Abstract
Background
It is essential to anticipate and limit the social, economic and sanitary cost of type 2 diabetes (T2D), which is in constant progression worldwide.
When blood glucose targets are not achieved with diet and lifestyle intervention, insulin is recommended whether or not the patient is already taking hypoglycaemic drugs. However, the benefit/risk balance of insulin remains controversial. Our aim was to determine the efficacy and safety of insulin vs. hypoglycaemic drugs or diet/placebo on clinically relevant endpoints.
Methods
A systematic literature review (Pubmed, Embase, Cochrane Library) including all randomised clinical trials (RCT) analysing insulin vs. hypoglycaemic drugs or diet/placebo, published between 1950 and 2013, was performed. We included all RCTs reporting effects on all-cause mortality, cardiovascular mortality, death by cancer, cardiovascular morbidity, microvascular complications and hypoglycaemia in adults ≥ 18 years with T2D. Two authors independently assessed trial eligibility and extracted the data. Internal validity of studies was analyzed according to the Cochrane Risk of Bias tool. Risk ratios (RR) with 95 % confidence intervals (95 % CI) were calculated, using the fixed effect model in first approach. The I2 statistic assessed heterogeneity. In case of statistical heterogeneity, subgroup and sensitivity analyses then a random effect model were performed. The alpha threshold was 0.05. Primary outcomes were all-cause mortality and cardiovascular mortality. Secondary outcomes were non-fatal cardiovascular events, hypoglycaemic events, death from cancer, and macro- or microvascular complications.
Results
Twenty RCTs were included out of the 1632 initially identified studies. 18 599 patients were analysed: Insulin had no effect vs. hypoglycaemic drugs on all-cause mortality RR = 0.99 (95 % CI =0.92–1.06) and cardiovascular mortality RR = 0.99 (95 % CI =0.90–1.09), nor vs. diet/placebo RR = 0.92 (95 % CI = 0.80–1.07) and RR = 0.95 (95 % CI 0.77–1.18) respectively. No effect was found on secondary outcomes either. However, severe hypoglycaemia was more frequent with insulin compared to hypoglycaemic drugs RR = 1.70 (95 % CI = 1.51–1.91).
Conclusions
There is no significant evidence of long term efficacy of insulin on any clinical outcome in T2D. However, there is a trend to clinically harmful adverse effects such as hypoglycaemia and weight gain. The only benefit could be limited to reducing short term hyperglycemia. This needs to be confirmed with further studies.
Similar content being viewed by others
Background
In 2030, according to the World Health Organization [1], 366 million people worldwide will live with type 2 diabetes (T2D). This increase is linked to aging of the population, the rise of obesity, the change in diagnostic criteria of diabetes and more extensive screening [2]. Compared with the non-diabetic population of the same age, all-cause mortality: (hazard ratio: 1.80 (95 % CI: 1.71 to 1.90) and cardiovascular mortality: 2.32 (95 % CI: 2.11–2.56) are increased in T2D [2].
Insulin is a natural vital treatment in type 1 diabetes, because of the total absence of insulin secretion. In T2D the lack of insulin is relative and endogenous insulin levels are very high. [3, 4] In T2D, insulin is often prescribed when blood glucose targets are not met despite maximal dosages and combinations of oral hypoglycaemic drugs.
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) published a consensus statement recommending insulin as a well-validated tier 1 option for the metabolic management of hyperglycaemia in T2D [5].
ADA/EASD [5] and the NICE [6] guidelines recommend metformin as the first line oral hypoglycaemic agent. However, although metformin is still considered as the first line drug, a recent meta-analysis has shown a controversial benefit/risk balance for metformin [7].
Another recent meta-analysis [8] demonstrated no efficacy of sulfonylureas on cardiovascular morbidity or mortality in T2D. Furthermore, studies analysing the efficacy of metformin or sulfonylureas on clinical endpoints are scarce.
For these reasons, our objective is to analyse the efficacy of insulin on clinically relevant endpoints in T2D.
To answer this question we performed this meta-analysis of randomized clinical trials (RCT) analysing the short, medium and long-terms effects of insulin on clinical outcomes in T2D. Clinical outcomes were defined as mortality, morbidity and main adverse effects (such as total and severe hypoglycaemic events).
Methods
Our methodology adheres to the PRISMA guidelines (see PRISMA checklist: Additional file 1)
Data sources
Clinical trials were identified searching: Pubmed, Embase and Cochrane Library. We included all trials, with no language resctriction, published from January 1950 to April 2013. Keywords used were: “type 2 diabetes”, “diabetes mellitus”; “mortality”; “sudden death”; “sudden death”, “cardiac”; “macrovascular”; “cardiovascular or coronary disease”; “stroke”; “peripheral vascular disease”; “microvascular”; “retinopathy”; “neuropathy”; “diabetic nephropathy”; “kidney disease”; “hypoglycaemia”; “hypoglycaemic agents”; “insulin”; “insulin, lente”; “insulin aspart”; “insulin lispro”; “short-acting insulin”, “long-acting”; “insulin isophane”; “insulin ultralente”. We restricted our search to randomized clinical trials (RCTs), systematic reviews and meta-analyses of RCTs. We manually searched the reference lists of systematic reviews to check they had all already been identified in our study.
Search strategy is illustrated in the Additional file 2.
Study selection
We included RCTs comparing insulin regimens vs. a hypoglycaemic drug or placebo/diet in T2D patients aged 18 to 80 years. By excluding patients over the age of 80, we excluded patients in which moderate hyperglcaemia is sometimes accepted, to reduce the risk of hypoglycaemia. Insulin was either administered alone or in combination with another hypoglycaemic agent. For example: Insulin vs. hypoglycaemic dug, (Insulin + hypoglycaemic drug) vs. (hypoglycaemic drug), Insulin vs. placebo, Insulin vs. diet.
Two investigators independently assessed eligibility (FR and EG). In case of discrepancy, a third observer adjudicated the eligibility (FG or SE). The extraction forms and the risk of bias assessments are attached as Additional file 2.
Quality assessment
Two authors (FR and EG) independently assessed trial quality. Internal validity was analyzed with the Cochrane Risk of Bias tool [9]. These articles were then rated according to methodological quality: good, moderate or low.
Outcomes
Primary outcomes were all-cause mortality and cardiovascular mortality. Secondary outcomes were non-fatal cardiovascular events, (such as myocardial infarction and stroke), hypoglycaemic events (total and severe), death from cancer and macro- or microvascular complications (such as blindness, or retinopathy). Hypoglycaemia requiring the intervention of a third party was considered as severe.
Two reviewers (FR and EG) independently extracted the data for all the outcomes of interest.
Principal summary measures and statistical analysis
Analyses were done using Revman software version 5 (www.cc-ims.net/revman).
For all studies we calculated risk ratios (RR) with 95 % confidence intervals, (95 % CI), using the fixed effect model in first approach. Heterogeneity was investigated with the I2 statistic. It measures the proportion of overall variation attributable to between study heterogeneity. I2 values of 25 %, > 50 % and > 75 % refers respectively to a low, substantial and considerable degree of heterogeneity. In case of statistical heterogeneity, we tried to explain this with subgroup and sensitivity analyses then with a random effect model. Statistical significance was defined with an alpha threshold at 0.05.
Results
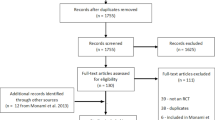
Figure 1 shows the selection of studies. The list of the trials that were excluded after reviewing the abstract is available on request (under reasons for exclusion). 20 trials were included in the final analysis, 8 were identified as citations from other meta-analyses. The 20 trials compared insulin regimens vs. hypoglycaemic drugs (Table 1). Four of the 20 studies also compared insulin versus placebo or diet alone (Table 2). UGDP [10] and UKPDS 33 [11] were the only studies analysing mortality as a primary outcome. Eleven trials were graded as moderate quality, 9 as good. For all other studies, primary and secondary outcomes were surrogate endpoints whereas morbidity, mortality and hypoglycaemic events were reported as adverse events. Study characteristics are shown in Tables 1 and 2.
Primary outcomes
Insulin regimens did not affect mortality compared to placebo or diet alone (RR: 0.92, 95 % CI: 0.80 to 1.07) (Fig. 2). Results remained non-significant when insulin regimens were compared to hypoglycaemic drugs (RR: 0.99, 95 % CI 0.92–1.06) (Fig. 3). Insulin regimens showed no efficacy on cardiovascular mortality versus placebo/diet (RR: 0.95, 95 % CI 0.77 to 1.18) (Fig. 4), nor versus hypoglycaemic drugs (RR: 0.99, 95 % CI 0.90 to 1.09) (Fig. 5).
Secondary outcomes
Compared to placebo or diet alone, insulin regimens did not affect sudden death (RR: 0.75, 95 % CI 0.45 to 1.27) (Fig. 6), myocardial infarction (RR: 1.07, 95 % CI 0.90 to 1.28) (Fig. 7), strokes (RR: 0.88, 95 % CI 0.59 to 1.32) (Fig. 8) or leg amputations (RR; 0.92, 95 % CI 0.48 to 1.74) (Fig. 9),
Results remained non-significant when insulin regimens were compared to hypoglycaemic drugs. (Figs. 10, 11, 12 and 13)
Insulin regimens did not affect blindness (RR: 1.10, 95 % CI: 0.76 to 1.60) (Fig. 14), or renal failure or doubling of serum creatinine level (RR: 0.68, 95 % CI, 0.43 to 1.06) (Fig. 15), compared to placebo or diet alone.
Regarding retinal photocoagulation, the only available data was from UKPDS 33 [11] which compared insulin versus diet. In the insulin group, there was a significant decrease in retinal photocoagulations (RR: 0.70, 95 % CI: 0.53 to 0.94).
No data was available for neuropathy.
Compared to oral hypoglycaemic drugs, the risk of hypoglycaemic events and major hypoglycaemia were significantly higher in the insulin group (RR: 2.62; 95 % CI 2.48 to 2.77 and RR: 2.78, 95 % CI 2.30 to 3.36 respectively) (Figs. 16 and 17)
Data from UGDP and UKPDS on these criteria have not been published and it was not possible to assess the effect of insulin vs. placebo/diet.
The only data available on death from cancer were from UKPDS [12] and UGDP [13], versus placebo/diet. Results were not significant, (RR: 0.82, 95 % CI 0.58 to 1.15) (Fig. 18). There is no data analysing insulin vs. hypoglycaemic drugs.
Discussion
This meta-analysis from 20 RCT analysing 18,599 T2D patients showed no benefit of insulin vs. hypoglycaemic drugs or vs. diet/placebo on all-cause mortality, cardiovascular mortality, micro and macro vascular complications, except for retinopathy requiring photocoagulation. This last result comes from a single open label trial, and it is not possible to test its reproducibility. Moreover, regarding all types of hypoglycaemic events, insulin is significantly more harmful than other active treatments. There is no increase in death by cancer with insulin therapy vs. placebo/diet. (Fig. 18)
Data comparing the efficacy of insulin versus diet/placebo on cardiovascular mortality are scarce. Only two studies (2,426 patients) analysed mortality as a primary outcome: UGDP [10] and UKPDS 33 [11]. The other studies were based on surrogate outcomes, like HbA1c or other forms of ‘metabolic control’. Neither of these studies showed significant results regarding cardiovascular or all-cause mortality.
The small number of trials accessing these outcomes could explain the lack of significant results regarding all-cause mortality and cardiovascular mortality. Among these studies, two compare insulin with agents that have been withdrawn from the market, (tolbutamide, a sulfonylurea and phenformin, in the University Group Diabetes Program study). The sensitivity analysis removing these two studies did not affect the results, but cancelled heterogeneity across trials.
Results of our comparison with placebo/diet should be taken with caution, as we cannot exclude a 20 % reduction or a 7 % increase in all-cause mortality. We cannot exclude a reduction of 23 % or an 18 % increase in cardiovascular mortality either.
Clinical efficacy of insulin needs to be demonstrated with long-term trials. Insulin is currently prescribed to millions of patients without a proven benefit. The only two long-term studies available have significant weaknesses. The first, the UKPDS trial was open label and treatment could be increased according to blood glucose levels in the diet control group. 16 % of patients received insulin in the diet alone group [11]. Moreover, this study has major methodological shortcomings (was not double-blind, endpoints were added and follow-up was lengthened during the trial after observing negative results) [12–14]. Finally, concomitant treatments were not reported in the publications [15]. The second study was interrupted, because of increased mortality in the tested agent group, tolbutamide, which has been withdrawn from the market. The UGDP trial included T2D patients, according to diagnostic criteria in use at the time of patient recruitment (i.e., from 1961 to 1965) and was open label (comparisons: standard dose insulin vs adapted insulin vs placebo). After a 10-year follow-up (12.5 years on average), there were 15 to 18 % dropouts. The insulin regimens and concomitant treatments were different to those used today.
Our meta-analysis demonstrates no efficacy of insulin vs. placebo/diet on macrovascular complications (fatal or global). Furthermore, insulin does not decrease relevant microvascular outcomes such as blindness or renal failure either. However, significant results are found for retinopathy requiring laser photocoagulation. This effect should be taken with caution since it is derived from the single UKPDS trial, whose limits we have already underlined. The number needed to treat is about 30 at 10 years.
We analysed all types of insulin regimens, although “biphasic insulin aspart” or biphasic insulins are reported to induce a better metabolic control (estimated from fasting blood glucose and HbA1c) than other regimens (conventional insulin or other analogue insulin) [15]. We made this choice because there is no evidence that any insulin regimen was better at preventing clinically relevant outcomes like mortality or macro- or microvascular complications; and because there was too little data to analyse each single insulin regimen separately.
As the efficacy of insulin has not been proven, the safety analysis is essential. Our meta-analysis confirms that compared to other hypoglycaemic drugs, there is a serious risk of hypoglycaemia with insulin. According to UKPDS [11] the NNH is about 5 to 6 at 10 years for hypoglycaemia and 91 each year for a major hypoglycaemic event.
Previous meta-analyses have reported that various insulin regimens increase hypoglycaemia equally [16].
The link between severe hypoglycaemia and mortality has been reported in several studies [17] or, indirectly, when comparing conventional to intensive treatments [18–21]. Insulin treatment could therefore be considered as a high risk treatment option.
It is noteworthy that insulin is the second cause of drug-related hospital admissions in patients over 65 [22]. In two U.S. representative surveys over a 4 year period, Geller and al. estimated there were nearly 100,000 emergency department visits per year for insulin-related hypoglycaemia and errors [23], among which almost one-third required hospitalization. The estimated rate of severe neurological sequelae was 60 %. Patients over 80 treated with insulin were more than twice as likely to visit the emergency department and nearly 5 times as likely to be hospitalized. Moreover there is some evidence that hypoglycaemia may increase the risk of dementia [24].
Another well-known side effect of insulin-based regimen is weight gain [25], which secondarily increases insulin resistance.
The fact that insulin has shown no impact on clinically relevant outcomes is of major importance. Theoretically, insulin has potential negative clinical consequences, due to the underlying cellular and molecular mechanisms [3, 4]. From a patho-physiological point of view it is understandable that insulin is vital in the case of absolute insulin deficiency, such as type 1 diabetes. However, in T2D, where insulin resistance and high circulating levels of endogenous insulin are key concepts, the role of exogenous insulin is unclear. Observational studies have shown an association between endogenous insulin levels and cardiovascular risk, [26] and do not seem to be in favour of exogenous insulin either. In a retrospective cohort study, insulin therapy was associated with an increase in total mortality, (adjusted hazard ratio [HR] = 1.75; 95%CI: 1.24 to 2.47 for low insulin exposure and HR = 2.79; 95%CI: 2.36 to 3.30 for high insulin exposure, compared to no exposure) [27]. Other observational studies suggest an increased risk of cancer [28]. Our meta-analysis of RCTs shows no increase in deaths by cancer and cancer cases were not reported in many of the included studies. However, these results are to be taken with caution due to the possible lack of power of our meta-analysis. In vitro, the mitogenic effect of insulin is well established [29, 30].
The underlying mechanism of the higher risk associated with insulin is unclear. Positive associations could be explained by confounding factors: patients with T2D using insulin are usually older, with a longer history of diabetes, more comorbidities, at higher cardiovascular risk and with greater insulin resistance. Although observational studies may partially adjust for these factors, residual confounding factors may be responsible for the reported associations. However, it is also possible that hypoglycaemia plays a role (via sympathoadrenal activation, abnormal cardiac repolarization, increased thrombogenesis, inflammation and vasoconstriction [3, 4]) or that a direct atherogenic/mitogenic effect exists (cell growth, differentiation and proliferation [29, 30]), or that there is another specific effect of insulin that remains unknown.
Implications for clinical practice
Insulin for T2D should only be used when no other treatment is available, to prevent short-term acute complications (such as hyperosmolar coma or ketoacidosis in case of an infection) or when the lack of insulin per se assigns patients in a high risk group.
This meta-analysis, as well as two other recent meta-analyses on metformin [7] and sulfonylureas [8], discredits blood glucose and HbA1c as valid surrogate outcomes for morbidity in T2D. The HbA1c target should be reconsidered since “the lower the better” model is censored by the increased mortality in the ACCORD study [18]. “The lower the better” and “treat to target” models, greatly increased requirements for insulin in patients with T2D (in the UK: 137,000 patients in 1991 vs. 421,000 in 2010 [31]).
The most appropriate treatment target in T2D is reduction in global cardiovascular risk. Although statins and angiotensin converting enzyme inhibitors have shown their efficacy to reduce cardiovascular mortality, for now, insulin has not.
Implications for research
Further long-term studies are needed to establish whether insulin is beneficial in T2D.
Conclusions
In T2D, insulin is recommended as an alternative or in combination with oral hypoglycaemic drugs when blood glucose targets are not achieved. Our meta-analysis does not support these recommendations, showing no long term benefit on cardiovascular risk or other clinical outcomes. Moreover our analysis has shown harmful adverse effects such as hypoglycaemia. The only benefit could be limited to reducing short term hyperglycaemia to improve symptoms (thirst, polyuria, asthenia, blurred sight) and to avoid acute complications (infection, hyperosmolar coma). Therefore, there is a great need for further studies.
Abbreviations
95%CI, 95 % confidence interval; ADA/EASD, American Diabetes Association/European Association for the Study of Diabetes; HR, hazard ratio; NICE, National Institute for Health and Care Excellence; RR, risk ratio; T2D, type 2 diabetes; UGDP, University Group Diabetes Program; UKPDS, United Kingdom Prospective Diabetes study
References
http://www.who.int/diabetes/ (accessed 12 Jun 2015)
The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Rensing KL, Reuwer AQ, Arsenault BJ, Von der Thûsen JH, Hoekstra JB, Kastelein JJ, Twickler TB. Reducing cardiovascular disease risk in patients with type 2 diabetes and concomitant macrovascular disease: can insulin be too much of a good thing? Diabetes Obes Metab. 2011;13:1073–87.
Currie CJ, Johnson JA. The safety profile of exogenous insulin in people with type 2 diabetes: justification for concern. Diabetes Obes Metab. 2012;14:1–4.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–42.
NICE Guidance: December 2015. www.nice.org.uk/guidance/ng28. Accessed 12 June 2015
Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel JP, Kassai B, Moreau A, Gueyffier F, Cornu C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med. 2012;9(4), e1001204.
Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938–53.
Higgins J P T, Altman D G, Gøtzsche P C, Jüni P, Moher D, Oxman A D, Savović J, Schulz K F, Weeks L, Sterne J A C, Cochrane Bias Methods Group, Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343 doi: http://dx.doi.org/10.1136/bmj.d5928.
UGDP. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VIII. Evaluation of insulin therapy: final report. Diabetes. 1982;31:1–81.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Nathan DM. Some answers, more controversy, from UKPDS. Lancet. 1998;352:832–3.
Ewart RM. The case against agressive treatment of type 2 diabetes: critique of the UK prospective diabetes study. BMJ. 2001;323:854–8.
McCormack J, Greenhalgh T. Seeing what you want to see in randomised controlled trials: versions and perversions of UKPDS data. BMJ. 2000;320:1720–3.
Boussageon R, Supper I, Erpeldinger S, Cucherat M, Bejan-Angoulvant T, Kassai B, Cornu C, Gueyffier F. Are concomitant treatments confounding factors in randomized controlled trials on intensive blood-glucose control in type 2 diabetes? A systematic review. BMC Med Res Methodol. 2013;13:107.
Halimi S, Raskin P, Liebl A, Kawamori R, Fulcher G, Yan G. Efficacy of biphasic insulin aspart in patients with type 2 diabetes. Clin Ther. 2005;27(suppl B):S57–74.
Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36:1107–10.
ACCORD study. Gerstein HC, Miller ME , Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
ADVANCE Study. Patel A, MacMahon S, Chalmers Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, De Galam BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
Zoungas S, Patel A, Chalmers J, de Galam BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S. Severe hypoglycaemia and risks of vascular events and death. N Eng J Med. 2010;363:1410–8.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–12.
Geller AI, Shebab N, Lovegrove MC, Kegler SR, Weidenbach KN, Ryan GJ, Budnitz DS. National estimates of insulin-related hypoglycaemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 2014;174:678–86.
Whitmer RA, Karter AJ, Yaffe K, Quesenberry Jr CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–72.
Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab. 2012;14:810–20.
Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation. 1998;97:996–1001.
Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab. 2010;12:47–53.
Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulphonylureas or insulin. Diabetes Care. 2006;29:254–8.
Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer. 2008;8:915–28.
Draznin B. Mitogenic action of insulin: friend, foe or “frenemy’? Diabetologia. 2010;53:229–33.
Holden SE, Gale EA, Jenkins-Jones S, Currie CJ. How many people inject insulin? UK estimates from 1991 to 2010. Diabetes Obes Metab. 2014;16:553–9.
Alvarsson M, Sundkvist G, Lager I, Henricsson M, Berntorp K, Ferngvist-Forbes E, Steen L, Westermark G, Westermark P, Orn T, Grill V. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231–7.
Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain M-P, Pilorget V, Echtay A. Fonseca: insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379:2262–9.
Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Ykri-Jârvinen H, Heine RJ. One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin treated type 2 diabetic patients. Diabetes Care. 2009;32:762–8.
Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11:1153–62.
Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–43.
Gallwitz B, Böhmer M, Segiet T, Môlle A, Milek K, Becker B, Helsberg K, Petto H, Peters N, Bachmann O. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34:604–6.
Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006;23:736–42.
Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Hartemann-Heurtier A, Halbron M, Golmard JL, Jacqueminet S, Bastard JP, Rouault C, Aved A, Pieroni L, Clement K, Grimaldi A. Effects of bed-time insulin versus pioglitazone on abdominal fat accumulation, inflammation and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes Res Clin Pract. 2009;86:37–43.
Heine RJ, Van Gaal LF, Johns D, Mihhm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2009;143:559–69.
Hollander P, Raslova K, Skjøth TV, Råstam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab. 2011;13:268–75.
Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–67.
Nauck M, Horton E, Andjelkovic M, Ampudia-Blasco FJ, Parusel CT, Boldrin M, Balena R, T-emerge 5 Study Group. Taspoglutide, a once-weekly glucagon-like peptide 1 analogue, vs. insulin glargine titrated to target in patients with Type 2 diabetes: an open-label randomized trial. Diabet Med. 2013;30:109–13.
Reynolds LR, Kingsley FJ, Karounos DG, Tannock LR. Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res Clin Pract. 2007;77:180–7.
Rosenstock J, Sugimoto D, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care. 2006;29:554–9.
United Kingdom Prospective Diabetes Study 24. a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. 1998;128:165–75.
Blicklé J-F, Hancu N, Piletic M, Profozic V, Shestakova M, Dain M-P, Jacqueminet S, Grimaldi A. Insulin glargine provides greater improvements in glycaemic control vs. intensifying lifestyle management for people with type 2 diabetes treated with OADs and 7-8 % A1c levels. The TULIP study. Diabetes Obes Metab. 2009;11:379–86.
Russel-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55.
The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes. 1975;24 Suppl 1:65–184.
Phenformin and vascular complications of diabetes. Med Lett Drugs Ther. 1972;14:1–2.
Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971;217:1671–5.
Cornfield J. The University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971;217:1676–87.
Feinglos MN, Bethel MA. Therapy of type 2 diabetes, cardiovascular death, and the UGDP. Am Heart J. 1999;138:S346–52.
Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:1400–10.
Acknowledgments
The authors would like to thank Bernd Richter for his commentary and proofreading the English. Permission was obtained for this acknowledgment.
SE and RB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
There was no funding for this work.
Availability of data and materials
All data and materials are from published papers and are available.
Authors’ contributions
SE, EG, FR, and FG conceived the study. SE, FR, EG, CC and RB extracted the data and reviewed the selected papers. SE, FR, EG, FG and RB performed the statistical analysis. SE, MR, CB, YB, IS, BK, FG and RB drafted the manuscript. SE, MR, CC, FG and RB interpreted the results and performed a critical review. All authors gave final approval of the version to be published; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare that they do not have any financial interests that may be relevant to the submitted work.
Consent for publication
not applicable
Ethics approval and consent to participate
Not required.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
PRISMA checklist. (DOC 62 kb)
Additional file 2:
Appendix with search strategy. (DOCX 28 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Erpeldinger, S., Rehman, M.B., Berkhout, C. et al. Efficacy and safety of insulin in type 2 diabetes: meta-analysis of randomised controlled trials. BMC Endocr Disord 16, 39 (2016). https://doi.org/10.1186/s12902-016-0120-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-016-0120-z