Abstract
Background
Type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease frequently coexist and share pathophysiological manifestations. This study aimed to explore the association between T2DM status and prevalence of liver steatosis and fibrosis, identified using the controlled attenuation parameter and liver stiffness measurement attained via liver ultrasound transient elastography.
Methods
This was a cross-sectional analysis of data collected in the National Health and Nutrition Examination Survey for 2017–2018. Multivariable logistic regression model was used to evaluate the association between T2DM and prevalence of liver steatosis and fibrosis. Subgroup analyses, stratified by sex age, race, and body mass index (BMI), were further performed.
Results
Of the 2,780 participants aged ≥ 40 years enrolled, 749 had T2DM, and 2,031 did not. After adjustment for potential confounders, T2DM was associated with a higher prevalence of liver steatosis (OR = 1.7, 95% CI, 1.3–2.1). This T2DM-related prevalence was higher among women (OR = 1.8, 95% CI, 1.3–2.5) and in the non-Hispanic Black (OR = 1.8, 95% CI, 1.1–3.0), other race (OR = 1.9, 95% CI, 1.2–3.0), and BMI < 25 kg/m2 (OR = 2.0, 95% CI, 1.1–3.8) groups. T2DM was also associated with a significantly higher prevalence of fibrosis (OR = 2.0, 95% CI, 1.5–2.7), with this association being more prominent for the other race (OR = 2.9, 95% CI, 1.5–5.5) and BMI < 25 kg/m2 (OR = 3.3, 95% CI: 1.3–8.8) groups.
Conclusions
Our findings indicated a positive association between T2DM status and prevalence of hepatic steatosis and fibrosis. This association was more prominent for individuals with a BMI < 25 kg/m2 and was influenced by race-specific effects.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and has become a major global health concern [1, 2]. In recent years, the prevalence of NAFLD has been rising progressively, along with type 2 diabetes mellitus (T2DM), which has reached epidemic levels [3]. T2DM is recognized as one of the strongest risk factors for the progression of NAFLD to non-alcoholic steatohepatitis, advanced fibrosis, or cirrhosis [4]. T2DM and NAFLD frequently coexist, with shared pathophysiological manifestations of excessive fat accumulation and insulin resistance [5].
The diagnosis of NAFLD is based on the detection of steatosis on liver biopsy and imaging techniques, after the exclusion of hepatic fatty infiltration and other causes of abnormal transaminase values via laboratory screening and medical history [6]. As a non-invasive imaging tool, liver ultrasound transient elastography (TE) provides excellent diagnostic accuracy for liver steatosis and advanced liver diseases in adults [7]. The latest cycle of the National Health and Nutrition Examination Survey (NHANES) includes liver ultrasound TE for the diagnosis of liver steatosis and advanced liver disease based on the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM). Herein, we explored the association between T2DM status and prevalence of liver steatosis and fibrosis, indicated by the CAP and LSM, among adults aged ≥ 40 years using the NHANES database.
Methods
Study population
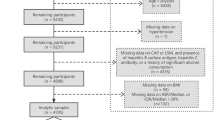
This cross-sectional study used data from the NHANES database (2017–2018 cycle). The NHANES is a program designed to provide objective health data of the population of the United States. The methodology and data collection for the NHANES are freely available (http://www.cdc.gov/nchs/nhanes.htm) and have been fully described [8]. Among 3,882 adults aged ≥ 40 years whose data were available in the database, the following were excluded: 441 for whom serum glucose or glycohemoglobin (HbA1c) data were unavailable; 234 without CAP or LSM data; 375 due to the presence of hepatitis B surface antigen, hepatitis C antibody, or a history of significant alcohol consumption (men: > 30 g/day; women: > 20 g/day) [9], 26 aged < 30 years at the time of diabetes mellitus (DM) onset; and 26 without body mass index (BMI) data. We included 2,780 participants in the final analysis.
The National Center for Health Statistics Research Ethics Review Board approved the survey protocol and all participants provided written informed consent for data collection and the use of their information for research.
Our study is compliant with the Guidelines for the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [10].
Study variables
The exposure for our study is the T2DM status, defined according to the following criteria: participants being informed that they had DM by their doctor, age at time of DM diagnosis ≥ 30 years; and/or a HbA1c level ≥ 6.5% [11]. Outcomes on liver ultrasound TE were measured using a FibroScan® system (model 502, V2 Touch) and included CAP, with a value ≥ 274 dB/m indicative of liver steatosis [12], and LSM, with a median value ≥ 8 kPa indicative of significant fibrosis [13], provided by the liver ultrasound TE on a FibroScan® model 502 V2 Touch equipped with a medium or extra large probe. The following demographic and clinical variables were also collected as covariates in our analyses: age; sex; race; level of education; ratio of family income to poverty; level of moderate recreational activities; history of smoking ≥ 100 cigarettes; BMI; and blood urea nitrogen (BUN) levels, total cholesterol, uric acid, gamma-glutamyl transpeptidase (GGT), aspartic acid transferase, alanine amino transferase (ALT), alkaline phosphatase (ALP), and serum glucose.
Statistical analysis
All analyses were performed using statistical software R (version 3.4.3) and EmpowerStats (X&Y Solutions, Boston, MA), with a P-value < 0.05 considered significant. Multivariable logistic regression model was used to evaluate the association between T2DM status and prevalence of liver steatosis and fibrosis. Three statistical models were constructed: model 1, no adjustment for covariates; model 2, adjusted for age, sex, and race; and model 3, adjusted for all covariates presented in Table 1. Subgroup analyses, stratified by sex, age, race and, BMI were further performed.
Results
The characteristics of the study sample, according to T2DM status, are presented in Table 1. Of the 2,780 participants enrolled, 749 had a diagnosis of T2DM, with the other 2,031 classified in the non-DM group. Compared to the non-DM group, participants with T2DM were older, had a higher BMI and levels of ALP, ALT, GGT, uric acid, and BUN, had higher CAP and LSM values, a higher proportion of liver steatosis and significant fibrosis, and a lower level of total cholesterol.
Association between T2DM status and CAP
After adjustment for potential confounding factors, T2DM status was positively associated with CAP (β = 16.8, 95% CI, 11.8–21.8; Table 2). On subgroup analyses, this positive association was more prominent among women (β = 19.7, 95% CI, 12.6–26.7) than it was among men (β = 12.2, 95% CI, 4.9–19.4), and in the non-hispanic black (β = 19.5, 95% CI, 9.1–29.9), other race (β = 19.4, 95% CI, 10.2–28.5), and BMI < 25 kg/m2 (β = 19.8, 95% CI, 8.7–31.0) groups.
Association between T2DM status and risk of liver steatosis
In the fully adjusted model (Table 3), T2DM status was positively associated with prevalence of liver steatosis (OR = 1.7, 95% CI, 1.3–2.1). On subgroup analyses, this positive association was more prominent among women (OR = 1.8, 95% CI, 1.3–2.5) than men (OR = 1.5, 95% CI: 1.0–2.1), and in the non-Hispanic Black (OR = 1.8, 95% CI, 1.1–3.0), other race (OR = 1.9, 95% CI, 1.2–3.0), and BMI < 25 kg/m2 (OR = 2.0, 95% CI, 1.1–3.8) groups.
Association between T2DM status and LSM
In the fully adjusted model, there was a positive association between T2DM status and LSM (β = 0.8, 95% CI, 0.2–1.3; Table 4). On subgroup analyses, this positive association was only identified among men (β = 0.9, 95% CI, 0.0–1.8) and in the 40–59 age (β = 1.0, 95% CI, 0.1–1.8), other race (β = 1.8, 95% CI, 0.8–2.9), and BMI ≥ 30 kg/m2 (β = 1.0, 95% CI, 0.1–1.9) groups.
Association between T2DM status and risk of significant fibrosis
In the fully adjusted model, T2DM status and prevalence of significant fibrosis were positively correlated (OR = 2.0, 95% CI, 1.5–2.7) (Table 5). On subgroup analyses, this positive association was more prominent among individuals in the other race (OR = 2.9, 95% CI, 1.5–5.5) and BMI < 25 kg/m2 (OR = 3.3, 95% CI, 1.3–8.8) groups.
Discussion
In this study, we evaluated the association between T2DM status and prevalence of liver steatosis and fibrosis among adults aged ≥ 40 years, and found that T2DM was associated with a significantly higher prevalence of liver steatosis, with this association being more prominent among women and the non-Hispanic Black, other race, and BMI < 25 kg/m2 groups. T2DM also positively correlated with the prevalence of significant fibrosis, which was more prominent in the other race and BMI < 25 kg/m2 groups.
The bidirectional and mutual relationship between T2DM and NAFLD has been highlighted by epidemiological studies, with NAFLD increasing the risk of T2DM incidence, and T2DM increasing the risk of NAFLD incidence and progression [14]. A recent meta-analysis showed that the pooled prevalence of NAFLD among adults with T2DM was around 60%, with this prevalence varying by age and by BMI [15]. Compared to non-diabetes patients, those with combined NAFLD and T2DM have a higher risk of NAFLD progression [16]. A previous NHANES study (NHANES III) revealed that diabetes was associated with all-cause and cardiovascular mortality among individuals with NAFLD [17].
Among the non-invasive tests for NAFLD, TE is the most widely used for the assessment of liver fibrosis [18]. A higher prevalence of advanced fibrosis assessed via TE was observed among patients with T2DM [19,20,21,22]. The results of a recent NHANES study reported high rates of hepatic steatosis and fibrosis, diagnosed by CAP and LSM, among patients with T2DM, but with race-dependent differences [23]. Similarly, in our study, the association between T2DM status and CAP or LSM was prominent in some races, but not in others, including a non-significant association among Mexican–American individuals.
The common pathophysiological mechanisms shared by NAFLD and T2DM include a series of metabolic changes; in particular, changes in the white adipose tissue may play a central role in the initiation of both NAFLD and T2DM [24]. In 2020, an international panel of experts from 22 countries proposed the novel term “metabolic dysfunction-associated fatty liver disease” to replace NAFLD, which further emphasizes the strong association between T2DM and NAFLD [25]. NAFLD and T2DM not only have almost the same risk factors, but also have synergistic effects on each other’s disease progression and complications. Therefore, routine screening for T2DM among individuals with NAFLD and lifestyle changes, including diet modifications and physical activity, are recommended for the prevention and management of both T2DM and NAFLD.
Our study had some limitations. First, as this was a cross-sectional study, no causality could be established. Second, we excluded participants with age of DM onset of < 30 years of age to minimize the number of participants with T1DM, as previously described [26, 27], as the NHANES database does not differentiate diabetes by type. Third, the values of CAP defining hepatic steatosis and LSM defining significant fibrosis are both inconsistent among different studies using NAHENS 2017–2018 database [13, 28, 29]. Thus, the sensitivity and specificity of TE test may vary depending on the cut-off values. Fourth, differences in measurements depending on the probe used in FibroScan have been demonstrated in previous reports [30, 31]. However, the elastography exams were performed by trained and certified technicians, according to the manufacturer guidelines [32]. Last, self-reported confounders may be susceptible to individual biases. This source of bias was minimized by the utilization of the NHANES data, which is collected by trained personnel through established procedures.
Conclusion
In conclusion, our findings indicate that T2DM is positively associated with prevalence of hepatic steatosis and fibrosis. This association was more prominent for individuals with a BMI < 25 kg/m2 and was influenced by race-specific effects. Routine screening for T2DM among individuals with NAFLD may contribute to the prevention and the management of both T2DM and NAFLD.
Availability of data and materials
The datasets generated and analysed during the current study are available in the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- T2DM:
-
Type 2 diabetes mellitus
- TE:
-
Transient elastography
- NHANES:
-
National Health and Nutrition Examination Survey
- CAP:
-
Controlled attenuation parameter
- LSM:
-
Liver stiffness measurement
- HbA1c:
-
Glycohemoglobin
- BMI:
-
Body mass index
- DM:
-
Diabetes mellitus
- BUN:
-
Blood urea nitrogen
- GGT:
-
Gamma-glutamyl transpeptidase
- ALT:
-
Alanine amino transferase
- ALP:
-
Alkaline phosphatase
References
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16.
Lee CH, Lui D, Lam K: Non-alcoholic fatty liver disease and type 2 diabetes - An Update. J Diabetes Invest 2022.
Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612.
Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009–2019. J Hepatol. 2021;75(4):795–809.
Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, Newton KP, Sawh MC, Hooker J, Sy EZ, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J Pediatr. 2019;207:64–70.
Ramírez-Vélez R, García-Hermoso A, Correa-Rodríguez M, Izquierdo M: Defining values for controlled attenuation parameter and liver stiffness in youth without liver disease. Pediatr Res 2021.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR: National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat Ser 2 Data Eval Methods Res2013(161):1–24.
Kim D, Konyn P, Cholankeril G, Ahmed A: Physical Activity Is Associated With Nonalcoholic Fatty Liver Disease and Significant Fibrosis Measured by FibroScan. Clin Gastroenterol Hepatol 2021.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England). 2007;370(9596):1453–7.
Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14-s31.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(6):1717–30.
Liu X, Shen H, Chen M, Shao J. Clinical Relevance of Vitamins and Carotenoids With Liver Steatosis and Fibrosis Detected by Transient Elastography in Adults. Front Nutr. 2021;8: 760985.
Muzica CM, Sfarti C, Trifan A, Zenovia S, Cuciureanu T, Nastasa R, Huiban L, Cojocariu C, Singeap AM, Girleanu I, et al. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Can J Gastroenterol Hepatol. 2020;2020:6638306.
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100(6):2231–8.
Wu W, Xiang J, Chen X. Association Between Diabetes Mellitus and All-Cause and Cardiovascular Mortality Among Individuals With Ultrasound-Defined Non-Alcoholic Fatty Liver Disease. Front Endocrinol. 2021;12: 773342.
Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline gastroenterology. 2014;5(3):211–8.
Sporea I, Mare R, Lupușoru R, Sima A, Sirli R, Popescu A, Timar R. Liver Stiffness Evaluation by Transient Elastography in Type 2 Diabetes Mellitus Patients with Ultrasound-proven Steatosis. J Gastrointest Liver Dis. 2016;25(2):167–74.
Chen K, Sng WK, Quah JH, Liu J, Chong BY, Lee HK, Wang XF, Tan NC, Chang PE, Tan HC, et al. Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PLoS ONE. 2020;15(8): e0236977.
Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34(8):1396–403.
Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, Portillo Romero J, Schmidt S, Chang KL, Samraj G, et al. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diab Care. 2021;44(2):399–406.
Ciardullo S, Monti T, Perseghin G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults With Type 2 Diabetes. Diab care. 2021;44(2):519–25.
Schröder B, Kahl S, Roden M. Non-alcoholic fatty liver disease in type 2 diabetes - A specific entity? Liver Int. 2021;41(Suppl 1):105–11.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Andary R, Fan W, Wong ND. Control of Cardiovascular Risk Factors Among US Adults With Type 2 Diabetes With and Without Cardiovascular Disease. Am J Cardiol. 2019;124(4):522–7.
Yao X, Xu X, Jin F, Zhu Z. The Correlation of Type 2 Diabetes Status with Bone Mineral Density in Middle-Aged Adults. Diab Metab Syndr Obesity Targets Ther. 2020;13:3269–76.
Gangireddy VGR, Pilkerton C, Xiang J, Tinajero R, Ashcraft AM. Hepatic Fibrosis and Steatosis in Metabolic Syndrome. J Obesity Metab Syndr. 2022;31(1):61–9.
Heredia NI, Zhang X, Balakrishnan M, Hwang JP, Thrift AP: Association of lifestyle behaviors with non-alcoholic fatty liver disease and advanced fibrosis detected by transient elastography among Hispanic/Latinos adults in the U.S. Ethnicity Health 2022. p. 1–14.
Oeda S, Takahashi H, Imajo K, Seko Y, Ogawa Y, Moriguchi M, Yoneda M, Anzai K, Aishima S, Kage M, et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan(®) M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J Gastroenterol. 2020;55(4):428–40.
Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5(1):76–85.
Liver Ultrasound Transient Elastography Procedures Manual. Centers for Disease Control and Prevention (2018). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2018_Liver_Ultrasound_Elastography_Procedures_Manual.pdf
Acknowledgements
The authors appreciate the time and effort given by participants during the data collection phase of the NHANES project.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
JC, PH, and YFW contributed to data collection, analysis and writing of the manuscript. ZXZ contributed to study design and editing of the manuscript. The author(s) read and approved the final manuscript..
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics review board of the National Center for Health Statistics approved all NHANES protocols and written informed consents were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Hu, P., Wang, Y. et al. Association between type 2 diabetes status and prevalence of liver steatosis and fibrosis among adults aged ≥ 40 years. BMC Endocr Disord 22, 128 (2022). https://doi.org/10.1186/s12902-022-01046-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01046-y