Abstract
Background
Lower urinary tract symptoms are highly prevalent and a large proportion of these symptoms are known to be associated with a dysfunction of the afferent pathways. Diagnostic tools for an objective and reproducible assessment of afferent nerve function of the lower urinary tract are missing. Previous studies showed first feasibility results of sensory evoked potential recordings following electrical stimulation of the lower urinary tract in healthy subjects and patients. Nevertheless, a refinement of the methodology is necessary.
Methods
This study is a prospective, randomized trial conducted at Balgrist University Hospital, Zürich, Switzerland. Ninety healthy subjects (forty females and fifty males) without lower urinary tract symptoms are planned to be included in the study. All subjects will undergo a screening visit (including standardized questionnaires, 3-day bladder diary, urinalysis, medical history taking, vital signs, physical examination, neuro-urological examination) followed by two measurement visits separated by an interval of 3 to 4 weeks. Electrical stimulations (0.5Hz-5Hz, bipolar, square wave, pulse width 1 ms) will be applied using a custom-made transurethral catheter at different locations of the lower urinary tract including bladder dome, trigone, proximal urethra, membranous urethra and distal urethra. Every subject will be randomly stimulated at one specific site of the lower urinary tract. Sensory evoked potentials (SEP) will be recorded using a 64-channel EEG cap. For an SEP segmental work-up we will place additional electrodes on the scalp (Cpz) and above the spine (C2 and L1). Visit two and three will be conducted identically for reliability assessment.
Discussion
The measurement of lower urinary tract SEPs elicited by electrical stimulation at different locations of the lower urinary tract has the potential to serve as a neurophysiological biomarker for lower urinary tract afferent nerve function in patients with lower urinary tract symptoms or disorders. For implementation of such a diagnostic tool into clinical practice, an optimized setup with efficient and reliable measurements and data acquisition is crucial. In addition, normative data from a larger cohort of healthy subjects would provide information on variability, potential confounding factors and cut-off values for investigations in patients with lower urinary tract dysfunction/symptoms.
Trial registration
Clinicaltrials.gov; Identifier: NCT02272309.
Similar content being viewed by others
Background
Lower urinary tract symptoms (LUTS) such as urinary urgency, frequency and incontinence, imply a massive impairment of quality of life [1, 2].
LUTS are highly prevalent and a large proportion of LUTS are found to be associated with afferent nerve dysfunction [1, 3–5]. Assessment of afferent pathways in patients with LUTS is however a challenge. Specific diagnostic tools for an objective and reproducible measurement of bladder and urethral afferent nerve function are missing. Yet, filling cystometry (FC) is the standard method used in clinical practice for the assessment of bladder sensations [6–10]. Nevertheless, FC largely depends on the subjective perceptions and collaboration of the patient and is hence not an objective measurement of bladder sensations. In addition, the reliability of the FC is questionable and its variability and outcome resolution is too large to detect differences smaller than 100 mL [11, 12]. Moreover, FC only covers sensory information from the bladder but not from the urethra.
Current perception threshold (CPT) testing is another method of assessing sensations from the lower urinary tract (LUT). CPT testing is performed by asking the subject to indicate the onset of sensation when an increasing electrical stimulus is applied [13]. It was shown that this method is safe and well tolerated by healthy subjects and patients, but still, it provides only semi-quantitative information on sensations of the LUT [14–16]. In addition, local factors such as distance of electrodes to the mucosa and the mucosal condition itself can significantly affect CPTs [14, 17, 18].
A more objective and qualitative assessment of afferent nerve function are sensory evoked potentials (SEP) that are routinely used in neurophysiology to detect afferent nerve conduction qualities and integrity from different parts of the human body. By analysing the latencies and amplitudes of the SEPs (Fig. 1), information on nerve fiber integrity, conduction velocity and fiber type can be obtained [19, 20]. SEPs from the LUT would be useful not only for an assessment of LUT sensory function, to amend findings from previous investigations (i.e. history, neurologic examination, urodynamic examination), but also as a surrogate marker and outcome measure for treatments targeting afferent LUT pathways [21]. However, SEP measurements stimulating the LUT are more challenging than SEP measurements for cutaneous sites due to less direct control of electrode placement and potential changes of bladder volume with time, which can influence the SEP measurement. Furthermore, bladder SEPs may be less synchronized as they are likely mediated by poorly or non-myelinated fibres leading to less distinct summation of sensory potentials. Nevertheless, previous studies reported first feasibility results of SEP recordings from the LUT following electrical stimulation in healthy subjects [22–26] and patients [20, 27–29]. However, due to heterogeneous measurement settings and study populations, a clear conclusion cannot be drawn from these data. Currently, there is no standard for SEP measurements for the LUT. Hence, optimal stimulation and recording procedures as well as parameters still need to be determined.
In this study we would like to advance the evaluation of viscero-sensory afferent pathways of the LUT and to refine the methodology of LUT SEPs in healthy subjects. We aim to get more knowledge on the impact of different stimulation parameters (e.g. stimulation frequency) on the reliability, shape, latency, amplitude and topographical distribution of SEPs recorded during electrical stimulation of the LUT. In a first step it is our goal to find a frequency that allows a faster acquisition of reliable SEPs than the previously used 0.5 Hz [25, 26] and to obtain normative LUT SEP data for the different localizations in the LUT from different gender groups. In a second step we aim to implement the optimized methodology into clinical practice to use it as an objective marker of pathological LUT conditions and to show distinction of healthy LUT neurophysiology and function.
Methods and design
Study design
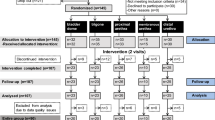
This study is a prospective, randomized trial conducted as a single center study at the Spinal Cord Injury Center & Research Lab, Balgrist University Hospital, University of Zürich, Zürich, Switzerland. The study comprises three visits of which the first will be a screening visit followed by two measurement visits separated by an interval of 3–4 weeks (Fig. 2). The site of stimulation will be indicated by random group assignment (in females and males: dome, trigone, proximal urethra, distal urethra; in males additionally: membranous urethra). Consequently, ten females and ten males will be allocated to one localization. In addition, the frequencies used for the stimulation of the LUT and thereafter the SSEP measurements will be randomly applied.
Study population and recruitment
The volunteers will be recruited via announcements at the University of Zürich, internet platforms (i.e. www.marktplatz.uzh.ch, www.tutti.ch, www.ronorp.net) and personal contacts. According to the inclusion- and exclusion criteria (Table 1), healthy female and male subjects without any LUTS will be included. Health is defined as the absence of any health troubles, as assessed by a complete medical history, standardized questionnaires, physical, neurological and neuro-urological examinations (Table 2). The absence of LUTS will be determined by uroflowmetry, a 3-day bladder diary and standardized urological questionnaires (FLUTS [30] /MLUTS [31]; Qualiveen [32]; IPSS [33]; Swiss German OAB [34]) (Table 2).
Investigations and procedures
Screening (visit one)
The content and purpose of the study will be explained in written and oral form to all recruited subjects. Those subjects providing written informed consent will be screened for in- and exclusion criteria by using the tests, questionnaires, and examinations listed in Table 2. Subjects who are eligible for study participation according to the in- and exclusion criteria (Table 1) will be invited for visit two and three (Fig. 2).
Measurement visits (visit two and three)
Prior to each measurement, the urine of the volunteers is analyzed to exclude signs suggestive for asymptomatic bacteriuria, microhaematuria, and (in women only) pregnancy. Both measurements consist of a resting electroencephalogram (EEG) measurement followed by recordings of SEPs elicited by transurethral electrical stimulation at a specific LUT site indicated by the group assignment. Each measurement includes recordings of the electrooculogram (left and right eye), electrocardiogram, and electroencephalogram using a 64 Ag/AgCl surface electrodes system comprising a cap-based extended international 10–20 montage (Easy cap, Easy cap GmbH, Herrsching, Germany). Electrode impedances are constantly kept below 20kΩ. Six additional electrodes are placed at Cpz (reference: Fz), C2 (reference: Fz) and L1 (reference: iliac crest), respectively, for segmental assessment. LUT electrical stimulation will be applied transurethrally with a custom-made 14 Ch catheter (Unisensor AG, Attikon, Switzerland), using frequencies between 0.5Hz and 5Hz (bipolar, square wave, pulse width 1 ms). Stimulation intensities are adapted to the 3 to 4× CPT, which is determined using the method of limits prior to each SEP measurement [13]. The catheter includes platinum electrodes and a radiopaque marker, which allows precise catheter positioning under fluoroscopic guidance. After each stimulation, the bladder will be emptied and filled with 60 mL of contrast medium (Ultravist® 150TM, Bayer AG, Switzerland). Consequent to LUT SEPs, SEPs elicited by transcutaneous stimulation of the tibial and pudendal nerves will be recorded in random order. These standard neurophysiological measurements will serve as comparators to the LUT SEPs. Visits two and three will be performed identically with an interval of 3 to 4 weeks (Fig. 3).
Follow-up
Two to three days after each measurement visit, a follow-up interview is performed to evaluate the general well-being of the volunteers. In case of any side effects, such as dysuria, the subjects are appointed to an extra medical visit for further evaluation, investigation and, if necessary, medical treatment.
Safety
During the first visit, all subjects are carefully screened to exclude subjects with neurological and/or urological pathology or any regular medication intake. At the beginning of every visit an urinalysis and pregnancy test (in females only) is performed to exclude signs suggestive for asymptomatic bacteriuria, microhaematuria and pregnancy, respectively. Pregnancy leads to study exclusion and referral to a gynecologist for further evaluation. In case of a positive urine dipstick test suspicious for asymptomatic bacteriuria or urinary tract infection (UTI), the measurement will be postponed until the dipstick test result becomes negative or UTI has been treated. In case of a dipstick test indicating microhaematuria, subjects can choose to repeat the test at a later time-point or to directly have the result verified by clean catheterization. If clean catheterization still indicates microhaematuria, subjects will be excluded from study participation and referred to their general practitioner or urologist for further evaluation.
Two or three days after the measurement visits, a follow-up interview will be conducted to assess general well-being and document possible adverse events or symptoms. In case of an adverse event, additional tests or medical interventions will be initiated as necessary and subjects might be referred to a general physician or medical specialist for further investigations and/or treatment. All responsible authorities will be informed about any adverse events (AE) or severe adverse events (SAE). These events will be observed and followed until complete cure. To decrease the radiation dose during visits two and three, we will not perform full radiographs but fluoroscopy with a reduced field of view focusing on the LUT only. For a protection of the male gonads, men will wear a gonad shielding.
Endpoints of the study
Primary endpoint: N1 responder rate / latency of N1 – as the most prominent peak of LUT SEPs.
Secondary endpoints: A) Latencies (P1, P2), amplitudes (P1, N1, P2, P1N1, N1P2), topographies and source localizations of LUT SEPs; B) CPTs; C) latencies, amplitudes, topographies and source localization of tibial, pudendal SSEPs; D) 3-day bladder diary, scores of questionnaires (i.e. ICIQ-FLUTS [30]/ICIQ-MLUTS [31], IPSS [33], IIEF [35] /FSFI [36], Swiss German OAB [34], ICIQ-LUTSqol [37], Qualiveen [32], HADS [38], MoCA [39]).
Determination of sample size
Based on data from a previous study [25], the sample size was determined using a non-parametric approach [40]. Non-parametric smoothing estimates (kernel smooth) were iteratively compared for subsets of individuals in order to establish the smallest subset (sample size) in which a significant outcome was observed. Determined using cross-validation [41], the smoothing parameter was set to 20. From the smoothed curves, the empirical second derivatives (as an expression of the 'information', i.e. latency, amplitude and dispersion, contained therein) were used, the estimates of which were computed and standardized by the absolute value of their mean. The difference between the two frequencies (0.5Hz and 3Hz) was taken into account, to allow for a standardized vector summary of the curve. Normality was tested using the Kolmogorov-Smirnov method and no evidence against was revealed. A t-test based on this standardized vector was subsequently conducted. Power analysis was performed using a bootstrapping technique [42, 43]. For each different sample size combination, the difference between the two frequencies, contained in the standardized vector, was analyzed. The bootstrap simulations were executed at the individual level. All elements of the standardized vector summary were first sub-sampled before results were presented based on the mean p-values for all possible combinations. To ensure robustness of the results, four different criteria – standard deviation, variance, total variance (sum of the absolute values) and wigglyness (sum of the absolute values of the second derivatives) – were applied to the standardized vector. The aforementioned procedure was repeated for the different study visits and simulation sites. This resulted in a required sample size of 50 male and 40 female subjects, taking into account potential dropouts.
Data management and analysis
All EEG data will be filtered and segmented using Brain Vision Analyzer 2 (Version 2.1.0.327, Brain Products, Gilching, Germany). The segments will be averaged and the P1, N1, P2 latencies as well as the P1N1 and N1P2 amplitudes will be determined.
Study data will be collected and managed using the Research Electronic Data Capture Tool (REDCap, Version 6.12.1, Vanderbilt University) electronic data capture tools hosted at Balgrist University Hospital [44]. REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Primarily, all data will be examined using exploratory data analysis (EDA) methods and described providing mean and standard deviation (or median and range where appropriate). ANOVAs or independent sample t-tests (or Kruskal-Wallis test or Mann-Whitney tests where appropriate) will be performed to compare participant characteristics between groups or to detect gender differences. Linear mixed effects models will be used to compare the two measurement visits. The level of significance will be 5% (alpha = 0.05). Regression techniques will be taken into account, if needed. All the statistical analyses will be performed with the software RStudio (Version 0.98.1083) [45].
Discussion
This clinical trial will investigate the effect of several stimulation frequencies at different locations of the LUT. Since it was already shown that SEPs could be reproducibly recorded from the LUT [25], we now aim to optimize the settings to achieve a faster acquisition of reliable SEPs, which is important for implementation into clinical diagnostics and to minimize measurement bias through changes that occur over time such as bladder volume.
The assessment of normative values of LUT SEPs in healthy male and female subjects will give us more knowledge on the variability of LUT SEPs, as well as potential factors that may influence the shape and the reliability of the SEPs. Cut-offs for amplitude and latency values can be defined for future investigations in patients with LUT dysfunction. The advancement of neurophysiological assessment methods for the LUT will significantly influence the evaluation of afferent nerve function in the LUT and has the potential to serve as a clinical diagnostic tool complementary to standard urodynamic investigations. After having refined our methodology, we would like to apply LUT SEPs with the optimized stimulation frequency in different patient groups suffering from LUTS, including patients with spinal cord injury and multiple sclerosis. Established cut-off amplitudes and latency values from this study should then be used to relate LUT symptoms and dysfunction in patients with LUT SEP data, thus amending FC findings with an objective evaluation of afferent LUT nerve function in these disorders.
Trial status
At the time of manuscript submission, first subjects have been recruited, included and investigated.
Abbreviations
- AE:
-
Adverse event
- CPT:
-
Current perception threshold
- EDA:
-
Exploratory data analysis
- EEG:
-
Electroencephalography / electroencephalogram
- FC:
-
Filling cystometry
- GCP:
-
Good clinical practice
- LUTS:
-
Lower urinary tract symptoms
- OAB:
-
Overactive bladder
- SAE:
-
Severe adverse event
- SDV:
-
Strong desire to void
- SEP:
-
Sensory evoked potential
- UTI:
-
Urinary tract infection
References
Coyne KS, Wein AJ, Tubaro A, Sexton CC, Thompson CL, Kopp ZS, Aiyer LP. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103 Suppl 3:4–11.
Irwin DE, Milsom I, Kopp Z, Abrams P, Cardozo L. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006;97(1):96–100.
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–8.
Yoshimura N. Lower urinary tract symptoms (LUTS) and bladder afferent activity. Neurourol Urodyn. 2007;26(6 Suppl):908–13.
Fowler CJ. Bladder afferents and their role in the overactive bladder. Urology. 2002;59(5 Suppl 1):37–42.
Schafer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, Sterling AM, Zinner NR, van Kerrebroeck P, International Continence S. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21(3):261–74.
Heeringa R, van Koeveringe GA, Winkens B, van Kerrebroeck PE, de Wachter SG. Degree of urge, perception of bladder fullness and bladder volume--how are they related? J Urol. 2011;186(4):1352–7.
Erdem E, Akbay E, Doruk E, Cayan S, Acar D, Ulusoy E. How reliable are bladder perceptions during cystometry? Neurourol Urodyn. 2004;23(4):306–9.
De Wachter S, Van Meel TD, Wnydaele JJ. Can a faked cystometry deceive patients in their perception of filling sensations? A study on the reliability of spontaneously reported cystometric filling sensations in patients with non-neurogenic lower urinary tract dysfunction. Neurourol Urodyn. 2008;27(5):395–8.
Groen J, Pannek J, Castro Diaz D, Del Popolo G, Gross T, Hamid R, Karsenty G, Kessler TM, Schneider M, t Hoen L, et al. Summary of European Association of Urology (EAU) guidelines on Neuro-Urology. Eur Urol. 2016;69(2):324–33.
Virseda M, Salinas J, Esteban M, Mendez S. Reliability of ambulatory urodynamics in patients with spinal cord injuries. Neurourol Urodyn. 2013;32(4):387–92.
Bellucci CH, Wollner J, Gregorini F, Birnbock D, Kozomara M, Mehnert U, Kessler TM. Neurogenic lower urinary tract dysfunction--do we need same session repeat urodynamic investigations? J Urol. 2012;187(4):1318–23.
Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20(2):198–204.
De Wachter S, Wyndaele JJ. Quest for standardisation of electrical sensory testing in the lower urinary tract: the influence of technique related factors on bladder electrical thresholds. Neurourol Urodyn. 2003;22(2):118–22.
De Laet K, De Wachter S, Wyndaele JJ. Current perception thresholds in the lower urinary tract: Sine- and square-wave currents studied in young healthy volunteers. Neurourol Urodyn. 2005;24(3):261–6.
Ulrich Mehnert, André Reitz, Maya Ziegler, Peter A. Knapp, Brigitte Schurch. Does Tolterodine Extended Release Affect the Bladder Electrical Perception Threshold? A Placebo Controlled, Double-Blind Study With 4 and 8 mg in Healthy Volunteers. The Journal of Urology. 2007;178(6):2495-2500.
Ukimura O, Ushijima S, Honjo H, Iwata T, Suzuki K, Hirahara N, Okihara K, Mizutani Y, Kawauchi A, Miki T. Neuroselective current perception threshold evaluation of bladder mucosal sensory function. Eur Urol. 2004;45(1):70–6.
Knupfer SC, Liechti MD, Gregorini F, De Wachter S, Kessler TM, Mehnert U. Sensory function assessment of the human male lower urinary tract using current perception thresholds. Neurourol Urodyn. 2016 [Epub ahead of print].
Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia-Larrea L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119(8):1705–19.
Deltenre PF, Thiry AJ. Urinary bladder cortical evoked potentials in man: suitable stimulation techniques. Br J Urol. 1989;64(4):381–4.
Chiappa KH, Ropper AH. Evoked potentials in clinical medicine (first of two parts). N Engl J Med. 1982;306(19):1140–50.
Ganzer H, Madersbacher H, Rumpl E. Cortical evoked potentials by stimulation of the vesicourethral junction: clinical value and neurophysiological considerations. J Urol. 1991;146(1):118–23.
Hansen MV, Ertekin C, Larsson LE. Cerebral evoked potentials after stimulation of the posterior urethra in man. Electroencephalogr Clin Neurophysiol. 1990;77(1):52–8.
Sarica Y, Karacan I, Thornby JI, Hirshkowitz M. Cerebral responses evoked by stimulation of vesico-urethral junction in man: methodological evaluation of monopolar stimulation. Electroencephalogr Clin Neurophysiol. 1986;65(2):130–5.
Gregorini F, Wollner J, Schubert M, Curt A, Kessler TM, Mehnert U. Sensory evoked potentials of the human lower urinary tract. J Urol. 2013;189(6):2179–85.
Flavia Gregorini, Stephanie C. Knüpfer, Martina D. Liechti, Martin Schubert, Armin Curt, Thomas M. Kessler, Ulrich Mehnert. Sensory evoked potentials of the bladder and urethra in middle-aged women: the effect of age. BJU International. 2015;115:18-25.
Sarica Y, Karatas M, Bozdemir H, Karacan I. Cerebral responses elicited by stimulation of the vesico-urethral junction (VUJ) in diabetics. Electroencephalogr Clin Neurophysiol. 1996;100(1):55–61.
Badr G, Carlsson CA, Fall M, Friberg S, Lindstrom L, Ohlsson B. Cortical evoked potentials following stimulation of the urinary bladder in man. Electroencephalogr Clin Neurophysiol. 1982;54(5):494–8.
Hansen MV, Ertekin C, Larsson LE, Pedersen K. A neurophysiological study of patients undergoing radical prostatectomy. Scand J Urol Nephrol. 1989;23(4):267–73.
Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol female lower urinary tract symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol. 2004;191(1):73–82.
Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schafer W. Scoring the short form ICSmaleSF questionnaire. International Continence Society. J Urol. 2000;164(6):1948–55.
Costa P, Perrouin-Verbe B, Colvez A, Didier J, Marquis P, Marrel A, Amarenco G, Espirac B, Leriche A. Quality of life in spinal cord injury patients with urinary difficulties. Development and validation of qualiveen. Eur Urol. 2001;39(1):107–13.
Barry MJ, Fowler Jr FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The measurement committee of the American Urological Association. J Urol. 1992;148(5):1549–57. discussion 1564.
Coyne KS, Thompson CL, Lai JS, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015;34(3):255–63.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–30.
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino Jr R. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997;104(12):1374–9.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Nasreddine ZS, Phillips N, Chertkow H. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2012;78(10):765–6. author reply 766.
Silverman BW. Some aspects of the spline smoothing approach to non-parametric regression curve fitting. J R Stat Soc B Methodol. 1985;47(1):1–52.
Abraham B, Ledolter J. Introduction To Regression Modeling. 2006.
Davidson AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge University Press. 1997;48.
Efron B. Bootstrap methods: another look at the jackknife. Annals of Statistics. 1979;7(1):1–26.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
R Development Core Team. R. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing;2015.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
International conference on harmonisation. Good clinical practice guideline.http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html.
Swiss Academy of Medical Sciences. Guideline-concerning scientific research involving human beings. 2009:http://www.samw.ch/de/Publikationen/Leitfaden-fuer-die-Praxis.html. Accessed 22 Nov 2016.
The Federal Authorities of the Swiss Confederation. Bundesgesetz über den Datenschutz (DSG) vom 19. Juni 1992, Stand. 01.01.2014. 1992: https://www.admin.ch/opc/de/classified-compliation/19920153/index.html#app1. Accessed 22 Nov 2016.
Funding
The study is made possible through funding from the Swiss National Science Foundation (grant number: 32003B_149628) and the Swiss Continence Foundation.
Authors’ contributions
SvdL, UM participated in designing protocol of the study. SvdL, MRS, MP, UM drafted the manuscript. MSt, RF, MDL, MS, TMK critically revised the manuscript. MS, TMK, UM obtained the funding of this study. SvdL, MSt, MRS collect the data of the study. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich, KEK-ZH-Nr. 2013-0518) and is performed according to the (World Medical Association) Declaration of Helsinki [46], the guidelines for Good Clinical Practice (GCP) [47], and the guidelines of the Swiss Academy of Medical Sciences [48]. Written informed consent is required for study participation. All personal data are handled according to the federal law of data protection in Switzerland [49].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
van der Lely, S., Stefanovic, M., Schmidhalter, M.R. et al. Protocol for a prospective, randomized study on neurophysiological assessment of lower urinary tract function in a healthy cohort. BMC Urol 16, 69 (2016). https://doi.org/10.1186/s12894-016-0188-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-016-0188-9