Abstract
Background
Although dozens of studies have investigated the relationship between the content of serum cystatin C (Cys-C) and diabetic nephropathy (DN), the results are still controversial. Hence, This study aims to explore the accuracy of serum Cys-C for diagnosing DN by meta-analysis.
Methods
The studies about serum Cys-C diagnosing DN were searched from six online databases from inception to September 22, 2020. The data were processed by Stata 15.0 statistic software. The corresponding diagnostic effect sizes, such as sensitivity and specificity, were obtained. We drew a summary receiver operating characteristic (SROC) curve. We assess the risk of literature bias was following the QUADAS-2 guidelines.
Results
Twenty-six published studies were identified. The results showed a pooled sensitivity of 0.86 (95% confidence interval (CI): 0.82–0.90), specificity of 0.89 (95%CI: 0.85–0.92), positive likelihood ratio of 7.59 (95%CI: 5.66–10.19), negative likelihood ratio of 0.16 (95%CI: 0.12–0.21), and diagnostic odds ratio of 48.03 (95%CI: 30.64–75.29). The area under the SROC curve was given a value of 0.94 (95%CI: 0.91–0.96).
Conclusion
Serum cystatin C has an excellent diagnostic value with good sensitivity and specificity for diabetic nephropathy.
Similar content being viewed by others
Introduction
A total of 425 million people suffered from diabetes worldwide based on the International Diabetes Federation (IDF) (2017). The incidence will be increased to 629 million in 2045 if not controlled. There are about 842,993 deaths from diabetes in China, of which 33.8% patients are younger than 60 years (IDF Diabetes Atlas. 8th Edition [EB / OL]. [2019 - 08 - 14] http://www.idf.org/e-library/epidemiologyresearch/diabetes-atlas.html). Diabetic nephropathy (DN) is one of the most common serious complications of diabetes [1]. DN refers to kidney damage caused by chronic hyperglycemia, which becomes the leading cause of an end-stage renal disease (ESRD) in China instead of glomerulonephritis-related chronic kidney disease (CKD) [2]. Due to its insidious onset and slow development, the entire course of the disease could be irreversible at diagnosis, which led to disability and death eventually [3]. Therefore, the early diagnosis of DN is of significance for its treatment and prognosis [4]. With the extensive development of kidney biopsy, studies have found that diabetic patients with albuminuria or abnormal renal function do not necessarily have DN [5], which indicates the difficulty of early diagnosis of DN and the complicated disease development.
At present, two main clinical indicators including urine albumin and estimated glomerular filtration rate (eGFR) are used to diagnose DN. Since 2002, Kidney Dialysis Outcomes Quality Initiative (KDOQI) guidelines have recommended the 24-h urine albumin as an indicator for evaluating kidney damage in the course of diabetes [6]. However, albuminuria has some deficiencies as an important diagnostic indicator. Albuminuria is neither a unique marker of diabetic kidney damage nor a unique marker of kidney damage. Additionally, 24-h urine microalbumin as a method for early diagnosis of kidney disease changes generally in the early stage of glomerulopathy [7]. Urine microalbumin is likely affected by menstrual period, urine retention, blood pressure, exercise, urinary tract infection, and other factors that cannot fully meet clinical requirements [8]. Albuminuria can’t be detected in about 30% of diabetic patients who have developed renal failure [9]. GFR is mainly estimated by the serum creatinine concentration, which is likely affected by many other factors, such as muscle content, gender, age, diet, and medication. Apart from glomerular filtration, part of urine creatinine comes from the secretion of renal tubules. Therefore, the GFR estimated by the creatinine clearance level may be overestimated [10]. Therefore, the identification of non-invasive diagnostic markers with good sensitivity and specificity is the development direction of clinical nephrology. With the increase of patients with DN, it is necessary to explore non-invasive markers that reflect predictable and therapeutic effects.
Cystatin C (Cys-C) is a non-glycosylated low-molecular-weight (13 kDa) protein, whose concentration in serum is closely related to the GFR [11]. It stably exists in almost all nucleated cells in the human body with no tissue specificity, independent of gender, age, inflammatory state, and activity. The kidney is the only organ that clears Cys-C from the circulatory system, and the GFR mainly determines the concentration of serum Cys-C [12,13,14]. Prior reports [15, 16] have demonstrated that Cys-C can serve as an indicator for kidney function with close relation to GFR and good sensitivity regardless of mild, moderate, or severe renal dysfunction, suggesting its promise as a diagnostic marker.
Although there are many investigations on the association of serum Cys-C with patients with DN, major investigations are of discrepancy. To explore more objective evidence of the serum of Cys-C for diagnosing DN, we comprehensively searched the relevant studies and performed this meta-analysis.
Methods
Retrieval strategy of the literature
Two researchers retrieved relative studies about serum Cys-C in the diagnosis of DN independently from databases including Embase, Cochrane Library, Web of Science, PubMed, China National Knowledge Infrastructure (CNKI) and WanFang database from inception to September 22, 2020, with no limitations on language. The literature search formula was as follows: ("cystatin C" OR "Cys-C") AND ("diabetic nephropathy" OR "DN" OR "diabetic kidney disease" OR "DKD" OR "kidney" OR "renal function").
Literature screening
Inclusion criteria:
(1) The data of serum Cys-C level could be collected; (2) The samples were enrolled from diabetic patients; (3) The enrolled diabetic patients were diagnosed with nephropathy; (4) The level of glomerular filtration rate (GFR), albumin-creatinine ratio (ACR), or albumin excretion rate (AER) in the patients with DN was provided.
Exclusion criteria:
(1) Case report, review, letter, conference abstract, or animal studies; (2) Insufficient data to extract to extract four-cell table data; (3) Duplicate data.
Literature quality assessment
Two researchers evaluated the bias risk of the included literature according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) [17]. The scoring system contains 11 items, covering several aspects of the case selection, trials assessment, gold standard, case processes, etc. According to the answers to the landmark questions included in each part of "yes," "no," or "unclear," the risk of bias can be judged as "low," "high," or "moderate." Disagreements between the two authors were settled through discussion.
Data retrieval
The information including the first author, year, region, type of diabetes, the method of Cys-C detection, number of participants, cut-off value, false negative, true negative, true positive, false positive, sensitivity (Sen), specificity (Spe), and diagnostic criteria for DN was extracted. Data retrieval was conducted independently by two researchers. A third author participated in the discussion in case of disagreement.
Statistical analysis
The data analysis was processed by the Stata 15.0 statistical software [18]. The I2 index and p-value were used to assess the heterogeneity. I2 > 50% (P < 0.05) means significant heterogeneity[19]. We combined a typical "shoulder-arm" shape in the summary receiver operating characteristic (SROC) curve with the spearman correlation coefficient of the logarithm of 1-specificity with the logarithm of sensitivity to determine the threshold effect. Adopting the statistic model of bivariate mixed effects, we analyzed the following diagnostic effect sizes from positive likelihood ratio (+ LR), negative likelihood ratio (-LR), sensitivity, and specificity to diagnostic odds ratio (DOR), obtaining the corresponding forest plots [20]. The area under the curve (AUC) value was estimated [21]. The sources of heterogeneity were analyzed using Meta-regression, and the stability of the conclusion was assessed via sensitivity analysis. The assessment of the publication bias was performed by the asymmetry test of Deeks’ funnel plot. P-value < 0.05 is considered significant.
Results
Results and characteristics of the included articles
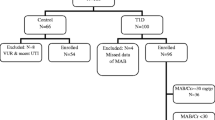
A total of 2521 published studies (PubMed 307, Cochrane Library 372, Embase 293, Web of Science 116, CNKI 391, and China WanFang 1042) were obtained after retrieval, among which 960 repeated ones. After reviewing the title and abstract, 1496 irrelevant articles were excluded, and after reviewing the whole text and complete data, 39 articles were excluded. Finally, 26 articles were included [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. There were 3993 samples included in these studies, containing 1828 in the DN group, and 2165 controls. The detailed screening procedures were given in Fig. 1.
Among the 26 articles, 18 articles were published in English [22,23,24,25,26,27,28,29,30,31,32,33,34, 36, 42, 43, 45, 46], the other 8 articles published in Chinese [35, 37,38,39,40,41, 44, 47]. The enrolled patients were diagnosed with DN according to the GFR, ACR, and AER values. The main characteristics of the identified research were given in Table 1.
QUADAS-2 scores
The results of bias risk assessment of the identified articles were provided (Fig. 2 A,B). Most of the included articles reached a medium-to-high quality level. All samples were selected continuously or randomly. The gold standards of all results were assessed blindly, and the gold standard can correctly distinguish the target disease state. Almost all studies avoided the case–control comparative study design. However, bias was found during experiment evaluation in terms of case processes and disease progression. For example, the duration of the research experiment was different. On the other hand, the threshold was not pre-specified.
Meta-analysis results
The heterogeneities were significant in the pooled analysis of sensitivity (P = 0.00, I2 = 86.68) (Fig. 3A), specificity (P = 0.00, I2 = 85.12%) (Fig. 3A), DOR (P = 0.00, I2 = 100%) (Fig. 3B), + LR (P = 0.00, I2 = 79.81%) (Fig. 3C), and -LR (P = 0.00, I2 = 87.49%) (Fig. 3C).
A typical "shoulder-arm" shape of SROC curve did not display, with the Spearman correlation coefficient 0.061 (P = 0.776), indicating no obvious threshold effect. The bivariate mixed-effects model results showed the pooled Sen of 0.86 (95% confidence interval (CI): 0.82–0.90), Spe of 0.89 (95%CI: 0.85–0.92), + LR of 7.59 (95%CI: 5.66 10.19), -LR of 0.16 (95%CI: 0.12–0.21), and DOR of 48.03 (95%CI: 30.64–75.29). The Fagan’s Nomogram plot suggested that, when the probability ratio pre-test was given a value of 20%, the + LR probability post-test was 66%, the -LR post-test being 4% (Fig. 3D). The AUC was given a value of 94% (95% CI: 0.91–0.96) (Fig. 4). This indicates that serum Cys-C has excellent diagnostic accuracy for DN.
Publication bias
No obvious publication bias existed in the asymmetry test of Deeks’ funnel plot (P = 0.38) (Fig. 5).
Meta-regression and subgroup analyses
The article publication year, language, type of diabetes, Cys-C detection method, sample size, cut-off value, and diagnostic criteria were enrolled in the meta-regression and subgroup analyses (Fig. 6). The results showed that these factors could lead to a significance (P < 0.05), which might be the source of heterogeneity. The subgroups with the publication before 2010, the publication in English, the sample not limited to the type 2 diabetes subgroup, the detection method of PENIA or PETIA, the sample size ≤ 120 patients, the cut-off value ≤ 1.1 mg/L, and GFR as diagnostic criteria had higher sensitivity of statistical significance than the corresponding subgroups.
Sensitivity analysis
Both goodness-of-fit and bivariate normality fit well (Fig. 7A, B). The impact analysis found a study [22] weight (Fig. 7C). The outlier detection showed that this study might be the sources of heterogeneity (Fig. 7D). After removing this abnormal article, the pooled sensitivity varied from 0.86 to 0.87; the specificity remained unchanged; the DOR increased from 48.03 to 51; the + LR increased from 7.59 to 7.6; the -LR decreased from 0.16 to 0.15. These data suggested that re-analysis was changed mildly compared with the combined results before exclusion. This indicates that the conclusion of this study are of robustness.
Discussion
The pathogenesis of DN is quite complicated, involving genetic factors and metabolic disorders. Metabolic abnormalities caused by hyperglycemia, abnormal metabolism of vasoactive substances during the progression of diabetes, changes in kidney hemodynamics, albuminuria after kidney damage are the factors that cause glomerular basement membrane thickening, mesangial cell proliferation, and glomerular sclerosis [48], and finally leading to end-stage renal failure and death [49]. The clinical onset of DN is generally insidious, and the disease progresses slowly, which brings great difficulties in the early treatment of patients [50]. Kidney disease can be reversed after timely and effective symptomatic treatment [51]. However, when patients have symptoms of edema or obvious albuminuria, the optimal treatment time is missed out [52]. In 2014, the American Diabetes Association (ADA) and the National Kidney Foundation (NKF) reached a consensus. DN is defined as chronic kidney disease caused by diabetes, with symptoms mainly including GFR lower than 60 mL/min/1.73 m2 or the urinary ACR higher than 30 mg/g for more than three months [53, 54]. The tubular interstitial diseases share a closer association with kidney damage caused by DN than glomerulus, and tubular damage appears in the early stage of DN, before the glomerular disease [55]. The use of eGFR alone for diagnosis of diabetes combined with CKD is only suitable for patients with advanced-stage (≥ stage III), early diagnosis of CKD requires the detection of other markers for early kidney damage [56]. The early detection of DN mainly focuses on the urine protein excretion rate. However, 20%-30% of patients with type 2 diabetes have already suffered kidney damage even when their urine protein excretion is normal [57]. With the continuous improvement of the Cys-C standardization system, CKD-EPI was published in 2012 based on the Cys-C or combined Cys-C and Cr eGFR formula. Many studies have shown that it evaluates glomerular filtration function more precisely [58]. Serum Cys-C, as a sensitive indicator of early kidney damage, can accurately reflect GFR [59].
The pooled Sen and Spe in the meta-analysis were 0.86 and 0.89, respectively, suggesting that serum Cys-C has good sensitivity and specificity for diagnosing DN. The + LR and -LR were 7.59 and 0.16, respectively, indicating that patients with DN were 7.59 times more likely to be correctly diagnosed as positive than misdiagnosed as positive, while the likelihood of patients being wrongly judged negative was 16% of the likelihood of being correctly judged negative. The + LR > 10, and the -LR < 0.1 indicate convincing diagnostic performance [60], suggesting that serum Cys-C is of limitation in the diagnosis of DN. An increasing DOR value (0 to infinity) means better diagnostic potential [61]. The DOR value in this study was 48.03, suggesting that serum Cys-C is a biomarker for diagnosing DN. AUC more than 0.9 means excellent diagnostic capabilities [62, 63]. The AUC in this meta-analysis was 0.94, suggesting that serum Cys-C has a promising diagnostic accuracy for DN, which was consistent with the findings of the reviews in 2016 [64, 65]. According to a more strict standard, new research after 2016 were enrolled in our study [64, 65].
Meta-regression and subgroup analyses suggested that publication year, publication language, type of diabetes, Cys-C detection method, sample size, cut-off value, and diagnostic criteria might be the sources of heterogeneity. Higher diagnostic value was found in the groups with publication year ≤ 2010 group, publication in English, samples not limited to type 2 diabetes, PENIA, or PETIA for detection of serum Cys-C, sample size ≤ 120, cut-off value ≤ 1.1 mg/L, and GFR as diagnostic criteria than that of the corresponding group. The sensitivity analyses and publication bias suggested that the findings were stable and credible in this meta-analysis.
There were also some limitations in our study. First of all, the included studies included comparative studies of Cys-C and other molecules in the diagnosis of DN and combined diagnostic value studies. The inclusion criteria used between the trials, the staging of DN, the presence of other combined diseases, sample size, detection methods, and the choice of the gold standard are different, leading to the heterogeneity of results. Secondly, some of the included studies did not describe in detail information, such as the trial randomization, blinded design, and quality control, which might affect the quality of this study. Finally, the included articles were from multiple countries, and the incidence and medical level were different among different countries and regions, which could affect the accuracy of the diagnosis and thus affect the results of this study. Therefore, the investigation of the correlation between Cys-C in the diagnosis of DN requires a large sample, random, blinded research design, using a unified gold standard and disease staging, so that the authenticity and reliability of the results are more clinically meaningful.
Conclusion
In summary, this meta-analysis indicates that serum cystatin C has an excellent diagnostic value with good sensitivity and specificity for patients with DN. This study reveals an association of serum Cys-C with patients with DN. Serum Cys-C is conducive to the diagnosis of this disease. Considering the limitations of this meta-analysis, the conclusions of this research are yet to be confirmed using high-quality clinical trials in the future.
Availability of data and materials
The datasets using in the current meta-analysis are available on reasonable request from the corresponding author.
Abbreviations
- Sen:
-
Sensitivity
- Spe:
-
Specificity
- DOR:
-
Diagnostic Odds Ratio
- + LR:
-
Positive Likelihood Ratio
- -LR:
-
Negative Likelihood Ratio
- AUC:
-
Area Under the Curve
- ROC:
-
Receiver Operating Characteristic Curve
- SROC:
-
Summary Receiver Operating Characteristic Curve
- PETIA:
-
Particle-Enhanced Turbidimetric Assay
- PENIA:
-
Particle Enhanced Nephelometry Assay
- ADA:
-
American Diabetes Association
- NKF:
-
National Kidney Foundation
- Cys-C:
-
Cystatin C
- DN:
-
Diabetic Nephropathy
- IDF:
-
International Diabetes Federation
- ESRD:
-
End-Stage Renal Disease
- CKD:
-
Chronic Kidney Disease
- eGFR:
-
Estimated Glomerular Filtration Rate
- KDOQI:
-
Kidney Dialysis Outcomes Quality Initiative
- ACR:
-
Albumin-Creatinine Ratio
- AER:
-
Albumin Excretion Rate
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- CNKI:
-
China National Knowledge Infrastructure
- TP:
-
True Positive
- FP:
-
False Positive
- FN:
-
False Negative
- TN:
-
True Negative
References
Shomali M. Add-on therapies to metformin for type 2 diabetes. Expert Opin Pharmaco. 2011;12(1):47–62.
Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in Chronic Kidney Disease in China. N Engl J Med. 2016;375(9):905–6.
Kim JW, Chae JY, Kim JW, Yoon CY, Oh MM, Kim JJ, et al. Survey on the Perception of Urogenital Complications in Diabetic Patients. World J Mens Health. 2012;30(3):172–6.
Hickey FB, Martin F. Diabetic kidney disease and immune modulation. Curr Opin Pharmacol. 2013;13(4):602–12.
Yan ST, Liu JY, Tian H, Li CL, Li J, Shao YH, et al. Clinical and pathological analysis of renal damage in elderly patients with type 2 diabetes mellitus. Clin Exp Med. 2016;16(3):437–42.
Association AD. 5. Glycemic Targets. Diabetes Care. 2016;39 Suppl 1:39–46.
Ma X, An L, Wang Q. Changes in Serum Nampt Levels and Its Significances in Diabetic Nephropathy Patients-The Potential Role of Nampt in T2DM with Diabetic Nephropathy. Endocr Metab Immune. 2017;17(2):114–24.
Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298(1):E117–26.
Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin—dependent diabetes. New Engl J Med. 1985;312(10):617–21.
Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The Effects of Weight Loss on Renal Function in Patients with Severe Obesity. J Am Soc Nephrol. 2003;14(6):1480–6.
Lee BW, Ihm SH, Choi MG, Yoo HJ. The comparison of cystatin C and creatinine as an accurate serum marker in the prediction of type 2 diabetic nephropathy. Diabetes Res Clin Pr. 2007;78(3):428–34.
Kazama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F. Serum Cystatin C Reliably Detects Renal Dysfunction in Patients with Various Renal Diseases. Nephron. 2002;91(1):13–20.
Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentation. Kidney Int. 2006;69(2):399–405.
Sjöström P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Inv. 2009;65(2):111–24.
Stevens KG. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40(2):221–6.
Asci-Buturovi B, Cavaljuga S. Cystatin C as a marker for detection of early renal failure in diabetes type 2 patients. Bosnian J Basic Med. 2005;5(4):68–71.
Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma J. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155(8):529–36.
Houwelingen VH, Arends L, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Statist Med. 2002;21(4):589–624.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90.
Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. 2009;28(21):2653–68.
Mojiminiyi OA, Abdella N, George S. Evaluation of serum cystatin C and chromogranin A as markers of nephropathy in patients with Type 2 diabetes mellitus. Scand J Clin Lab Inv. 2000;60(6):483–99.
Oddoze C, Morange S, Portugal H, Berland Y, Dussol B. Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis. 2001;38(2):310–6.
Christensson AG, Grubb AO, Nilsson JA, Norrgren K, Sterner G, Sundkvist G. Serum cystatin C advantageous compared with serum creatinine in the detection of mild but not severe diabetic nephropathy. J Intern Med. 2004;256(6):510–8.
Bicik Z, Bahcebasi T, Kulaksızoglu S, Yavuz O. The efficacy of cystatin C assay in the prediction of glomerular filtration rate. Is it a more reliable marker for renal failure. Clin Chem Lab Med. 2005;43(8):855–61.
Beauvieux MC, Le Moigne F, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, et al. New Predictive Equations Improve Monitoring of Kidney Function in Patients With Diabetes. Diabetes Care. 2007;30(8):1988–94.
MacIsaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, et al. The accuracy of cystatin C and commonly used creatinine-based methods for detecting moderate and mild chronic kidney disease in diabetes. Diabetic Med. 2007;24(4):443–8.
Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, et al. Cystatin C and estimates of renal function searching for a better measure of kidney function in diabetic patients. Clin Chem. 2007;53(3):480–8.
Kimura T, Ikeda H, Fujikawa J, Nomura K, Aoyama T, Wada Y, et al. Usefulness of serum cystatin C in Japanese patients with type 2 diabetes mellitus and nephropathy. Diabetes Res Clin Pr. 2008;83(2):e58-61.
Rigalleau V, Beauvieux MC, Le Moigne F, Lasseur C, Chauveau P, Raffaitin C, et al. Cystatin C improves the diagnosis and stratification of chronic kidney disease, and the estimation of glomerular filtration rate in diabetes. Diabetes Metab. 2008;34(5):482–9.
Iliadis F, Didangelos T, Ntemka A, Makedou A, Moralidis E, Gotzamani-Psarakou A, et al. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine- or cystatin C-based equations. Diabetologia. 2011;54(12):2987–94.
Jeon YK, Kim MR, Huh JE, Mok JY, Song SH, Kim SS, et al. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci. 2011;26(2):258–63.
Bevc S, Hojs R, Ekart R, Završnik M, Gorenjak M, Puklavec L. Simple Cystatin C Formula for Estimation of Glomerular Filtration Rate in Overweight Patients with Diabetes Mellitus Type 2 and Chronic Kidney Disease. Exp Diabetes Res. 2012;2012:1–8.
Chae HW, Shin JI, Kwon AR, Kim HS, Kim DH. Spot Urine Albumin to Creatinine Ratio and Serum Cystatin C are Effective for Detection of Diabetic Nephropathy in Childhood Diabetic Patients. J Korean Med Sci. 2012;27(7):784–7.
Wang H. Diagnostical value of serum Cystatin C and lopoprotein A for early diabetic nephropathy. J Zhengzhou Univ. 2012;47(3):388–90.
Assal HS, Tawfeek S, Rasheed EA, El-Lebedy D, Thabet EH. Serum Cystatin C and Tubular Urinary Enzymes as Biomarkers of Renal Dysfunction in Type 2 Diabetes Mellitus. Clin Med Insights Endocrinol Diabetes. 2013;6:7–13.
Tan T, Cheng L, Wei H, Xu Z, Zhang K. Diagnostic value of HbA1c, urinary AAG and serum Cyt C for early diabetic nephropathy. Chin J Clin Lab Sci. 2015;33(11):827–9.
Cao Y, Jiang Y. Diagnostic efficiency of cystatin C and other biomarkers on early diabetic nephropathy. Mod Prev Med. 2015;42(14):2669–71.
Yang N, Liu L, Wei R. Diagnostic value of serum cystatin C, β2-microglobulin and homocysteine in the diagnosis of diabetic nephropathy. Chin J Health Lab Tec. 2017;27(6):836–41.
Zhang H, Yang H, Liu L. Application value of serum neutrophil gelatinase-associated lipocalin, cystatin C, urinary albumin creatinine rate and N-acetyl-β-D-glucosaminidase in early diabetic kidney disease. Chin J Diabetes. 2018;26(4):309–15.
Zhang R, Wang H, Peng X. Combined determination of serum amyloid A protein, cystatin C and urinary albumin-to-creatinine ratio in the diagnosis of early diabetic nephropathy. Lab Med. 2018;33(2):97–100.
Mohammed R, El-Shazely A. Diagnostic Values Of Serum Cystatin C And Urinary Fetuin-A As Early Biochemical Markers In Predicting Diabetic Nephropathy Among Patients With Type 2 Diabetes Mellitus. Res J Pharm Biol Chem Sci. 2019;10(6):237–44.
Xu W, Tang S, Xiang M, Peng J. Serum Homocysteine, cystatin C as Biomarkers for Progression of Diabetic Nephropathy. Pteridines. 2019;30(1):183–8.
Wang H, Guo L, Lin J. Diagnostic value of homocysteine, Cystatin C and hign-sensitivity C-reactive protein in the diagnosis of early diabetic nephropathy. Chin J Lab Diagn. 2019;23(12):2048–51.
Salem NA, El Helaly RM, Ali IM, Ebrahim HAA, Alayooti MM, El Domiaty HA, et al. Urinary Cyclophilin A and Serum Cystatin C as Biomarkers for Diabetic Nephropathy in Children with Type 1 Diabetes. Pediatr Diabetes. 2020;21(5):846–55.
Wang S, Yan X. The value of serum cystatin C combined with miR-16-5p in the early diagnosis of diabetic kidney disease (DKD). Acta Medica Mediterr. 2020;36:1581–5.
Guang S, Zhai R, Wang L. The value of serum homocysteine, Cystatin C and superoxide dismutase in the early diagnosis of type 2 diabetic nephropathy. Chin J Lab Diagn. 2020;25(7):1114–7.
Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A Glimpse of Various Pathogenetic Mechanisms of Diabetic Nephropathy. Annu Rev Pathol. 2011;6:395–423.
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann Intern Med. 2003;139(2):137–47.
Khan IA, Nasiruddin M, Haque S, Khan R. Comparative evaluation of efficacy and safety profile of rhubarb and α-keto analogs of essential amino acids supplementation in patients with diabetic nephropathy. Saudi J Kidney Dis Transpl. 2016;27(4):710–6.
Srivastava A, Adams-Huet B, Vega GL, Toto RD. Effect of losartan and spironolactone on triglyceride-rich lipoproteins in diabetic nephropathy. J Invest Med. 2016;64(6):1102–8.
Kim Y, Park CW. AMP-Activated Protein Kinase in Diabetic Nephropathy. Kidney Res Clin Pract. 2016;35(2):69–77.
Kdoqi K. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2, supplement 2):12–154.
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, De Boer IH, Goldstein-Fuchs J, et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–33.
Tang SCW, Leung JCK, Lai KN. Diabetic Tubulopathy: An Emerging Entity. Diabetes and the Kidney. 2011;170:124–34.
Foundation NK. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–86.
de F Olivarius N, Andreasen AH, Keiding N, Mogensen CE. Epidemiology of renal involvement in newly-diagnosed middle-aged and elderly diabetic patients. Cross-sectional data from the population-based study “Diabetes Care in General Practice” Denmark. Diabetologia. 1993;36(10):1007–16.
Inker L, Schmid C, Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9.
Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603.
Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. A likelihood ratio approach to meta-analysis of diagnostic studies. J Med Screen. 2003;10(1):47–51.
Glas A, Lijmer J, Prins M, Bonsel G, Bossuyt P. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
Jones C, Athanasiou T. Summary Receiver Operating Characteristic Curve Analysis Techniques in the Evaluation of Diagnostic Tests. Ann Thorac Surg. 2005;79(1):16–20.
Walter S. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2010;21(9):1237–56.
Zhou B, Zou H, Xu G. Clinical Utility of Serum Cystatin C in Predicting Diabetic Nephropathy Among Patients with Diabetes Mellitus: a Meta-Analysis. Kidney Blood Press R. 2016;41(6):919–28.
Yang SK, Liu J, Zhang XM, Hu C, Zhang W, Sun L, et al. Diagnostic Accuracy of Serum Cystatin C for the Evaluation of Renal Dysfunction in Diabetic Patients: A Meta-Analysis. Ther Apher Dial. 2016;20(6):579–87.
Acknowledgements
The authors appreciate the reviewers for their helpful comments on this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LXL,ZY,XC: Significant contribution to the conception and design of the research; LXL,ZY: manuscript drafting;LXL,ZY,XC: literature acquisition, analysis and interpretation of the data;LXL,ZY,XC: Crucial revision of the manuscript; LXL,ZY,XC: Revising the manuscript comprehensively, final approval of the version to publish. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval and consent to participate were not needed, for this is a meta-analysis.
Consent for publication
Not applicable.
Competing interests
All the authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, X., Zhu, Y. & Xue, C. Diagnostic value of serum cystatin C for diabetic nephropathy: a meta-analysis. BMC Endocr Disord 22, 149 (2022). https://doi.org/10.1186/s12902-022-01052-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01052-0