Abstract
Background
Recent studies have shown that dietary carbohydrate quantity and quality as well as genetic variants may contribute to determining the metabolic rate and general and central obesity. This study aimed to examine interactions between melanocortin 4 receptor gene (MC4R) rs17782313 and dietary carbohydrate intake, glycemic index (GI), and glycemic load (GL) on body mass index (BMI), waist circumferences (WC), basal metabolic rate (BMR), and BMR/kg in overweight/obese women.
Methods
A total of 282 Iranian women (BMI ≥ 25) aged 18–56 years were enrolled in this cross-sectional study. All participants were assessed for blood parameters, body composition, BMR, and dietary intake. Dietary carbohydrate intake, GI, and GL were determined using a valid, reliable 147-item food frequency questionnaire. MC4R rs17782313 was genotyped by the restriction fragment length polymorphism (PCR-RFLP) method.
Results
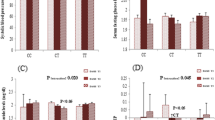
After adjustment for age and energy intake, significant interactions were observed between carbohydrate intake and MC4R rs17782313 in terms of BMI (P Interaction = 0.007), WC (P Interaction = 0.02), and BMR/kg (P Interaction = 0.003) in this way that higher carbohydrate intake, compared with lower intake, was associated with an increase in BMI and WC for individuals with C allele carriers (TC + CC genotypes), while related to an increase in BMR/kg for those carrying the TT genotype. No significant interaction was found between MC4R rs17782313 and GI and GL on BMI, WC, BMR/kg, and BMR.
Conclusions
Interactions between the MC4R rs17782313 and carbohydrate intake probably can have an effect on BMI, WC, and BMR/kg in overweight/obese women.
Similar content being viewed by others
Background
Obesity has become a leading issue globally [1, 2] that threatens both psychosocial and physical health, contributing to a higher incidence of many chronic complications, such as nonalcoholic fatty liver disease, diabetes, cardiovascular diseases, cancer, psychosocial disorders, and so on [3,4,5,6]. Unlike general obesity, defined as elevated total body adiposity irrespective of its distribution, central obesity represent a high level of abdominal or visceral fat, which is a stronger predictor of obesity-related diseases [7]. Obesity is a multifactorial phenomenon, resulting from an interaction between genetic variants and environmental factors, including diet, physical inactivity, low sleep quality, obesogens, and even the diversity and composition of gut microbiota [8, 9]. Also, a low basal metabolic rate (BMR), as a leading component of total daily energy expenditure, is one of the main metabolic predictors for the development of body weight gain [10].
Recently, the contribution of carbohydrate quality and quantity as measured by the glycemic index (GI) and glycemic load (GL) to obesity has received remarkable attention [11]. Diets with a high GI or GL are quickly digested, absorbed, and raise blood glucose more quickly, which this process accelerates the fluctuations of insulin and glucose, leading to the early return of hunger and excessive calorie intake [12]. In contrast, low GI and GL diets, because of the slower release of glucose and insulin after consumption, stimulate prolonged satiety, decrease food consumption, reduce fat storage by increasing fat oxidation and decreasing lipogenesis [13], limit the reduction in BMR in the fasting condition [14], and in turn may reduce the risk of obesity [15]. Nevertheless, the relation of the quality and quantity of carbohydrate on obesity is currently unknown. Studies investigating the association between measures of obesity and carbohydrates, GI or GL in the diet so far have reported inconsistent results, with positive, null, or negative relationships [13, 16,17,18,19,20]. The diversity of these associations is possibly due to variations in genetic background and gene-diet interactions [21, 22]. Mutations in the Melanocortin-4-receptor (MC4R) gene is the most prevalent monogenic cause of obesity [23], with a prevalence of 1.7–3.0% among obese people [24]. In this sense, variants in the rs17782313 near the MC4R have been strongly related to obesity [25] and showed a significant association with the dietary intake [26], total energy intake [27], increased snacking, as well as hunger [28]. The quality and quantity of dietary carbohydrates affect serum levels of insulin [29]. It is well recognized that brain insulin has a specific role in feeding behavior and satiety [30] and genetic variations in an obesity risk MC4R rs17782313 affect cerebrocortical insulin signaling, resulting in an impaired insulin response in the human brain [31], indicating a possible interaction. It remains, however, to explore the effect of MC4R rs17782313 polymorphism on the association of dietary carbohydrate quantity and quality with obesity and energy expenditure. Although, there is no evidence related to gender impacts on the MC4R gene, gender affects the metabolic rate [32]. A low metabolic rate has been suggested as a likely cause for the elevated adiposity commonly observed in women compared with men [33]. Thus, to precisely investigate and remove gender-specific associations, the present study was undertaken to assess the interactions of MC4R rs17782313 and dietary carbohydrate, GI, and GL on BMR and general and central obesity in overweight/obese women.
Methods
Study population
A total of 282 overweight/obese women aged18-56 (mean: 36.49 ± 8.38) years with BMI 25.2–49.60 (mean: 31.04 ± 4.31) were recruited for the present cross-sectional study. The study was performed from October 2014 to January 2015 from women who attended health centers in Tehran, Iran. The inclusion criteria were: body mass index (BMI) 25 or more, age ≥ 18, absence of any acute or chronic infection, no drug/ alcohol abuse, and not being lactating or pregnant at the time of the study. Subjects with an inflammatory disease or with a history of diabetes, thyroid disease, cardiovascular disease, stroke, cancer, liver or kidney disease, and sustained hypertension were excluded because of possible disease-associated alterations in their diet. We also excluded subjects taking vitamin or mineral supplements, hormone therapy, herbal medicines, and corticosteroids, and participants with total daily energy intake ˂800 kcal/ day or ˃ 4200 kcal/day based on the food frequency questionnaire (FFQ). The study protocol has approved by the ethics committee of Endocrinology and Metabolism Research Center of Tehran University of Medical Sciences (TUMS) (ID: IR.TUMS.VCR. REC 97-03-161-41155) and written informed consent was obtained from all participants.
Sample size calculation
The sample size was calculated based on the method of Peduzzi et al. [34] using the following formula, in which, p is the proportion of cases in the population and k the number of independent variables, then the minimum number of cases to include is: N = 10 k / p. Considering that we had 4 independent variables to include in the model and the proportion of obesity in the Iranian population was 0.22 [35], the minimum number of participants required is: N = 10 × 4 / 0.22 = 182. Because of the availability of participants in the health centers, we included a total of 282 subjects in the study.
BMR, body composition, physical activity, and anthropometric measurements
The body composition of all subjects was measured using a Body Composition Analyzer (InBody770 scanner; InBody, Seoul, Korea) according to the manufacturer’s protocol as reported previously in detail [2]. BMR was measured by an expert nutritionist with the use of a Fitmate TM calorimeter (Cosmed Company, Rome, Italy). After 10–12 h overnight fasting and resting, the BMR was registered with the person lying awake and completely at rest. Physical activity was evaluated using a reliable and validated International Physical Activity Questionnaire (IPAQ) [36]. Height and weight were measured in participants with shoes off in standing position and light clothing to the nearest 0.5 cm and 0.1 kg, respectively. BMI was then calculated as body weight (kg) divided by height squared (m2). Waist circumference was assessed at a point midway between the iliac crest and lower rib margin with the use of a Seca tape to the nearest 0.5 cm. The hip circumference of subjects was also measured in the largest part of the hip. Blood pressure (BP) of all subjects was assessed following a 15- min rest by a standard calibrated mercury sphygmomanometer on the right arm. All assessments were carried out by one trained nutritionist to prevent the individual error.
Dietary assessment
The usual dietary intake of participants during the past year was assessed using a 147-items of FFQ, which its validity and reliability have already been approved [37]. Portion sizes of consumed food items were reported in household measures and were then converted to grams of intake. Nutritionist 4 software was applied to compute nutrient and energy contents of consumed foods. The GI for carbohydrate-containing food items (foods with ≥ 5 g carbohydrate per 100 g or 100 mL) was calculated with the use of average GI values from the GI table reported by Foster-Powell et al. [38], considering glucose as the reference food (GI = 100). The average daily dietary GI was calculated by multiplying the GI of individual foods by the percentage of total energy contributed by carbohydrate {\(\sum\) [GI of food item * (grams carbohydrate of consumed food/ total grams of carbohydrate consumed per day)]}. Dietary GL was calculated as [(daily GI * grams carbohydrate consumed per day) /100].
Laboratory assays
Serum samples (5 mL) were obtained following 10 to12 hours of overnight period. Fasting blood sugar (FBS) was measured using the glucose oxidase phenol 4-Aminoantipyrine Peroxidase (GOD/PAP) method. Serum level of high-sensitive C-reactive protein (hs-CRP) was assessed by immunoturbidimetric assay. Lipid profile of participants was assessed using enzymatic methods. All measurements were done by an automatic analysis system (Autoanalyzer; Hitachi Ltd, Tokyo, Japan) with Randox laboratories kits.
DNA extraction and determination of MC4R rs17782313 variants
The extraction of genomic DNA from blood samples was performed with the use of the GeneALL DNA extraction kit (Type G Exgene; Genall; Korea) based on the manufacturer’s protocol. The concentration and purity of the extracted DNA were assessed using the Nano Drop spectrophotometer (Thermo Scientific Company, USA). The extracted DNA was stored at 4 C until sequencing was performed. The MC4R rs17782313 single nucleotide polymorphism (SNP) (major allele: T; minor allele: C) was genotyped by polymerase chain reaction-restricted length polymorphism (PCR–RFLP) technique using forward (5-AAGTTCTACCTACCATGTTCTTGG-3) and reverse (5-TTCCCCCTGAAGCTTTTCTTGTCATTTTGAT-3) primers. PCR reactions were performed in a final volume of 20 µl contains 50 ng/µl extracted DNA, 10 pmol/µl from each primer (0.5 µl), 10 µl Permix (Amplicon, Germany), and 8 µl Distilled water with the following conditions in a DNA thermocycler: primary denaturation at 95 °C for 2 min; 35 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 30 s; with a final extension at 72 °C for 5 min; 4- final step at 4 °C. Amplified DNA (7 µl) was digested with 0.5 µl of BCII restriction enzyme at 56 °C overnight. All products were visualized by electrophoresis in agarose gel. Fragments containing three likely genotypes were then distinguished: CC, CT, and TT.
Statistical analyses
MC4R genotypes were recoded based on risk allele: code 0 for TT and 1 for TC + CC. Carbohydrate intake, GI, and GL were stratified into low and high categories based on the medians (category 1: lower than the median, category 2: higher than the median), in which, subjects in the category 2 were considered as individuals adhering to a diet with a high GI, GL, or high intake of carbohydrate. Chi-square test was applied to compare qualitative variables across MC4R genotypes (TT vs. TC + CC) and to assess the Hardy–Weinberg equilibrium (HWE). Comparing the quantitative variables between MC4R genotypes was conducted using independent sample test and re-analyzed by analysis of covariance (ANCOVA) to adjust for age, BMI, and physical activity; Notably, for anthropometric and body composition variables, BMI considered as collinear and these variables were adjusted for age and physical activity. Age and energy intake-adjusted generalized linear model (GLM) analysis was used to assess the potential interactions between MC4R genotypes and GI, GL, and carbohydrate intake on BMR, BMR/kg, WC, and BMI. Furthermore, the Pearson correlation coefficient was used to determine the correlation between serum levels of insulin to dietary carbohydrate intake. All analyses were carried out by a statistical Package for Social Science (Version 22.0; SPSS Inc., Chicago IL, USA). P < 0.05 was considered statistically significant.
Results
Genotype and allele frequencies of MC4R rs17782313 SNP among 282 Iranian women who participated in this study are reported in Table 1. The prevalence of the T allele was 54.04%, while the C allele was 45.96%. Participants were categorized based on MC4R rs17782313 genotypes and divided into two groups: TT genotype (n = 153) and TC + CC genotype (n = 129) (Table 2). Genotypes were not in HWE (p ≤ 0.001). All participants were generally (BMI ˃25 kg/m2) and centrally (WC ˃ 80 cm) obese. The mean BMI, WC, BMR, and BMR/kg of the TT genotype carriers were 31.06 ± 4.66 kg/m2, 99.16 ± 10.31 cm, 1391.22 ± 119.50 kcal/day, and 17.35 ± 1.74, respectively, while corresponding values for TC + CC carriers were 30.78 ± 3.90 66 kg/m2, 98.53 ± 9.81 cm, 1368.69 ± 120.51 kcal/day, and 17.29 ± 1.50, respectively, with no significant difference between two groups. There was no significant difference between genotypes in terms of demography, anthropometry, body composition, and insulin and other blood parameters, as well as physical activity, marital status, smoking status, and education level. After adjustment for covariates including age and physical activity, a significant difference in height (p = 0.04) was observed between the two groups (Table 2). After adjustment for calorie intake, no significant difference in food intake was revealed between the MC4R rs17782313 genotypes (Table 3). Moreover, no significant correlation was found between serum insulin levels and dietary carbohydrate intake (r = − 0.04, p = 0.52).
Comparing women who consumed a diet with GI, GL, or carbohydrate less than the median with women who consumed higher than the median, there were no differences in BMI, WC, and BMR (p > 0.05), but women with higher carbohydrate intake, compared with those with lower intake, had a height BMR/kg (low intake: 17.16 ± 1.53 vs. high intake: 17.41 ± 1.74; p = 0.01). After adjustment for age and energy intake, significant interactions were observed between dietary carbohydrate intake and MC4R rs17782313 in terms of BMI (P Interaction = 0.007), WC (P Interaction = 0.02), and BMR/kg (P Interaction = 0.003) in this way that higher carbohydrate intake, compared with lower intake, was associated with an increase in BMI and WC for individuals with C allele carriers (TC + CC genotypes), while related to an increase in BMR/kg for those carrying the TT genotype (Fig. 1). No significant interaction was found between MC4R rs17782313 and GI (Fig. 2) and GL (Fig. 3) on BMI, WC, BMR/kg, and BMR.
Discussion
Dietary intake is one of the leading contributing factors to obesity; besides, genetic variants potentially affect daily energy expenditure and obesity. In an attempt to recognize factors involved in susceptibility to being overweight/obese, this study aimed to examine the interaction between dietary carbohydrate intake, GI, and GL and MC4R rs17782313 on BMR and measures of central and general obesity in overweight/obese women. The main finding of this study is that MC4R SNP can modify the association between dietary carbohydrate intake and BMR, BMI, and WC. Women with high carbohydrate intake and the TT genotype of MC4R rs17782313 had a higher BMR/kg; moreover, higher carbohydrate intake was associated with an increase in BMI and WC in individuals with risk allele (TC + CC genotypes).
For MC4R rs17782313, most studies, in agreement with or findings, found no significant association between this SNP and dietary carbohydrate and energy intake [39, 40]. Although, limited evidence revealed that carriers of the CC genotype may have a higher energy intake and a lower carbohydrate intake, compared with individuals with the TT genotype [27]. Our results regarding higher BMR/kg in persons consuming a high carbohydrate diet and TT genotype of MC4R is supported by previous studies showing that energy expenditure is higher after carbohydrate overfeeding [41]. The metabolic rate is decreased after adherence to a low-carbohydrate diet via mechanisms associated with substrate availability, and autonomic and hormonal activity [14, 42]. Clinical trial studies also identified that the reduced effectiveness of low-carbohydrate diets over time is because of metabolic depression [43]. Tentolouris et al. [44] and Potter et al. [45] showed that higher energy expenditure after a high-carbohydrate meal is due to increased activity of the sympathetic nervous system, which is not seen following a high-fat meal.
The relation of carbohydrate amount and quality in promoting obesity has been debating, with inconclusive epidemiologic results for the contribution of dietary carbohydrate intake, GL, and GI to long-term weight gain [13, 16,17,18,19,20]. Earlier reports from Asian [46] and Mediterranean [47] populations showed that carbohydrate-rich diets are not positively related to any measure of obesity. In contrast, in another study, higher dietary total carbohydrate and GL were related to a diminished probability of being obese (BMI ≥ 30 kg/m2), while GI did not relate to obesity [48]. In agreement with our findings, a cross-sectional study from the Iranian population identified no significant relationship between dietary GI and abdominal as well as general obesity; however, an inverse link was reported between GL and BMI and WC [49]. Another cross-sectional study on 979 adults, in line with our study, reported no association between GI and GL with either WC or BMI [17]. In contrast, some studies from the US [16] and Japan [50] reported a positive link between GI and BMI. In spite of this, a negative relationship between dietary GI or GL and measures of obesity was reported in some other investigations [18, 51]. Based on our findings, it is probable that, at least in the present population, only the quantity of carbohydrate is determinant for the prediction of obesity rather than quality. One of the reasons for the lack of association between GI and measures of obesity in the present study could be that high-carbohydrate diets may override any impacts of GI [52]. Moreover, heterogeneity in the findings may result from the discrepancy in the subject’s characteristics, dietary assessment methods used, study design, study sample size, and lack of controlling for covariates. Further studies are needed to investigate this area of research.
Our study revealed that the relation of carbohydrate intake to BMR and measures of obesity depends on MC4R polymorphism. The MC4R is a fundamental regulator of energy balance via functionally divergent central melanocortin neuronal pathways, affecting energy expenditure and food intake [53]. Rs17782313 is shown to be significantly related to visceral fat accumulation, WC, and BMI so that each additional C allele of rs17782313 displayed an elevated weight by 2.45 kg [54]. Animal evidence has shown that mice with MC4R knockout mutations have elevated food intake and decreased energy expenditure [55]. Mice lacking MC4R also have lower energy expenditure in comparison with leptin knockout mice [56]. MC4R knockout mice progress obesity possibly as a result of reduced energy expenditure even in case of stable food intake [57]. This shows that the MC4R gene could control both energy intake and energy expenditure, supporting the evidence that, in rodents, MC4R contributes to mediating energy homeostasis, food intake, and obesity. Though evidence on the relation of MC4R SNP to energy expenditure is partial in humans, we found that its mutated variants are associated with indices of obesity and decreased energy expenditure by having an interaction with dietary carbohydrate intake. Iranians consume a large amount of carbohydrate in their usual diets [49], which in case of being the carrier of MC4R rs17782313 risk allele, may be at higher risk for general and central obesity. Dietary carbohydrate is the most potent inducer of insulin release, which substantially stimulates the synthesis, uptake, and storage of fat in adipose tissue [46]; this underlies the hypothesis that high-carbohydrate diets induce accumulation of excess adiposity and obesity. Modulation of the relation of carbohydrate to WC and BMI by MC4R SNP may partly justify the inconsistent results of previous studies investigating the association of carbohydrate intake with obesity risk. The potential clinical importance of gene-diet interaction observed in our study is to develop personalized dietary approaches for the prevention of obesity and its related metabolic disorders according to the genetic background that is modified to meet individual’s requirements.
Insulin is a neuromodulator of energy homeostasis, a marker of energy stores, a mediator of energy balance, and an adiposity signal [30]. Hypothalamic or intra-cerebroventricular delivery of insulin, as a leptogenic and anorexigenic signal, decreases food intake, and yields a continuing weight loss in both primates [58] and rodents [59, 60]. Also, neuron-specific insulin receptor “knockout” mice display an elevation in food intake and body weight [61]. Extracellular concentrations of insulin in the hypothalamus are increased in response to a carbohydrate meal [30]. On the other side, it has been shown that insulin response in the human brain is impaired in C allele carriers (TC/CC) of the MC4R rs17782313 [31]. Therefore, mechanistically, higher carbohydrate intake contributes to an increase in obesity indices in individuals with the C allele (TC + CC genotypes), because brain insulin concentration is elevated in response to carbohydrate [30], while the C allele of MC4R rs17782313 is associated with cerebrocortical insulin resistance [31], resulting in a reduction in its anorexigenic effects, which ultimately their interactive effect leads to the increased body weight. These justify the interaction observed between MC4R rs17782313 variants and dietary carbohydrate intake on indices of obesity. As strength points, to the best of our knowledge, this is the first study to identify an interaction between dietary carbohydrate and MC4R rs17782313 for the susceptibility to increased measures of central and general obesity as well as metabolic rate. Also, several measurable covariates such as energy intake, age, and physical activity were measured and adjusted for. Some limitations of this study should be considered. First, because of the cross-sectional nature of the present study, causality cannot be inferred; moreover, it was not possible to determine the mechanism of the relationship between carbohydrate intake and the rs17782313 genotype. Thus, further investigations, particularly clinical trials or prospective studies, are required to confirm our results. Second, this study included a relatively small number of subjects and restricted participants to women, which limits the generalizability of findings. Accordingly, it is proposed to conduct similar investigations with a larger sample size on both genders. Third, we assessed dietary intake using a validated FFQ; though, FFQ is prone to measurement error and recall bias. Forth, genotype frequencies did not follow the HWE. Our study is a part of a larger study, in which data regarding MC4R rs17782313 variants was available in 282 subjects; we included these participants, and deviation from HWE might result from this reason that the study participants are a part of a larger study. Deviation from the Hardy-Weinberg equilibrium has been reported in many gene-disease association studies [62,63,64]. Nevertheless, this deviation may limit the generalizability of the findings. Finally, in the present study, only the most common SNP in the MC4R gene was examined and other SNPs are suggested to be investigated by future similar studies.
Conclusions
In conclusion, this study for the first time provided preliminary evidence that there may be an interactive effect between carbohydrate intake and the MC4R rs17782313 genotypes on BMR, BMI, and WC. We found that adherence to a high-carbohydrate diet in subjects with the TT genotype of MC4R rs17782313 is related to a higher BMR/kg. These data also emphasize that individuals with the C allele of rs17782313 in MC4R following a high-carbohydrate diet had higher BMI and WC; indeed, these people are more susceptible to being overweight/obese with high- carbohydrate diets. Additional studies are required by including the clinical level to clarify the biology of MC4R rs17782313 and its impact on the relationship between dietary carbohydrate intake, metabolic rate, and degree of obesity.
Availability of data and materials
The Data are not publicly available because of containing information that could compromise the privacy of research.
Abbreviations
- MC4R:
-
melanocortin 4 receptor
- GI:
-
glycemic index
- GL:
-
glycemic load
- BMI:
-
body mass index
- WC:
-
waist circumferences
- BMR:
-
basal metabolic rate
- BMR/kg:
-
basal metabolic rate/kilogram
- PCR-RFLP:
-
polymerase chain reaction-restriction fragment length polymorphism
- FFQ:
-
food frequency questionnaire
- IPAQ:
-
international Physical Activity Questionnaire
- FBS:
-
Fasting blood sugar
- GOD/PAP:
-
glucose oxidase phenol 4-Aminoantipyrine Peroxidase
- hs-CRP:
-
high-sensitive C-reactive protein
- BP:
-
Blood pressure
- SNP:
-
single nucleotide polymorphism
- ANCOVA:
-
analysis of covariance
- GLM:
-
generalized linear model
- WHR:
-
waist-to-hip ratio
- FFM:
-
fat free mass
- HDL-C:
-
low density lipoprotein cholesterol
- TBW:
-
Total body water
- TG:
-
triacylglycerol
- TC:
-
total cholesterol
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
References
Maddahi NS, Yarizadeh H, Setayesh L, Nasir Y, Alizadeh S, Mirzaei K. Association between dietary energy density with mental health and sleep quality in women with overweight/obesity. BMC Res Notes. 2020;13(1):1–6.
Alizadeh S, Mirzaei K, Mohammadi C, Keshavarz SA, Maghbooli Z. Circulating omentin-1 might be associated with metabolic health status in different phenotypes of body size. Arch Endocrinol Metabol. 2017;61(6):567–74.
Askarpour M, Alizadeh S, Hadi A, Symonds ME, Miraghajani M, Sheikhi A, et al. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm Metab Res. 2020;52(04):207–15.
Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg. 2019;29(8):2631–47.
Janmohammadi P, Sajadi F, Alizadeh S, Daneshzad E. Comparison of Energy and Food Intake Between Gastric Bypass and Sleeve Gastrectomy: A Meta-analysis and Systematic Review. Obes Surg. 2019;29(3):1040–8.
Alizadeh S, Esmaeili H, Alizadeh M, Daneshzad E, Sharifi L, Radfar H, Radaei MK. Metabolic phenotypes of obese, overweight, and normal weight individuals and risk of chronic kidney disease: a systematic review and meta-analysis. Arch Endocrinol Metabol. 2019;63:427–37.
Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metabolism. 2008;93(11_supplement_1):s57–63.
Mollahosseini M, Rahimi MH, Yekaninejad MS, Maghbooli Z, Mirzaei K. Dietary patterns interact with chromosome 9p21 rs1333048 polymorphism on the risk of obesity and cardiovascular risk factors in apparently healthy Tehrani adults. Eur J Nutr. 2020;59(1):35–43.
Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–47.
Sepandar F, Rashidbeygi E, Maghbooli Z, Khorrami-Nezhad L, Hajizadehoghaz M, Mirzaei K. The association between resting metabolic rate and metabolic syndrome May Be mediated by adipokines in overweight and obese women. Diabetes Metabol Syndr Clin Res Rev. 2019;13(1):530–4.
Murakami K, McCaffrey T, Gallagher AM, Neville CE, Boreham CA, Livingstone MB. Dietary glycemic index and glycemic load in relation to changes in body composition measures during adolescence: Northern Ireland Young Hearts Study. Int J Obes. 2014;38(2):252–8.
Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23.
Silva KC, Nobre LN, Vicente SEdCF, Moreira LL, do Carmo Lessa A, Lamounier JA. Influence of glycemic index and glycemic load of the diet on the risk of overweight and adiposity in childhood. Revista Paulista de Pediatria (English Edition). 2016;34(3):293–300.
Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low–glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292(20):2482–90.
Augustin L, Franceschi S, Jenkins D, Kendall C, La Vecchia C. Glycemic index in chronic disease: a review. Eur J Clin Nutr. 2002;56(11):1049–71.
Ma Y, Olendzki B, Chiriboga D, Hebert JR, Li Y, Li W, et al. Association between dietary carbohydrates and body weight. Am J Epidemiol. 2005;161(4):359–67.
Liese AD, Schulz M, Fang F, Wolever TM, D’Agostino RB, Sparks KC, et al. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28(12):2832–8.
Gaesser GA. Carbohydrate quantity and quality in relation to body mass index. J Am Diet Assoc. 2007;107(10):1768–80.
Park S, Park MS, Ko JA. The association between carbohydrate intake and waist circumference. Korean J Obes. 2008;17(4):175.
Youn S, Woo HD, Cho YA, Shin A, Chang N, Kim J. Association between dietary carbohydrate, glycemic index, glycemic load, and the prevalence of obesity in Korean men and women. Nutr Res. 2012;32(3):153–9.
Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90(5):1418–25.
Uusitupa M. Gene–diet interaction in relation to the prevention of obesity and type 2 diabetes: evidence from the Finnish Diabetes Prevention Study. Nutr metabolism Cardiovasc Dis. 2005;15(3):225–33.
Kohlsdorf K, Nunziata A, Funcke J-B, Brandt S, von Schnurbein J, Vollbach H, et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int J Obes. 2018;42(9):1602–9.
Muller YL, Thearle MS, Piaggi P, Hanson RL, Hoffman D, Gene B, et al. Common genetic variation in and near the melanocortin 4 receptor gene (MC4R) is associated with body mass index in American Indian adults and children. Hum Genet. 2014;133(11):1431–41.
Beckers S, Zegers D, de Freitas F, Mertens IL, Van Gaal LF, Van Hul W. Association study of MC4R with complex obesity and replication of the rs17782313 association signal. Mol Genet Metab. 2011;103(1):71–5.
Martins MC, Trujillo J, Freitas-Vilela AA, Farias DR, Rosado EL, Struchiner CJ, et al. Associations between obesity candidate gene polymorphisms (fat mass and obesity-associated (FTO), melanocortin-4 receptor (MC4R), leptin (LEP) and leptin receptor (LEPR)) and dietary intake in pregnant women. Br J Nutr. 2018;120(4):454–63.
Khalilitehrani A, Qorbani M, Hosseini S, Pishva H. The association of MC4R rs17782313 polymorphism with dietary intake in Iranian adults. Gene. 2015;563(2):125–9.
Stutzmann F, Cauchi S, Durand E, Calvacanti-Proenca C, Pigeyre M, Hartikainen A, et al. Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes. 2009;33(3):373–8.
Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr. 2009;63(1):78–86.
Gerozissis K. Brain insulin and feeding: a bi-directional communication. Eur J Pharmacol. 2004;490(1–3):59–70.
Tschritter O, Haupt A, Preissl H, Ketterer C, Hennige AM, Sartorius T, et al. An obesity risk SNP (rs17782313) near the MC4R gene is associated with cerebrocortical insulin resistance in humans. J Obesity. 2011;2011.
Molnar D, Schutz Y. The effect of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur J Pediatrics. 1997;156(5):376–81.
Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol. 1993;75(6):2514–20.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Ayatollahi S, Ghoreshizadeh Z. Prevalence of obesity and overweight among adults in Iran. Obes Rev. 2010;11(5):335–7.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010;20(2):150–8.
Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–3.
Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90(4):951–9.
Hasselbalch AL, Angquist L, Christiansen L, Heitmann BL, Kyvik KO, Sørensen TI. A variant in the fat mass and obesity-associated gene (FTO) and variants near the melanocortin-4 receptor gene (MC4R) do not influence dietary intake. J Nutr. 2010;140(4):831–4.
Dirlewanger M, Di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, et al. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes. 2000;24(11):1413–8.
Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–34.
Winwood-Smith HS, Franklin CE, White CR. Low-carbohydrate diet induces metabolic depression: a possible mechanism to conserve glycogen. Am J Physiology-Regulatory Integr Comp Physiol. 2017;313(4):R347-R56.
Tentolouris N, Tsigos C, Perea D, Koukou E, Kyriaki D, Kitsou E, et al. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. 2003;52(11):1426–32.
Potter J, Heseltine D, Hartley G, Matthews J, MacDonald I, James O. Effects of meal composition on the postprandial blood pressure, catecholamine and insulin changes in elderly subjects. Clin Sci. 1989;77(3):265–72.
Kim H-N, Song S-W. Association between carbohydrate intake and body composition: the Korean National Health and Nutrition Examination Survey. Nutrition. 2019;61:187–93.
REGICOR, es HiMMAhieCMIMJVJSHhi. Glycemic load, glycemic index, and body mass index in Spanish adults. Am J Clin Nutr. 2009;89(1):316–22.
Kaartinen NE, Knekt P, Kanerva N, Valsta LM, Eriksson JG, Rissanen H, et al. Dietary carbohydrate quantity and quality in relation to obesity: a pooled analysis of three Finnish population-based studies. Scand J Public Health. 2016;44(4):385–93.
Fuseini A-M, Rahimi MH, Mollahosseini M, Yekaninejad MS, Maghbooli Z, Mirzaei K. The association between dietary glycemic index and glycemic load and a body shape and fat distribution among apparently healthy Iranian adults. J Am Coll Nutr. 2018;37(5):415–22.
Murakami K, Sasaki S, Okubo H, Takahashi Y, Hosoi Y, Itabashi M. Dietary fiber intake, dietary glycemic index and load, and body mass index: a cross-sectional study of 3931 Japanese women aged 18–20 years. Eur J Clin Nutr. 2007;61(8):986–95.
Rossi M, Bosetti C, Talamini R, Lagiou P, Negri E, Franceschi S, et al. Glycemic index and glycemic load in relation to body mass index and waist to hip ratio. Eur J Nutr. 2010;49(8):459–64.
Hui L-L, Nelson EAS, Choi K-C, Wong GW, Sung R. Twelve-hour glycemic profiles with meals of high, medium, or low glycemic load. Diabetes Care. 2005;28(12):2981–3.
Adamska-Patruno E, Goscik J, Czajkowski P, Maliszewska K, Ciborowski M, Golonko A, et al. The MC4R genetic variants are associated with lower visceral fat accumulation and higher postprandial relative increase in carbohydrate utilization in humans. Eur J Nutr. 2019;58(7):2929–41.
García-Solís P, Reyes-Bastidas M, Flores K, García OP, Rosado JL, Méndez-Villa L, et al. Fat mass obesity-associated (FTO)(rs9939609) and melanocortin 4 receptor (MC4R)(rs17782313) SNP are positively associated with obesity and blood pressure in Mexican school-aged children. Br J Nutr. 2016;116(10):1834–40.
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505.
Krakoff J, Ma L, Kobes S, Knowler WC, Hanson RL, Bogardus C, et al. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes. 2008;57(12):3267–72.
Rutanen J, Pihlajamäki J, Karhapää P, Vauhkonen I, Kuusisto J, Mykkänen LM, et al. The Val103Ile polymorphism of melanocortin-4 receptor regulates energy expenditure and weight gain. Obes Res. 2004;12(7):1060–6.
Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–5.
McGowan MK, Andrews KM, Kelly J, Grossman SP. Effects of chronic intrahypothalamic infusion of insulin on food intake and diurnal meal patterning in the rat. Behav Neurosci. 1990;104(2):373.
Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr. Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13(3):387–414.
Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–5.
Uçar F, Çelik Ş, Yücel B, Sönmez M, Celep F, Erkut N. MTHFR C677T polymorphism and its relationship to myocardial infarction in the Eastern Black Sea region of Turkey. Arch Med Res. 2011;42(8):709–12.
Nasiri M, Roostaei A, Ehsanian Z. Association of methylenetetrahydrofolate reductase (MTHFR) Gene C677T and A1298C polymorphisms with myocardial infarction from North of Fars Province. Res Mol Med. 2014;2(3):36–40.
Salazar-Sánchez L, Chaves L, Cartin M, Schuster G, Wulff K, Schröder W, et al. Common polymorphisms and cardiovascular factors in patients with myocardial infarction of Costa Rica. Rev Biol Trop. 2006;54(1):1–11.
Acknowledgements
We would like to thank the Tehran University of Medical Sciences. This work was supported financially by the Tehran University of Medical Sciences (TUMS), Tehran, Iran (Grant ID: 97-03-161-41144).
Funding
This work was supported financially by the Tehran University of Medical Sciences (TUMS) (Grant ID: 97-03-161-41144).
Author information
Authors and Affiliations
Contributions
KM, and AM designed the research and collected the samples; SHA and HH wrote the paper; SHA analyzed data; KM conducted research and had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval for the study protocol was granted by The Human Ethics Committee of Tehran University of Medical Sciences (ID: IR.TUMS.VCR. REC 97-03-161-41155). All participants signed written informed consent forms.
Consent for publication
This is formally to submit the article entitled “Interaction of MC4R rs17782313 variants and dietary carbohydrate quantity and quality on basal metabolic rate and general and central obesity in overweight/obese women: a cross-sectional study” prepared by the Tehran University of Medical Sciences for review and, hopefully, publication in your prestigious journal. The authors would like to advise that all authors listed have contributed to the work. All authors have agreed to submit the manuscript to BMC Endocrine Disorders. No part of the work has been published before. There is no conflict of interest in this paper.
Competing interests
All authors declared that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alizadeh, S., Pooyan, S., Mirzababaei, A. et al. Interaction of MC4R rs17782313 variants and dietary carbohydrate quantity and quality on basal metabolic rate and general and central obesity in overweight/obese women: a cross-sectional study. BMC Endocr Disord 22, 121 (2022). https://doi.org/10.1186/s12902-022-01023-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01023-5