Abstract
Background
The food and drug administration approved many drugs to treat diabetes mellitus, but those drugs do not have a noticeable effect on weight management. Recently, glucagon-like peptide 1 agonist known as Cotadutide serve as a potent drug in treating type 2 diabetes by reducing blood glucose levels and body weight indices. This study aimed to explore the safety and efficacy of Cotadutide as a treatment for type 2 diabetes individuals.
Methods
A comprehensive literature search was done on different databases, including PubMed, Scopus, Web of Science, and Cochrane Library to capture all relevant articles using an established search strategy. The inclusion criteria were randomized controlled trials that assessed the safety and efficacy of Cotadutide versus placebo or any anti-diabetes drugs in patients with type 2 diabetes mellitus and a BMI between 22 kg/m2 and 40 kg/m2. We conducted the analysis using Revman software version 5.4.
Results
We found 663 relevant articles. From which nine studies were included and subjected to qualitative analysis and eight for quantitative analysis. The pooled effect showed that Cotadutide was better than placebo in reducing body weight (kg) (Mean difference (MD) = 3.31, p < 0.00001), glycated hemoglobin (HbA1c) (MD = 0.68, p > 0.00001), glucose area under the plasma concentration curve (AUC [0-4 h]) (MD = 30.15, p < 0.00001), and fasting plasma glucose over time (mg/dl) (MD = 31.31, p < 0.00001).
Conclusion
Cotadutide is safe and effective in reducing plasma glucose levels, HbA1c and body weight in individuals with type 2 diabetes.
Trial registration
The study protocol was registered on PROSPERO (CRD: CRD42021257670).
Similar content being viewed by others
Background
Type 2 diabetes mellitus is one of the most common endocrine disorders worldwide, according to the International Diabetes Federation (IDF) its prevalence has surged rapidly to include more than 400 million individuals over the past three decades [1]. Type 2 diabetes mellitus is a long-term disease characterized by chronic insulin resistance and hyperglycemia that increases over time, resulting in increasing of insulin resistance leading to weight gain [2, 3]. Therefore, reducing body weight will prevent more insulin resistance and better control of the body weight condition.
Many medications with different mechanisms are available to control type 2 diabetes mellitus as (a) metformin which acts through various trajectories to inhibit gluconeogenesis and reduce the level of lipopolysaccharide, (b) insulin secretagogues, (c) alpha-glucosidase inhibitors, (d) dipeptidyl peptidase 4 inhibitors, and (e) sodium-glucose co-transporter-2 inhibitor [4]. However, none of them is significant in reducing body weight at doses approved for blood glucose reduction. Therefore, weight loss remains an unmet medical need for these people [5].
Glucagon-like peptide-1 (GLP-1) receptor agonist's therapy known as Cotadutide seems to be effective in glycemic control and weight loss. The impact of GLP-1 drugs varies depending on the pharmacokinetic profile [6]. Lorenz M et al. 2013 showed that short-acting GLP-1 receptor agonists (lixisenatide) at a dose of 20 μg daily lowers postprandial hyperglycemia excursions in individuals with type 2 diabetes mellitus, probably caused by the continuous slowing of stomach emptying [7]. In the same way, J van Can et al. 2014 investigated the effects of long-acting GLP-1 receptor agonists (liraglutide) on gastric emptying and the result indicated that liraglutide at 3 mg significantly delays the gastric emptying [8]. In addition, Daniel R et al. reported in 2020 that lixisenatide reduced the gastric emptying rate more than liraglutide [9].
Based on the above-mentioned data, GLP-1 receptor agonists are useful in treating individuals with type 2 diabetes and obesity by controlling hyperglycemia and delaying stomach emptying. They also help people lose weight by reducing the appetite and increasing energy expenditure by optimizing metabolic reactions such as amino acid catabolism, and fatty acid oxidation [10].
Many studies have investigated the effect of Cotadutide (GLP-1 receptor agonist) on type 2 diabetes mellitus. In this study, we aim to summarize, review, and analyze those studies to understand the safety and efficacy profiles of this new medication in controlling type 2 diabetes mellitus and its effect on weight reduction.
Methods
Study design and registration
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline and Cochrane Handbook of Systematic Reviews of Intervention [11, 12]. The study protocol was registered on PROSPERO (CRD: CRD42021257670).
Literature search
PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and Web of Science were searched for articles conducted from 1 January 1979 to 1 June 2021 without any other restrictions. We used Mesh database to generate the search strategy used. The search strategy is formed of a combination of the following keywords and their relative words (Cotadutide) AND (Diabetes) AND (body weight). The detailed search strategy can be found in supplementary file 1.
Eligibility criteria and studies selection
The inclusion criteria included Randomized Controlled Trials (RCT) evaluating the efficacy and safety of the drug Cotadutide on men or women aged 18 to 65 years with controlled type 2 diabetes and a BMI between 22 kg/m2 and 40 kg/m2. Only English studies were included which provide full text online accessible to us. No restrictions regarding the date of publication. Protocols published in clinicaltrials.gov were included if they contain results and sufficient information to assess their quality.
We excluded studies with insufficient data for extraction. Reviews, book chapters, thesis, editorial, letters, conference papers, and non-English studies. Animal or In vitro studies, cohort, case–control, non-clinical studies, literature reviews, and meta-analysis were excluded.
Two independent authors screened the articles retrieved from the four electronic databases by title, abstract, and full text on an excel sheet for eligibility. Another independent author resolved any disagreements between the other two authors.
Quality assessment
Cochrane risk-of-bias tool for randomized trials (RoB 2) was applied to assess the quality of the selected RCTs [13]. The Rob2 tool consists of six domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and other biases. The evaluators' responses were categorized as yes, probably yes, probably no, no, and no information. Following that, all disputes were discussed and resolved.
Data extraction and study outcomes
Two independent auhrors extracted data in a pre-defined excel sheet. The excel sheet items were categorized as a summary of the included trials' key features, characteristics of the participants, and Cotadutide safety and efficacy outcomes. Any disagreements were solved by a discussion between the reviewers.
Outcome definition
Treatment efficacy was assessed by frequency of positive Anti-drug antibodies to Cotadutide, Percent Change from Baseline in Body Weight, Change from Baseline in Glycated Hemoglobin (HbA1c), Mean Percentage Change from Baseline in Glucose Area Under the Plasma Concentration Curve (AUC [0-4 h]) as Measured by (MMTT). The safety outcomes included Treatment-Emergent Adverse Events (TEAEs), and Treatment-Emergent Serious Adverse Events (TESAEs).
Data synthesis and assessment of heterogeneity
We performed all statistical analyses using Revman software Version 5.4.1. The present meta-analysis estimated the pooled risk ratio (RR) for dichotomous data, mean difference (MD) for continuous data with 95% confidence intervals (CI). The significance point was set at p-value less than 0.05.
We assessed the heterogeneity using the I-square and p-value. The analysis was considered heterogeneous if it had a p-value less than 0.05 or an I-square less than 50%. A random-effect model was applied if heterogeneity was detected and a leave one out test was performed to determine which study was causing the heterogeneity [14].
Results
Data collection and study selection
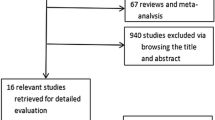
Our search retrieved 655 records from PubMed, Scopus, Web of Science, and Cochrane library. There were 75 duplicates. After title and abstract screening, we eliminated 557 records. Afterward, we screened 31 studies for eligibility, 22 studies were excluded. Eleven studies were protocols without results, six were without full texts available, and five were meeting abstracts. Finally, nine records were included in our study: four published clinical trials and five registered protocols from Clinicaltrials.gov, and only eight studies were included in the meta-analysis (Fig. 1).
The total sample size for this meta-analysis was 1259 participants (259 persons received a placebo, 890 participants received Cotadutide and 110 participants received other interventions). There were no concomitant treatment modalities except in two studies. In NCT03235050, participants in all study groups received metformin tablets and a separate group was treated with liraglutide to compare it with Cotadutide and placebo. Moreover, during the treatment period of the study NCT03444584, participants were on metformin and dapagliflozin as well. Table 1 elucidates the full summary of the included studies. The baseline characteristics of the participants are illustrated in Table 2.
Quality assessment results
The risk of bias summary is illustrated in Figs. 2 and 3. Regarding the Randomization process bias, all the studies were of low risk in terms of the randomization process except for NCT03550378, which was judged as some concerns because there was inadequate information about the allocation concealment, randomization, and baseline balance.
Regarding the intended interventions bias, most of the included trials had a low risk of bias in terms of deviations from the intended interventions except for NCT03645421 and NCT03745937, which were judged as some concerns. This is because there was no information about the statistical analysis used to estimate the effect of assignments in both of them despite blinding the personnel.
Regarding the missing outcome data bias, most of the included trials had a low risk of bias in terms of the missing outcome data due to applying the intention to treat analysis. We judged NCT03645421 and P.D. Ambery et al. as high risk of bias because the authors applied as-treated analysis [15].
Regarding the measurement outcome bias, we judged the risk of bias in the measurement of the outcome as low risk of bias in most of the studies due to blinding of all outcome assessors and using appropriate methods in measuring the outcomes. We judged NCT03645421 and NCT03550378 as some concerns due to the lack of information about blinding the outcome assessor.
For the selection of the reported results bias, the risk of bias due to the selection of the reported results ranged between low and some concerns. We judged all the registered protocols as some concerns because there is no published data yet to compare it with the protocols. The published studies [6, 15,16,17] were of low risk as all outcomes mentioned in the results were present in the protocols.
For other sourced of bias, we judged almost all the studies as high risk in terms of other potential sources of bias as most of them are registered protocols without any published papers yet. Parker et al. [17] stated the lack of statistical power to draw inferences between cohorts and the absence of validated questionnaires as a limitation in their study and so we judged it as having a high risk of bias. Accordingly, Ambery et al. [6] had a relatively small population size which we considered as high-risk potential. Only [15, 16] showed no other potential sources of bias.
Efficacy endpoints
Percentage decrease in body weight
The pooled effect estimates of five studies favored Cotadutide 300 mcg over placebo (MD = 3.31, 95% CI [2.76, 3.38], p > 0.00001). Pooled data were homogenous under a fixed effect model (p = 0.80, I2 = 0%); Fig. 4.
Decrease in glycated hemoglobin (HbA1c)
The pooled effect estimate of five studies showed that Cotadutide is significantly better than placebo (MD = 0.68, 95% CI [0.58, 0.79], p > 0.00001). Pooled data were homogenous under a fixed effect model (p = 0.05, I2 = 55%); Fig. 5.
Percentage decrease in glucose area under the plasma concentration curve (AUC [0-4 h])
The pooled effect estimates of six studies favored Cotadutide 300 mcg over placebo (MD = 30.15, 95% CI [23.18, 37.12], p > 0.00001). Pooled data were heterogeneous (p = 0.0002, I2 = 77%) under a random effect model and the heterogeneity was best resolved by leaving out NCT03596177 (p = 0.08, I2 = 48%); Fig. 6.
Decrease from baseline in fasting plasma glucose over time (mg/dl)
The pooled effect estimate of four studies favored Cotadutide over the placebo (MD = 31.31, 95% CI [22.59, 40.04], p > 0.00001). Pooled data were heterogeneous (p = 0.03, I2 = 63%) under a random effect model and the heterogeneity was best resolved by leaving out NCT03645421 (p < 0.17, I2 = 40%); Fig. 7.
Anti-drug antibodies (ADA)
Nahra et al. [16] reported a statistically significant increase in the number of participants with ADA in the Cotadutide group over the placebo (155 out of 256 in the Cotadutide group and three out of 256 in the placebo group); NCT03444584, NCT03550378, and NCT03596177 reported non-significant results on the number of participants having ADA. NCT03444584 reported 2 out of 24 and 1 out of 24 in the Cotadutide group and the placebo group, respectively. In NCT03550378, two out of 21 participants in the Cotadutide group had ADA in comparison to the 20 persons on placebo in which none of them developed ADA. In NCT03596177, three out of 14 and zero out of seven participants experienced ADA in the Cotadutide group and the placebo group, respectively.
Safety endpoints
Treatment-emergent adverse events (TEAEs)
The pooled effect estimate of six studies showed a statistically significant increased risk of TEAEs in the Cotadutide group compared to placebo (RR = 1.40, 95% CI [1.15, 1.70], p = 0.0007). Pooled data were homogenous under a fixed-effect model (p = 0.23, I2 = 26%); Fig. 8.
Treatment-emergent serious adverse events (TESAEs)
We didn’t do a meta-analysis for this outcome because none of the participants suffered any TESAEs in three out of six studies in both the Cotadutide and the placebo group. Only three studies reported some participants having TESAEs and they are relatively very low. In terms of TESAEs, NCT03550378 reported two out of 21 persons in the Cotadutide group and two out of 20 in the placebo group. On the other hand, NCT03596177 reported two persons out of 18 and zero out of seven in the Cotadutide group and the placebo group, respectively. In the MAD portion of the study by P. Ambery et al., they reported one out of seven participants having TESAEs in the Cotadutide 200 mcg group and none out of 19 participants in the placebo group [6].
Discussion
This meta-analysis on 1258 participants with type 2 diabetes revealed that efficacy outcomes, including body weight, fasting blood glucose, HbA1c, and AUC [0-4 h], were significantly better in people receiving Cotadutide treatment than placebo. The number of participants with positive ADA to Cotadutide was high but without a significant difference compared to placebo. Furthermore, no significant difference was observed between the Cotadutide group and placebo in TESAEs. Hence, Cotadutide is safe and effective as a hypoglycemic drug in people with controlled type 2 diabetes.
In ten years, more than half of individuals with type 2 diabetes mellitus switch from oral monotherapy (usually Metformin) to insulin therapy to control their blood glucose levels [18]. Multiple combination therapies are routinely used before insulin is initiated. Insulin use causes weight gain, which can exceed 6 kg 20 in the first year after starting insulin medication [19]. The overall gain in weight can cause an increase in insulin resistance which is associated with high blood pressure, dyslipidemia, and a high risk of cardiovascular mortalities and morbidities such as non-fatal myocardial infarction or stroke, both before and after diagnosis of diabetes [20, 21]. Pre-clinical findings further suggest that the balance of activities at GLP-1 receptors and glucagon receptors was appropriate for both weight reduction and glycemic management [22]. These activities are supposed to be balanced by stimulating insulin release mediated by glucose, delayed gastric emptying, and enhanced oxidation of fatty acids [23, 24]. GLP-1, including Cotadutide (MEDI0382) and glucagon receptor dual agonists, may have central impacts on appetite as glucagon receptor agonist has been found in animal and human studies to increase energy expenditure [25].
Cotadutide (5–300 µg) corrected the glucose levels to the normal range in phase one of the first human trial which was conducted on healthy volunteers, with a pharmacokinetic profile that included once-daily treatment [6]. Similar to these previous findings [6, 15], in phase 2a, Cotadutide (100–300 μg) significantly lowered blood glucose levels and body weight indices in overweight or obese Japanese people with type 2 diabetes throughout a 48-day treatment period compared to placebo. Parker et al. found a substantial decline in glucose AUC (0-4 h) by − 21.52% with up titrated Cotadutide (50–300 μg) in comparison to + 6.32% with placebo. Similarly, a decline in body weight was reported by − 3.41% versus − 0.08% for Cotadutide versus placebo, respectively [17]. Nevertheless, in different study [26], with a lower BMI (26.3–28.8 kg/m2) than Parker et al. (31.5 kg/m2) [17], blood glucose and weight reduction with Cotadutide 300 μg remained significant compared to placebo at − 37.86% versus + 2.45% and − 3.34% versus − 0.82%, respectively.
Cotadutide therapy reduced body weight in a dose-dependent approach, and the highest reductions occurred at 300 μg. Moreover, Cotadutide improved fasting plasma glucose, fructosamine, HbA1c, percentage of time in hyperglycemia, insulin secretion, and resistance. After 6 weeks of Cotadutide medication, significant decreases in HbA1c were found, with efficacy remaining constant [26].
Cotadutide has also been known to significantly reduce hepatic glycogen and steatosis, as well as having a beneficial effect on hepatic inflammation and fibrosis markers [26, 27]. The decrease in hepatic glycogen contrasts with what would be expected from a GLP-1 mono-agonist, which would cause glycogen accumulation and exhibit glucagon receptor interaction [28]. Furthermore, the degree of liver fat loss with Cotadutide (39% reduction) was comparable to that shown in a small study of women three months following bariatric surgery (42% reduction) [29]. This decrease in liver fat found with Cotadutide was larger than would be expected from weight loss alone—for example, in individuals with documented non-alcoholic fatty liver disease, a 5% decrease in BMI results in a 25.5 percent relative decline in liver fat [30].
MEDI0382 had a linear pharmacokinetic profile in the first human study on healthy volunteers (phase 1), and no participants tested positive for ADA [15]. In a previous study, participants were given Cotadutide for a year and had a significant ADA incidence. Only 16% of participants acquired ADAs over a titer of 80, at which point the influence on pharmacokinetics was around two times higher than the population average [16] (ClinicalTrials.gov identifier NCT04019561).
In the Harmony Outcomes study, albiglutide outperformed placebo in terms of serious adverse cardiovascular problems in people with type 2 diabetes and cardiovascular morbidities with a hazard ratio of 0.78, which implies that GLP-1 agonists can improve cardiovascular outcomes according to these data [31]. Due to GLP-1 and glucagon receptor agonism on the heart and vascular system, an increase in heart rate was expected. The rise in heart rate by 6.8 beats per minute observed with Cotadutide was not significantly greater than that seen with the GLP-1 receptor agonist liraglutide which increased by 6 to 9 beats from baseline. Furthermore, the drop in blood pressure was comparable to that seen with GLP-1 receptor agonists [23, 32].
Cotadutide plasma concentrations increased in agreement with the anticipated dose titration at all dose levels, with no TEAEs linked to immunogenicity observed [6]. In this study, Cotadutide had a higher rate of gastrointestinal co-morbidities such as nausea and vomiting compared to placebo. This outcome is also seen with the GLP-1 receptor mono-agonists [33, 34]. In addition, Cotadutide's safety profile was equivalent to that of previous global trials [6, 15], with a greater incidence of gastrointestinal adverse events.
To lower the gastrointestinal adverse events associated with Cotadutide 300 μg, dose escalation was required upon which a phase 2 study in obese type 2 diabetes participants reported that Cotadutide was effective and well-tolerated with starting doses of 50 μg for 7 days, then gradual dose escalation up to 300 μg [17, 35]. Despite causing more gastrointestinal upset than placebo, escalated dosages of Cotadutide of up to 300 μg which were given once daily were generally tolerated because the symptoms were mild or moderate in severity [15].
This study comprehensively evaluated the efficacy and safety of Cotadutide for people with type 2 diabetes. Nine RCTs were included in the study, resulting in a valuable evidence level. The included trials varied from low to high quality. The majority of the identified heterogeneity was resolved. Our analysis also has certain limitations, including the small sample size and the small number of included studies. We faced some limitations in our study, which include the following. Publication bias could not be detected due to the small number of included studies. Exclusion of studies published in the non-English language. The short follow-up period and lack of placebo were the major drawbacks of the study. Most of the included studies were protocols with published results, not articles. Cotadutide medication should also be evaluated for its effects on stomach emptying, energy intake, and energy expenditure in larger studies.
Conclusions
Over a short dosage period, Cotadutide provided considerable metabolic benefits to overweight and obese participants with type 2 diabetes. Cotadutide’s safety and pharmacokinetics allow once-daily administration of dosages less than 150 μg, which can be followed by dose escalation. Cotadutide's promising impacts on glycemic control, body weight, and liver fat suggest that it might be a helpful agent for type 2 diabetes individuals with longer-term treatment.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Abbreviations
- MD:
-
Mean difference
- HbA1c :
-
Glycated hemoglobin
- IDF:
-
International Diabetes Federation
- GLP-1:
-
Glucagon-like peptide-1
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- RCT:
-
Randomized Controlled Trials
- RoB 2:
-
Cochrane risk-of-bias tool for randomized trials
- TEAEs:
-
Treatment-Emergent Adverse Events
- RR:
-
Risk Ratio
- CI:
-
Confidence Intervals
- AUC [0-4 h]:
-
Percentage decrease in glucose area under the plasma concentration curve
- ADA:
-
Anti-drug antibodies
- TEAEs:
-
Treatment-emergent adverse events
- TESAEs:
-
Treatment-emergent serious adverse events
References
Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes. 2019;20(1):5–9.
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87.
Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–95.
Baretić M, Troskot R. How to fight obesity with antidiabetic drugs: targeting gut or kidney? Minerva Endocrinol. 2014;40(1):71–83.
Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607–18.
Lorenz M, Pfeiffer C, Steinsträßer A, Becker RHA, Rütten H, Ruus P, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes — Relationship to postprandial glycemia. Regul Pept. 2013;185:1–8.
van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–93.
Quast DR, Schenker N, Menge BA, Nauck MA, Kapitza C, Meier JJ. Effects of lixisenatide versus liraglutide (short- and long-acting GLP-1 receptor agonists) on esophageal and gastric function in patients with type 2 diabetes. Diabetes Care. 2020;43(9):2137–45.
Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Metab. 2003;284(4):E671–8.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. 1–694 p.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, Phase 1 study. Br J Clin Pharmacol. 2018;84(10):2325–35.
Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care. 2021;44(6):1433–42.
Parker VER, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. J Clin Endocrinol Metab. 2020;105(3):803–20.
Turner RC. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281(21):2005.
Douek IF, Allen SE, Ewings P, Gale EAM, Bingley PJ. Continuing metformin when starting insulin in patients with Type 2 diabetes: a double-blind randomized placebo-controlled trial. Diabet Med. 2005;22(5):634–40.
Wilcox R, Kupfer S, Erdmann E. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: Results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10). Am Heart J. 2008;155(4):712–7.
Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure. JAMA. 1998;280(2):140–6.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29.
Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006;49(3):452–8.
Lynch AM, Pathak N, Flatt YE, Gault VA, O’Harte FPM, Irwin N, et al. Comparison of stability, cellular, glucose-lowering and appetite supressing effects of oxyntomodulin analogues modified at the N-terminus. Eur J Pharmacol. 2014;743:69–78.
Habegger KM, Stemmer K, Cheng C, Muller TD, Heppner KM, Ottaway N, et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62(5):1453–63.
Asano M, Sekikawa A, Kim H, Gasser RA, Robertson D, Petrone M, et al. Pharmacokinetics, safety, tolerability and efficacy of cotadutide, a glucagon-like peptide-1 and glucagon receptor dual agonist, in phase 1 and 2 trials in overweight or obese participants of Asian descent with or without type 2 dia. Diabetes, Obes Metab. 2021;23(8):1859–67.
Guzman CB, Zhang XM, Liu R, Regev A, Shankar S, Garhyan P, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes, Obes Metab. 2017;19(11):1521–8.
Boland ML, Laker RC, Mather K, Nawrocki A, Oldham S, Boland BB, et al. Resolution of NASH and hepatic fibrosis by the GLP-1R and GCGR dual-agonist cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2(5):413–31.
Heath ML, Kow L, Slavotinek JP, Valentine R, Toouli J, Thompson CH. Abdominal adiposity and liver fat content 3 and 12 months after gastric banding surgery. Metabolism. 2009;58(6):753–8.
Patel NS, Doycheva I, Peterson MR, Hooker J, Kisselva T, Schnabl B, et al. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13(3):561-568.e1.
Green JB, Hernandez AF, D’Agostino RB, Granger CB, Janmohamed S, Jones NP, et al. Harmony Outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus—Rationale, design, and baseline characteristics. Am Heart J. 2018;203:30–8.
Kumarathurai P, Anholm C, Fabricius-Bjerre A, Nielsen OW, Kristiansen O, Madsbad S, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on 24-h ambulatory blood pressure in patients with type 2 diabetes and stable coronary artery disease. J Hypertens. 2017;35(5):1070–8.
Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes. JAMA. 2015;314(7):687.
Nauck MA, Petrie JR, Sesti G, Mannucci E, Courrèges J-P, Lindegaard ML, et al. A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(2):231-41. https://doi.org/10.2337/dc15-0165.
Robertson D, Parker VE, Ambery P, Petrone M, Wang T, Heise T, et al. 988-P: MEDI0382, an Oxyntomodulin-Like Peptide with Targeted GLP-1/Glucagon Receptor Activity, Promotes a Dose-Dependent Increase in Gastric Emptying Time. Diabetes. 2019;68(Supplement 1):988-P.
Code availability
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mahmoud M Ali leads the team, performed the search strategy and data collection step, solved any conflict in the screening phase, performed the meta-analysis part, and solved any conflict in the quality assessment part, took part in the data extraction phase. Ahmed Hafez took part in the screening process, data extraction, and meta-analysis in addition to writing the results section and edited the whole manuscript. Mahmoud Shaban took part in the screening process, data extraction, quality assessment, and drafting the tables. Mohammed Tarek Hasan took part in data extraction, and meta-analysis. Mohammed Magdy El-Ghannam took part in quality assessment and writing the introduction section. Osama M Ghogar tokk part in study selection and writing the methods section. Asmaa Ahmed Elrashedy wrote the discussion section and edited the manuscript. Mohamed Abd-ElGawad supervised the authors in all steps and performed peer-review. All authors reviewed the final manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Abd-ElGawad has nothing to disclose. All the authors also declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Code availability
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ali, M.M., Hafez, A., Abdelgalil, M.S. et al. Impact of Cotadutide drug on patients with type 2 diabetes mellitus: a systematic review and meta-analysis. BMC Endocr Disord 22, 113 (2022). https://doi.org/10.1186/s12902-022-01031-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01031-5