36, 66]. In contrast, Abbasiana et al. [67] revealed a significantly meaningful correlation between the oxidative stress indicators such as total antioxidant capacity and malondialdehyde and number of Mets components among 167 adult participants.
This study was not without limitations. At first, the study’s cross-sectional design restricts the casual inference, longitudinal investigations are required to clarify the cause-effect relationships. Second, this study was conducted in apparently healthy people aged 20–50 in the two centers of the provinces of Iran, Tabriz and Tehran, so generalizing the results to people with metabolic disorders /other age groups / other parts of the country should be done with caution. Not only the FFQ was not originally designed to assess POS, but the use of questionnaires can increase recall errors and create errors in data collection related to consumption reports. It is also better in further studies to measure the levels of pro-oxidant and antioxidant enzymes such as NADPH oxidase, xanthine oxidase and advanced glycation end products (AGE), superoxide dismutase, catalase, etc. be taken. Nevertheless, the present study also had several strengths. According to the knowledge of authors, this is the first cross-sectional study to examine the association between dietary consumption of pro-oxidants and cardiometabolic risk factors. Also three models were used to modify the multivariate multinomial logistic regression for a large number of potential confounding variables, which increased the reliability of the findings.
Conclusions
The results of the current study showed that lower intake of pro-oxidants is associated with reduced risk of general and central obesity. Nutritional clinical trainings with emphasis on following a diet with less content of dietary prooxidants can be effective in improving weight status and body mass index and waist circumference. Longitudinal or interventional studies are recommended to find the causal relationships between dietary pro-oxidants and metabolic risk factors.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical considerations, but can be available from the corresponding author on reasonable request.
References
Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative medicine and cellular longevity. 2013;2013.
Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19.
Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21–22):705–12.
Gallegos-Gonzalez G, Pineda-García G, Serrano-Medina A, Martinez AL, Ochoa-Ruiz E. Association between stress and metabolic syndrome and its mediating factors in university students. Am J Health Behav. 2021;45(6):1091–102.
Raut SK, Khullar M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2022:1–12.
Reisinger C, Nkeh-Chungag BN, Fredriksen PM, Goswami N. The prevalence of pediatric metabolic syndrome—A critical look on the discrepancies between definitions and its clinical importance. Int J Obes. 2021;45(1):12–24.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):1–8.
Farmanfarma KK, Kaykhaei MA, Adineh HA, Mohammadi M, Dabiri S, Ansari-Moghaddam A. Prevalence of metabolic syndrome in Iran: a meta-analysis of 69 studies. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13(1):792–9.
Fusco D, Colloca G, Monaco MRL, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Interv Aging. 2007;2(3):377–87.
Sánchez-Rodríguez MA, Martínez-Cruz M, Correa-Muñoz E, Mendoza-Núñez VM. Relationship between metabolic syndrome components and oxidative stress in elderly community-dwelling Mexicans. Annals of Nutrition and Metabolism. 2010;56(4):302–7.
Hopps E, Noto D, Caimi G, Averna M. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metabolism Cardiovasc Dis. 2010;20(1):72–7.
Guéraud F, Taché S, Steghens J-P, Milkovic L, Borovic-Sunjic S, Zarkovic N, et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic Biol Med. 2015;83:192–200.
Valenzuela R, Rincón-Cervera M, Echeverría F, Barrera C, Espinosa A, Hernández-Rodas MC, et al. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition. 2018;45:49–58.
Syrovatka P, Kraml P, Potockova J, Fialova L, Vejrazka M, Crkovska J, et al. Relationship between increased body iron stores, oxidative stress and insulin resistance in healthy men. Annals of Nutrition and Metabolism. 2009;54(4):268–74.
Albert BB, Cameron-Smith D, Hofman PL, Cutfield WS. Oxidation of marine omega-3 supplements and human health. BioMed research international. 2013;2013.
Oarada M, Tsuzuki T, Gonoi T, Igarashi M, Kamei K, Nikawa T, et al. Effects of dietary fish oil on lipid peroxidation and serum triacylglycerol levels in psychologically stressed mice. Nutrition. 2008;24(1):67–75.
Filaire E, Massart A, Portier H, Rouveix M, Rosado F, Bage AS, et al. Effect of 6 weeks of n-3 fatty-acid supplementation on oxidative stress in Judo athletes. Int J Sport Nutr Exerc Metab. 2010;20(6):496–506.
Walters JM, Hackett TB, Ogilvie GK, Fettman MJ. Polyunsaturated fatty acid dietary supplementation induces lipid peroxidation in normal dogs. Veterinary Medicine International. 2010;2010.
Xavier J, Farias CP, Soares MSP, Silveira GdO, Spanevello RM, Yonamine M, et al. Ayahuasca prevents oxidative stress in a rat model of depression elicited by unpredictable chronic mild stress. Archives of Clinical Psychiatry (São Paulo). 2021;48:90–8.
Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8(1):2–17.
Hyder KM, Mohan J, Varma V, Sivasankaran P, Raja D. Effects of muscle–specific exercises compared to existing interventions on insulin resistance among Prediabetes Population of South India. J Nat Sci Biology Med. 2021;12(2):230.
Al-Obaidi ZMJ, Abdul-Rasheed OF, Mahdi MF, Raauf AM. Biological evaluation of newly synthesized spebrutinib analogues: potential candidates with enhanced activity and reduced toxicity profiles. Int J Drug Delivery Technol. 2019;9(03):339–46.
Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells: a role for cardiolipin. J Biol Chem. 2003;278(34):31861–70.
Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291(1):H38–H44.
Malhi H, Gores GJ, editors. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Seminars in liver disease. © Thieme Medical Publishers; 2008.
Şenormancı G, Turan Ç, Çelik SK, Çelik A, Edgünlü TG, Bilgi C, et al. Gene variants and serum levels of synaptic vesicle and presynaptic plasma membrane proteins in alcohol dependence and their relationship with impulsivity and temperament. Archives of Clinical Psychiatry (São Paulo). 2021;48:99–104.
Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol. 2008;295(4):H1514–H21.
Abbasalizad Farhangi M, Vajdi M, Nikniaz L, Nikniaz Z. The interaction between dietary inflammatory index and 6 P21 rs2010963 gene variants in metabolic syndrome. Eating and Weight Disorders-Studies on Anorexia. Bulimia and Obesity. 2020;25:1049–60.
Khodarahmi M, Asghari-Jafarabadi M, Abbasalizad Farhangi M. A structural equation modeling approach for the association of a healthy eating index with metabolic syndrome and cardio-metabolic risk factors among obese individuals. PLoS ONE. 2019;14(7):e0219193.
Asghari A, Saed F, Dibajnia P. Psychometric properties of the Depression anxiety stress Scales-21 (DASS-21) in a non-clinical iranian sample. Int J psychol. 2008;2(2):82–102.
Flint A, Raben A, Blundell J, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48.
Washburn RA. Assessment of physical activity in older adults. Res Q Exerc Sport. 2000;71(sup2):79–87.
Guerra ZC, Moore JR, Londoño T, Castro Y. Associations of acculturation and gender with obesity and physical activity among Latinos. Am J Health Behav. 2022;46(3):324–36.
Zelenović M, Kontro T, Dumitru RC, Aksovic N, Bjelica B, Alexe DI et al. Leisure-time physical activity and all-cause mortality: a systematic review. Revista de Psicología del Deporte. 2022;31(1).
Goodman M, Bostick RM, Gross M, Thyagarajan B, Dash C, Flanders WD. Combined measure of pro-and anti-oxidant exposures in relation to prostate cancer and colorectal adenoma risk: an update. Ann Epidemiol. 2010;20(12):955–7.
Noruzi Z, Jayedi A, Farazi M, Asgari E, Dehghani Firouzabadi F, Akbarzadeh Z et al. Association of oxidative balance score with the metabolic syndrome in a sample of Iranian Adults. Oxidative Medicine and Cellular Longevity. 2021;2021.
Leman MA, Claramita M, Rahayu GR. Predicting factors on modeling health behavior: a systematic review. Am J Health Behav. 2021;45(2):268–78.
Rifai N. Tietz textbook of clinical chemistry and molecular diagnostics-e-book. Elsevier Health Sciences; 2017.
van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, et al. A saturated fatty acid–rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90(6):1656–64.
Wuni R, Adela Nathania E, Ayyappa AK, Lakshmipriya N, Ramya K, Gayathri R, et al. Impact of lipid genetic risk score and saturated fatty acid intake on central obesity in an asian indian population. Nutrients. 2022;14(13):2713.
Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr. 2011;141(12):2219–25.
Gonzalez CA, Pera G, Quiros JR, Lasheras C, Tormo MJ, Rodriguez M, et al. Types of fat intake and body mass index in a Mediterranean country. Public Health Nutr. 2000;3(3):329–36.
Williams DE, Prevost AT, Whichelow MJ, Cox BD, Day NE, Wareham NJ. A cross-sectional study of dietary patterns with glucose intolerance and other features of the metabolic syndrome. Br J Nutr. 2000;83(3):257–66.
Brunner E, Wunsch H, Marmot M. What is an optimal diet? Relationship of macronutrient intake to obesity, glucose tolerance, lipoprotein cholesterol levels and the metabolic syndrome in the Whitehall II study. Int J Obes. 2001;25(1):45–53.
Coelho OGL, da Silva BP, Rocha DMUP, Lopes LL, Alfenas RdCG. Polyunsaturated fatty acids and type 2 diabetes: impact on the glycemic control mechanism. Crit Rev Food Sci Nutr. 2017;57(17):3614–9.
Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105(3):428–40.
Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Experimental biology and medicine. 2008;233(6):674–88.
Grundt H, Nilsen D, Mansoor M, Nordøy A. Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (PUFAs) following an acute myocardial infarction. Eur J Clin Nutr. 2003;57(6):793–800.
Suzuki N, Sawada K, Takahashi I, Matsuda M, Fukui S, Tokuyasu H, et al. Association between polyunsaturated fatty acid and reactive oxygen species production of neutrophils in the general population. Nutrients. 2020;12(11):3222.
Kamolthip R, Fung XC, Lin C-Y, Latner JD, O’Brien KS. Relationships among physical activity, health-related quality of life, and weight stigma in children in Hong Kong. Am J Health Behav. 2021;45(5):828–42.
Shantakumari N, Eldeeb RA, Ibrahim SAM, Sreedharan J, Otoum S. Effect of PUFA on patients with hypertension: a hospital based study. Indian Heart J. 2014;66(4):408–14.
Colussi G, Catena C, Novello M, Bertin N, Sechi L. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: relevance for cardiovascular outcomes. Nutr Metabolism Cardiovasc Dis. 2017;27(3):191–200.
Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27(7):885–96.
Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17.
Dickson KB, Zhou J. Role of reactive oxygen species and iron in host defense against infection. Front Bioscience-Landmark. 2020;25(8):1600–16.
Zhao T, Guo X, Sun Y. Iron accumulation and lipid peroxidation in the aging retina: implication of ferroptosis in age-related macular degeneration. Aging and disease. 2021;12(2):529.
Molz P, de Freitas BS, Uberti VH, da Costa KM, Kist LW, Bogo MR, et al. Effects of lipoic acid supplementation on age-and iron-induced memory impairment, mitochondrial DNA damage and antioxidant responses. Eur J Nutr. 2021;60:3679–90.
Chen S, Zhou Z, Ren K. Influence of Sports Value on adolescent participation and preference of sci-tech experience activities. Revista de Psicología del Deporte. 2021;30(4).
dos Santos Vieira DA, Hermes Sales C, Galvão Cesar CL, Marchioni DM, Fisberg RM. Influence of haem, non-haem, and total iron intake on metabolic syndrome and its components: a population-based study. Nutrients. 2018;10(3):314.
Esfandiar Z, Hosseini-Esfahani F, Mirmiran P, Habibi-Moeini A-S, Azizi F. Red meat and dietary iron intakes are associated with some components of metabolic syndrome: Tehran lipid and glucose study. J translational Med. 2019;17(1):1–9.
Ding J, Liu Q, Liu Z, Guo H, Liang J, Zhang Y. Associations of the dietary iron, copper, and selenium level with metabolic syndrome: a meta-analysis of observational studies. Front Nutr. 2022;8:1300.
Ferreira EdÁ, Hatta M, Takeda Y, Horikawa C, Takeuchi M, Kato N, et al. Higher Iron Intake is independently Associated with obesity in younger japanese Type-2 diabetes Mellitus Patients. Nutrients. 2022;14(1):211.
Gillum R. Association of serum ferritin and indices of body fat distribution and obesity in mexican american men—the Third National Health and Nutrition Examination Survey. Int J Obes. 2001;25(5):639–45.
Oshaug A, Bugge K, Bjønnes C, Borch-Iohnsen B, Neslein I. Associations between serum ferritin and cardiovascular risk factors in healthy young men. A cross sectional study. Eur J Clin Nutr. 1995;49(6):430–8.
Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among US adults. Diabetes Care. 1999;22(12):1978–83.
Gupta A, Gupta S, Mani R, Durgapal P, Goyal B, Rajput D et al. Expression of human epidermal growth factor receptor 2, survivin, enhancer of zeste homolog-2, Cyclooxygenase-2, p53 and p16 molecular markers in Gall bladder carcinoma. J Carcinog. 2021;20.
Abbasian M, Delvarianzadeh M, Ebrahimi H, Khosravi F, Nourozi P. Relationship between serum levels of oxidative stress and metabolic syndrome components. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2018;12(4):497–500.
Acknowledgements
The authors wish to thank all the study participants for the sincere collaboration. We also thank from Research Undersecretary of Tabriz University of Medical Sciences for their financial support (Grant number: 72153).
Funding
The present study was financially supported by a grant from Tabriz University of Medical Sciences. (Code: IR.TBZME-D.REC.1400.454 and grant number: 72153). The funders had no role in hypothesis generation, recruiting and designing the study.
Author information
Authors and Affiliations
Contributions
All authors approved the final version of the article. BH and FJ were supervisors and had major role in hypothesis generation. MAF, BH and FJ contributed to study design, statistical analysis, and manuscript writing. NN was involved in revision. She also performed the statistical analysis. AS, FJ and BH were also involved in patients’ recruitment. ZR and RJ were involved in data collection, manuscript revision and patients’ recruitment. All of the authors have read and approved the final version of the article and were involved in revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All subjects provided a written informed consent before participation in the study. The study protocol was approved and registered by the ethics committee of Tabriz University of Medical Sciences (registration code: IR.TBZME-D.REC.1400.454). We confirm that methods were performed in accordance with declaration of Helsinki’s guidelines and regulations. Also, legal guardians of the illiterate participants provided a written informed consent.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nikrad, N., Shakarami, A., Rahimi, Z. et al. Dietary pro-oxidant score (POS) and cardio-metabolic panel among obese individuals: a cross-sectional study. BMC Endocr Disord 23, 144 (2023). https://doi.org/10.1186/s12902-023-01395-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01395-2