Abstract
Background
Normoalbuminuric diabetic kidney disease (NADKD) is a newly defined DKD, the clinical features and pathogenesis for which are still being understood. This study aimed to investigate the features and risk factors for NADKD in patients with type 2 diabetes mellitus (T2DM).
Methods
A retrospective cross-sectional study was conducted. The related clinical and laboratory data of patients with T2DM hospitalized between August 2012 and January 2020 were collected for statistical analysis. We classified the patients with T2DM into four groups on the basis of the presence or absence of albuminuria and reduced estimated glomerular filtration rate (eGFR). Analysis of variance, the Kruskal–Wallis test, and the chi-square test were used to compare the groups. Binary logistic regression analyses with a forward stepwise method were performed to explore the risk factors for renal dysfunction in hospitalized patients with normoalbuminuric T2DM.
Results
Among the 1620 patients evaluated, 500 (30.9%) had DKD, of which 9% had NADKD. The prevalence of stroke, cardiovascular events, carotid plaque, and peripheral arterial disease in NADKD was significantly higher than in a non-DKD control group (normoalbuminuric T2DM patients with eGFR of ≥60 ml/min/1.73 m2). Regression analyses revealed that three significant independent factors were associated with NADKD: age (OR = 1.089, confidence interval [CI] 95% [1.055–1.123], p < 0.001), previous use of renin−angiotensin system inhibitors (RASIs; OR = 2.330, CI 95% [1.212–4.481], p = 0.011), and glycated hemoglobin (HbA1c; OR = 0.839, CI 95% [0.716–0.983], p = 0.03).
Conclusions
NADKD is mainly associated with macrovascular rather than microvascular complications. NADKD is more common in patients with normoalbuminuric T2DM with older age, previous use of RASIs, and good glycemic control.
Similar content being viewed by others
Background
Being the most common microvascular complication of diabetes mellitus (DM), diabetic kidney disease (DKD) is presently the leading cause of end-stage renal disease [1]. In the past, the main indicator for the diagnosis of and treatment for DKD was albuminuria, which was quantified by urinary albumin-to-creatinine ratio (UACR); however, this belief has been challenged recently [2]. In 2019, the American Diabetes Association (ADA) stated that a clinical diagnosis of DKD is usually made on the basis of the presence of albuminuria and/or reduced estimated glomerular filtration rate (eGFR) in the absence of signs or symptoms of other primary causes of kidney damage [3], which is consistent with the 2019 Chinese clinical practice guidelines for DKD [4]. According to the diagnostic criteria, normoalbuminuric DKD (NADKD) is an emerging type of DKD and features a normal UACR and reduced eGFR.
The prevalence of NADKD—in which the prevalence of albuminuria declines while the prevalence of eGFR increases—has significantly increased in recent decades [5, 6]. In China, NADKD accounted for 47.6% of DKD in outpatients and 7.0% in inpatients, meaning that NADKD has not attracted sufficient attention in clinical work [7, 8]. Before the diagnostic criteria for DKD were determined, An et al. proposed that NADKD is an early renal complication for those with a shorter duration of diabetes and a lower prevalence of diabetic retinopathy (DR) [9]. Mottl et al. showed that NADKD is more common in women than men and in patients with diabetes who have well-controlled blood pressure (BP) and glycemia [10]. Recently, Du et al. suggested that type 2 diabetes mellitus (T2DM) patients with NADKD presented with an older age and well-controlled glycemia but had poorly controlled BP and serum lipids, whereas Gong et al. showed that NADKD was more common in younger patients with T2DM who had a shorter diabetes duration, higher body mass index (BMI), and better controlled hypertension and hyperlipidemia [7, 8]. The clinical features and pathogenesis of NADKD remain controversial; however, some conclusions are no longer applicable after updating the guidelines and determining the DKD diagnostic criteria. This study aimed to explore the clinical features and risk factors of NADKD in patients with T2DM and provides a clinical research basis for further studies on its pathogenesis and treatment.
Methods
Study population
A retrospective cross-sectional study was designed and implemented in patients with T2DM who were hospitalized in the Department of Endocrinology at the Eighth Affiliated Hospital of Sun Yat-sen University between August 2012 and January 2020. Inpatients with available details of serum creatinine (Scr) and UACR were enrolled in this study. For patients who met all selection criteria and had multiple hospitalization records, only the first hospitalization record was entered. Exclusion criteria were as follows: (1) patients with type 1 diabetes mellitus; (2) patients who had been rehospitalized; (3) patients with ketoacidosis, hyperosmolar coma, acute infection, pregnancy, acute cardiovascular and cerebrovascular diseases, and other stressors; and (4) patients with other types of nephropathy such as primary nephrotic syndrome and hypertensive nephropathy. A total of 1620 patients were ultimately enrolled in this study (Fig. 1). This study was examined and approved by the Ethics Committee of the Eighth Affiliated Hospital of Sun Yat-Sen University, and written informed consent was obtained from all participants.
Flow diagram for an overview of the study population. T2DM type 2 diabetes mellitus, UACR urinary albumin-to-creatinine ratio, Scr serum creatinine, eGFR estimated glomerular filtration rate, non-DKD: normal control group: (UACR< 30 mg/g and eGFR≥60 ml/min/1.73m2), ADKD: ADKD with albuminuria but without reduced eGFR (UACR≥30 mg/g and eGFR≥60 ml/min/1.73m2), NADKD: DKD with normoalbuminuria and reduced eGFR (UACR< 30 mg/g and eGFR< 60 ml/min/1.73m2), AGDKD: patients with albuminuria and reduced eGFR (UACR≥30 mg/g and eGFR< 60 ml/min/1.73m2)
Clinical data collection
We collected the following clinical data through face-to-face interviews: age, sex, smoking status, use of insulin, previous use of RASIs, previous treatments with lipid-lowering medication, histories of hypertension and diabetes, and adverse cardiovascular events.
Next, we physically examined all participants; height and weight, waist and hip circumferences, and BP were assessed. BMI was calculated as weight (kg) divided by height (m) squared (kg/m2) [11]. The difference between systolic BP (SBP) and diastolic BP (DBP) was pulse pressure (PP) [12].
Finally, imaging and laboratory test results were collected. Blood samples were obtained from patients after overnight fasting. Fasting plasma glucose (FPG), Scr, blood urea nitrogen, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (ApoAI), apolipoprotein B (ApoB), and serum uric acid (SUA) were directly measured using colorimetric and turbidimetric assays with the Beckman AU 5800 analyzer (Beckman Coulter, Shenzhen, China). Glycated hemoglobin (HbA1c) levels were examined using a fully automated glycosylated hemoglobin analyzer HLC-723 G7 (Tosoh, Shenzhen, China). Fasting morning urine samples were collected for the determination of albumin (UAlb) and creatinine (Ucr) using an A15 Automatic Biochemistry Analyzer (Biosystems, Shenzhen, China), and urinary albumin-to-creatinine ratio (UACR) was calculated as UAlb (mg) divided by Ucr (g). Ultrasonography of bilateral carotid and bilateral lower-extremity arteries were performed with a Sequoia 512 (Acuson, Shenzhen, China) for the evaluation of plaque and stenotic severity. We calculated the eGFR using the Chronic Kidney Disease Epidemiology Collaboration’s formula recommended in the ADA [3].
Diagnostic criteria
T2DM was diagnosed according to the 1999 World Health Organization diagnostic criteria [13]. DKD was defined as diabetes with UACR of ≥30 mg/g and/or eGFR of < 60 ml/min/1.73 m2 in the absence of signs or symptoms of other primary causes of kidney damage according to the ADA guidelines and Chinese clinical practice guidelines [3, 4]. DR was diagnosed by ophthalmologists using standardized fundoscopic examination according to the International Clinical Diabetic Retinopathy Disease Severity Scale [14]. Diabetic peripheral neuropathy (DPN) was evaluated by electromyography. Hypertension was defined as taking medication for hypertension or SBP of ≥140 mmHg and/or a DBP of ≥90 mmHg according to the 2010 Chinese Guidelines for the Management of Hypertension [15]. The carotid plaque score was evaluated according to a semiquantitative scale score that included the following: one site with plaques having thickness of < 2 mm (score = 1), two sites with plaques having both thickness of ≤2 mm or one site with plaques having thickness of > 2 mm (score = 2), two sites with plaques including at least one plaque with thickness of > 2 mm (score = 3), and two sites with plaques having both thickness of > 2 mm or annular plaque (score = 4), with the final score being the sum of bilateral carotid plaque scores [16]. Peripheral arterial disease (PAD) was defined as ≥50% luminal narrowing of a lower-extremity artery [17]. Anemia was defined as the adult men with hemoglobin (Hb) level of <120 g/L and adult women with Hb of < 110 g/L according to the Chinese criteria [18]. Smoking history was defined by the World Health Organization (WHO) in 1997 as those who had smoked for at least 6 months or had smoked at least 100 cigarettes in their lifetime.
Reduced eGFR was defined as eGFR of < 60 ml/min/1.73 m2; albuminuria was defined as UACR of ≥30 mg/g; and normoalbuminuria was defined as UACR of < 30 mg/g [3]. We classified the T2DM patients into four groups on the basis of the presence or absence of albuminuria and reduced eGFR:
-
Control group: non-DKD (UACR < 30 mg/g and eGFR ≥60 ml/min/1.73 m2);
-
ADKD group: DKD with albuminuria but without reduced eGFR (UACR ≥30 mg/g and eGFR ≥60 ml/min/1.73 m2);
-
AGDKD group: DKD with albuminuria and reduced eGFR (UACR ≥30 mg/g and eGFR < 60 ml/min/1.73 m2); and
-
NADKD group: DKD with normoalbuminuria and reduced eGFR (UACR < 30 mg/g and eGFR < 60 ml/min/1.73 m2).
Statistical analysis
All statistical analyses were conducted using SPSS version 26.0. Continuous data with normal distribution are expressed as mean ± standard deviation (x̄ ± s), whereas non-normally distributed data are presented as median and interquartile interval [M(QL, QU)]. Count data are expressed as n (%), and comparisons among groups were performed using Pearson’s chi-square test. One-way analysis of variance with least significant difference was applied for intergroup comparisons when the data showed normal distribution and homogeneity of variance. The Kruskal−Wallis test was used to compare variables with skewed distribution. Variates with a p-value of < 0.1 in the univariate analysis and the risk factors reported in prior literature were included in the binary logistic regression analyses with forward stepwise method to define independent risk factors of NADKD. Significance level was set at p < 0.05, and confidence interval (CI) was 95%.
Results
General clinical features and prevalence of diabetic complications
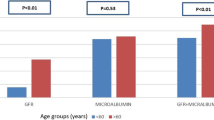
Of the 1620 eligible participants, 500 (30.9%) had DKD and 45 (2.8%) had NADKD, which accounted for 9.0% of all DKD. The characteristics of the participants are presented in Table 1. The average age of all participants was 57.0 ± 12.4 years, with 34.4% of them being female. In patients with normoalbuminuric T2DM, compared with the control group (non-DKD), NADKD was significantly associated with older age; female sex; higher prevalence and longer duration of diagnosed hypertension; higher PP, higher SUA; higher use of insulin before hospital admission; higher prevalence of anemia; higher prior use of antihypertensive drugs and lipid-lowering drugs; higher prior use of RASIs; and lower levels of DBP, HbA1c, TC, ApoB, and ApoB/ApoAI. In addition, patients with NADKD had a higher prevalence of macrovascular complications than did the control group, which was mainly manifested as a higher prevalence of stroke, adverse cardiovascular event, carotid plaque, and PAD. However, there were no significant differences in the prevalence of DR and DPN. The prevalence of diabetic complications in the four groups is presented in Fig. 2. Among patients with T2DM who had a reduced eGFR, those with NADKD had a shorter duration of diabetes and a lower level of SBP, DBP, PP, TC, ApoB, ApoB/ApoAI, and prevalence of anemia than patients with AGDKD (Table 1). Furthermore, patients with NADKD had a lower prevalence of PAD, DR, and DPN, except for the prevalence of stroke, adverse cardiovascular event, and carotid plaque (Fig. 2).
Logistic regression analysis of NADKD and non-DKD groups
Normoalbuminuric T2DM patients were selected as the study population. Considering reduced eGFR as the dependent variable, the potential risk factors of univariate logistic regression analysis and previous research reports were entered into binary logistic regression analyses with forward stepwise method to define independent risk factors of NADKD. Ultimately, sex and age; duration of diabetes; BMI; hypertension; previous use of antihypertensive drugs, RASIs, and lipid-lowering drugs; smoking; PP; duration of hypertension; and FPG, HbA1c, TG, TC, HDL-C, LDL-C, and ApoB/ApoAI levels were considered as independent variables. Table 2 showed that age (95% CI: 1.055–1.123, p < 0.001), HbA1c level (95% CI: 0.716–0.983, p = 0.03), and previous use of RASIs (95% CI: 1.212–4.481, p = 0.011) were the significant factors that independently predicted NADKD.
Logistic regression analysis of reduced eGFR
All T2DM patients were selected as the study population. Considering reduced eGFR as the dependent variable, binary logistic regression analyses using forward stepwise method were performed. Results demonstrated that independent risk factors associated with reduced eGFR were age (95% CI: 1.041–1.081, p < 0.001), duration of diabetes (95% CI: 1.011–1.065, p < 0.01), hypertension (95% CI: 1.656–4.573, p < 0.001), and UACR (95% CI: 1.001–1.002, p < 0.001; Table 3).
Discussion
This study revealed that the occurrence of NADKD is closely related to previous use of RASIs, and NADKD is more common in older patients with good glycemic control. RASIs can significantly reduce albuminuria while possibly leading to an acute decrease in eGFR, resulting in the conversion of some non-DKD patients into those with NADKD [19,20,21]. A total of 44.4% patients with NADKD were previously treated with RASIs in our study. This study shows that the prevalence of hypertension in patients with NADKD was significantly higher than in patients without DKD. Furthermore, our analyses revealed that hypertension as a risk factor for reduced eGFR in patients with T2DM. Hypertension and DM responses act synergistically in promoting kidney injury, which may be mediated by increased intraglomerular pressure. The synergistic effects of hypertension and DM to promote structural kidney injury may be mediated by increased intraglomerular pressure and flow, which ultimately contributes to glomerular hyperfiltration and albuminuria [22]. Holtkamp et al. showed that an acute decline in eGFR occurs in the initial phase of treatment with losartan, and the process is an acute reversible hemodynamic change rather than a structural renal function decline [20]. Clase et al. observed that eGFR decreased to a different extent after starting a RASI blockade, and 50% of the patients with a large initial decline improved to near baseline but 14% of patients showed a decline in eGFR on subsequent evaluation [19]. Therefore, RASIs are antihypertensive drugs that dilate the efferent arteriole more than the afferent arteriole, increasing the prevalence of NADKD by reducing intraglomerular pressure and flow.
We found no differences in SBP between the NADKD non-DKD groups after antihypertensive drug treatment in our study. However, DBP in the NADKD group was significantly lower than that in the non-DKD group. Consequently, the PP in the NADKD group was significantly higher than that in the non-DKD group. Several studies have reported that intensive control of BP did not universally succeed in reducing the slope of eGFR decline, and a significant increase in PP may lead to increased diastolic load and decreased renal perfusion pressure [23, 24]. Researchers have found that PP is significantly correlated with ankle brachial index and pulse wave velocity, and it has been used to indicate atherosclerosis and renal functional decline [12, 25]. Consequently, the PP and eGFR changes in patients with hypertension who were treated with antihypertensive drugs (particularly RASIs) should attract sufficient attention.
The age of patients in the NADKD group was significantly higher than that of those in the non-DKD group in our study; however, the difference in age between patients in the NADKD and AGDKD groups was not significant. In addition to hypertension, independent risk factors for reduced eGFR in patients with T2DM also include increasing age, UACR, and duration of diabetes. Age is a key determinant of prognosis in CKD [26]. O’Hare et al. pointed out that eGFR decreased with advancing age, and older patients had higher mortality rates than young patients when eGFR levels at baseline were comparable [27]. Murussi et al. speculated that the decrease in eGFR with advancing age is caused by the accumulation of mesangial matrix matter, and their study found that patients with microalbuminuria showed a higher eGFR fall rate than those with persisting normoalbuminuria [28]. Our results also showed that the decrease in eGFR was negatively associated with age and UACR.
Interestingly, HbA1c is significantly lower in the NADKD group (8.3 ± 2.5) than in the non-DKD group (9.3 ± 2.3) (p < 0.05) in our study. Mottl et al. has reported that NADKD is more common in patients with well-controlled glycemia [10]. Two reasons might explain this phenomenon. First, with the deterioration of renal function, the correlation between HbA1c and FPG is weakened, which is more obvious in patients with anemia [29]. HbA1c cannot accurately reflect recent blood glucose control in such situation. Anemia as a common metabolic complication of CKD can result in low HbA1c, which may be related to decreased erythropoietin synthesis and shortened red blood cell lifespan because of impaired renal function [30, 31]. Therefore, glycated albumin should be combined to determine recent blood glucose control [32, 33]. Second, the significant positive effect of HbA1c on eGFR in each stage of CKD suggests that hyperfiltration exists in all stages, and hyperglycemia induces glomerular hyperfiltration by increasing ultrafiltration coefficient and membrane permeability to low-molecular-size dextran [34, 35]. Furthermore, hyperglycemia promotes SUA excretion in states of hyperfiltration, and SUA level is inversely associated with HbA1c [36]. Although SUA could be an index of renal function, its increase may be the result of a decrease in eGFR [37, 38]. Therefore, we did not include SUA in our analyses.
Compared with patients in the non-DKD group, more patients in the NADKD group were treated with insulin before admission to our study. We found no significant differences in FPG between these groups, which suggested that HbA1c in the NADKD group decreased because of strict blood glucose control. Hyperfiltration caused by long-term hyperglycemia is relieved, which leads to reduced eGFR and elevated SUA. We believe that strict control of blood glucose is the main reason underlying lower HbA1c levels in the NADKD group than those in the non-DKD and ADKD groups.
In this study, the incidence of macrovascular complications in NADKD was significantly higher than that in the non-DKD group, whereas there was no significant difference in microvascular complications. Seo et al. suggested that carotid plaque is a significant independent predictor of renal function decline [39]. Roumeliotis et al. mentioned carotid intima-media thickness as a powerful and independent predictor of morbidity and mortality owing to DKD and a valuable tool for the stratification of patients with DKD [40]. Our study revealed that the semiquantitative scale score of carotid plaque in the NADKD group was significantly higher than that in the non-DKD group (p < 0.001), meaning that carotid atherosclerosis is more serious in NADKD. Wu et al. pointed out that renal function decline is an independent risk factor in the development of incident PAD in patients with a certain eGFR baseline [41]. In addition, several studies have demonstrated that the phenotype of NADKD might be primarily associated with macroangiopathy rather than microangiopathy, and NADKD can increase the risk of cardiovascular events and stroke [6, 8]. Thus, we believe that NADKD is different from previous diabetic nephropathy, which may be a new phenotype closely related to macroangiopathy.
Previous research has shown that a good correlation exists between reduced eGFR and albuminuria in some patients with DKD, whereas recent studies have revealed that the progress of albuminuria, which cannot fully reflect the impairment of renal function, is not synchronous with reduced eGFR, which might lead to DKD through a different pathogenesis [2, 42, 43]. First, during DKD pathogenesis, a proportion of patients with a rapid eGFR decline exhibited a normoalbuminuria, which might be related to ischemic renal changes caused by atherosclerosis of the intrarenal arteries [39]. Second, during the treatment course, albuminuria was controlled while eGFR showed a continuous decline in patients treated with RASIs [6], which is the main cause for the increased prevalence of NADKD in our study. Finally, patients with T2DM with increased albuminuria are associated with thickening of the basal membranes, mesangial expansion, and podocyte damage, whereas patients with NADKD more commonly have predominant interstitial and vascular changes [6, 44]. Therefore, sufficient attention should be paid to reduced eGFR in patients with T2DM after intensive hypoglycemic and antihypertensive therapy.
The strength of our study lies in the complete clinical data of the patients, including careful history taking, physical examination, laboratory findings, and imaging analysis. However, some limitations exist. First, this was a single-center, retrospective, observational study, which may have recall bias. We also could not identify a causal relationship between NADKD and macrovascular complications. Second, we used the eGFR values instead of directly measuring GFR. Finally, coronary angiography was not performed in all participants as patients had good enough renal function, and a large number of patients did not undergo head magnetic resonance imaging or computed tomography because of the associated high costs.
Conclusions
Our study demonstrated that the high prevalence of NADKD is closely related to the widespread use of RASIs, and NADKD is primarily associated with macrovascular complications rather than microvascular complications. Additionally, NADKD is more common in patients with normoalbuminuric T2DM who are of an older age, have a previous use of RASIs, and have good glycemic control. Therefore, it is necessary to pay attention to decreased renal function in patients with T2DM after intensive hypoglycemic and antihypertensive therapy.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- DKD:
-
Diabetic kidney disease
- ESRD:
-
End-stage renal disease
- UACR:
-
Urinary albumin-to-creatinine ratio
- ADA:
-
The American Diabetes Association
- eGFR:
-
Estimated glomerular filtration rate
- NADKD:
-
Normoalbuminuric diabetic kidney disease
- RASIs:
-
Renin-angiotensin system inhibitors
- T2DM:
-
Type 2 diabetes mellitus
- T1DM:
-
Type 1 diabetic patients
- WC:
-
Waist circumference
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PP:
-
Pulse pressure
- FPG:
-
Fasting plasma glucose
- Scr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- ApoAI:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- SUA:
-
Serum uric acid
- HbA1c:
-
Glycated hemoglobin
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- WHO:
-
World Health Organization
- DR:
-
Diabetic retinopathy
- DPN:
-
Diabetic peripheral neuropathy
- PAD:
-
Peripheral arterial disease
- Hb:
-
Hemoglobin
- ADKD:
-
Diabetic kidney disease with albuminuria but without reduced eGFR
- AGDKD:
-
Diabetic kidney disease with albuminuria and reduced eGFR
- ANOVA:
-
One-way analysis of variance
- ABI:
-
Ankle brachial index
- PWV:
-
Pulse wave velocity
- CKD:
-
Chronic kidney disease
- EPO:
-
Erythropoietin
- RBC:
-
Red blood cell
- CIMT:
-
Carotid intima-media thickness
References
Chen C, Wang C, Hu C, Han Y, Zhao L, Zhu X, et al. Normoalbuminuric diabetic kidney disease. Front Med. 2017;11(3):310–8.
Jiang W, Wang J, Shen X, Lu W, Wang Y, Li W, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care. 2020;43(4):925–33.
American Diabetes A. 11. Microvascular complications and foot care: standards of medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124–38.
The Microvascular Complications Group of Chinese Diabetes A. Chinese clinical practice guideline of diabetic kidney disease. Chinese J Diab Mellitus. 2019;11(1):15–28.
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with Diabetes, 1988-2014. JAMA. 2016;316(6):602–10.
Klimontov VV, Korbut AI. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetol Metab Syndr. 2019;13(1):474–9.
Gong W, He M, Yu Y, Wang M, Yang Y, Sun F, et al. Clinical features and risk factors of normoalbuminuric chronic kidney disease in hospitalized patients with type 2 diabetes mellitus. Chinese J Diab Mellitus. 2018;10(4):269–73.
Du R, Zhang Y, Li Q, Li L. Clinical features of normoalbuminuric diabetic kidney disease in patients with type 2 diabetes. Chinese J Diab Mellitus. 2017;9(8):494–8.
An JH, Cho YM, Yu HG, Jang HC, Park KS, Kim SY, et al. The clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: a possible early stage renal complication. J Korean Med Sci. 2009;24(Suppl):S75–81.
Mottl AK, Kwon KS, Mauer M, Mayer-Davis EJ, Hogan SL, Kshirsagar AV. Normoalbuminuric diabetic kidney disease in the U.S. population. J Diabetes Complicat. 2013;27(2):123–7.
Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 Diabetes. Diabetes. 2013;62(9):3224–31.
Kose E, An T, Kikkawa A, Matsumoto Y, Hayashi H. The association between the increase in pulse pressure and renal function in chronic kidney disease patients with dyslipidemia. Pharmazie. 2016;71(9):510–3.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Liu LS. Writing Group of Chinese Guidelines for the Management of H: [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615.
Zureik M, Bureau JM, Temmar M, Adamopoulos C, Courbon D, Bean K, et al. Echogenic carotid plaques are associated with aortic arterial stiffness in subjects with subclinical carotid atherosclerosis. Hypertension. 2003;41(3):519–27.
Choi KH, Park TK, Kim J, Ko YG, Yu CW, Yoon CH, et al. Sex differences in outcomes following endovascular treatment for symptomatic peripheral artery disease: An analysis from the K- VIS ELLA registry. J Am Heart Assoc. 2019;8(2):e010849.
Red Blood Cell Diseases Group CSoHCMA. Chinese expert consensus on the application of intravenous iron (2019). Zhonghua Xue Ye Xue Za Zhi. 2019;40(5):358–62.
Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017;91(3):683–90.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJL, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–7.
Weir MR. Acute changes in glomerular filtration rate with renin-angiotensin system (RAS) inhibition: clinical implications. Kidney Int. 2017;91(3):529–31.
Wang Z, do Carmo JM, da Silva AA, Fu Y, Hall JE. Mechanisms of synergistic interactions of diabetes and hypertension in chronic kidney disease: role of mitochondrial dysfunction and er stress. Curr Hypertens Rep. 2020;22(2):15.
Peralta CA, Jacobs DR Jr, Katz R, Ix JH, Madero M, Duprez DA, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis. 2012;59(1):41–9.
Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–29.
Wang Y, Hu Y, Li Y, Li H, Chu S, Zhu D, et al. Association of renal function with the ambulatory arterial stiffness index and pulse pressure in hypertensive patients. Hypertens Res. 2012;35(2):201–6.
Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. Jama. 2015;314:6.
O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–65.
Murussi M, Gross JL, Silveiro SP. Glomerular filtration rate changes in normoalbuminuric and microalbuminuric type 2 diabetic patients and normal individuals. J Diabetes Complicat. 2006;20(4):210–5.
Bloomgarden Z, Handelsman Y. How does CKD affect HbA1c? J Diabetes. 2018;10(4):270.
Sinha N, Mishra TK, Singh T, Gupta N. Effect of iron deficiency anemia on hemoglobin A1c levels. Ann Lab Med. 2012;32(1):17–22.
Hannedouche T, Fouque D, Joly D. Metabolic complications in chronic kidney disease: hyperphosphatemia, hyperkalemia and anemia. Nephrologie Ther. 2018;14(6):6S17–16S25.
Gan T, Liu X, Xu G. Glycated albumin versus HbA1c in the evaluation of glycemic control in patients with Diabetes and CKD. Kidney Int Rep. 2018;3(3):542–54.
Kim IY, Kim MJ, Lee DW, Lee SB, Rhee H, Song SH, et al. Glycated albumin is a more accurate glycaemic indicator than haemoglobin A1cin diabetic patients with pre-dialysis chronic kidney disease. Nephrology. 2015;20(10):715–20.
Lee CL, Li TC, Lin SY, Wang JS, Lee IT, Tseng LN, et al. Dynamic and dual effects of glycated hemoglobin on estimated glomerular filtration rate in type 2 diabetic outpatients. Am J Nephrol. 2013;38(1):19–26.
Remuzzi A, Viberti G, Ruggenenti P, Battaglia C, Pagni R, Remuzzi G. Glomerular response to hyperglycemia in human diabetic nephropathy. Am J Phys. 1990;259(4 Pt 2):F545–52.
Wei F, Chang B, Yang X, Wang Y, Chen L, Li WD. Serum uric acid levels were dynamically coupled with hemoglobin A1c in the development of type 2 Diabetes. Sci Rep. 2016;6:28549.
Chang Y-H, Lei C-C, Lin K-C, Chang D-M, Hsieh C-H, Lee Y-J. Serum uric acid level as an indicator for CKD regression and progression in patients with type 2 diabetes mellitus-a 4.6-year cohort study. Diabetes Metab Res Rev. 2016;32(6):557–64.
Tanaka K, Hara S, Hattori M, Sakai K, Onishi Y, Yoshida Y, et al. Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J Diabetes Investig. 2015;6(1):98–104.
Seo DH, Kim SH, Song JH, Hong S, Suh YJ, Ahn SH, et al. Presence of carotid plaque is associated with rapid renal function decline in patients with type 2 Diabetes mellitus and Normal renal function. Diabetes Metab J. 2019;43(6):840–53.
Roumeliotis A, Roumeliotis S, Panagoutsos S, Theodoridis M, Argyriou C, Tavridou A, et al. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren Fail. 2019;41(1):131–8.
Wu Z, Wang X, Jia J, Li Y, Jiang Y, Li J, et al. Kidney function predicts the risk of asymptomatic peripheral arterial disease in a Chinese community-based population. Int Urol Nephrol. 2020;52(3):525–32.
Zhao W, Wang Y, Shuai Y, Wang N, Zhang J, Zhang Y, et al. Correlation between microalbuminuria and decreased glomerular filtration rate in type 2 diabetic patients. Chinese J Diab Mellitus. 2018;10(8):542–7.
Liyanage P, Lekamwasam S, Weerarathna TP, Srikantha D. Prevalence of normoalbuminuric renal insufficiency and associated clinical factors in adult onset diabetes. BMC Nephrol. 2018;19(1):200.
Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, et al. Renal structure in Normoalbuminuric and Albuminuric patients with type 2 Diabetes and impaired renal function. Diabetes Care. 2013;36(11):3620–6.
Acknowledgements
We wish to acknowledge all participants and the healthcare technicians for their contribution to this study. We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by grants from the Science and Technology Planning Project of Shenzhen Municipality of China (JCYJ20140416094256882) and the Health Public Welfare Scientific Research Project in Futian District of Shenzhen City of China (FTWS20160015).
Author information
Authors and Affiliations
Contributions
ZYL and QD carried out the study design; QD, NC, LZ, XJL and FXJ collected and analyzed the data; ZYL, NC and XJZ revised the paper and made substantial contributions to the study conception and framework; QD was the main contributor in composing the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the Helsinki Declaration and approved by the Ethics Committee of the Eighth Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Q., Chen, N., Zeng, L. et al. Clinical features of and risk factors for normoalbuminuric diabetic kidney disease in hospitalized patients with type 2 diabetes mellitus: a retrospective cross-sectional study. BMC Endocr Disord 21, 104 (2021). https://doi.org/10.1186/s12902-021-00769-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-021-00769-8