Abstract
Background
Pseudomyxoma peritonei (PMP) is a disease involving the peritoneum characterized by the production of large quantities of mucinous ascites. PMP has a low incidence, is difficult to diagnose, and has a guarded prognosis. PMP induced by low-grade appendiceal mucinous neoplasm is extremely rare, and PMP accompanied by rectal cancer is even rarer.
Case presentation
We present a unique case of a 70-year-old male with PMP induced by low-grade appendiceal mucinous neoplasm accompanied by rectal cancer. The patient’s clinical, surgical, and histologic data were reviewed. The patient had persistent distended abdominal pain without radiating lower back pain, abdominal distension for 1 month, and no exhaustion or defecation for 4 days. A transabdominal ultrasound-guided biopsy was performed on the first day. The patient received an emergency exploratory laparotomy because of increased abdominal pressure. We performed cytoreductive surgery, enterolysis, intestinal decompression, special tumour treatment and radical resection of rectal carcinoma. The postoperative course was uneventful. The postoperative histological diagnoses were PMP, low-grade appendiceal mucinous neoplasm and rectal medium differentiated adenocarcinoma. At the 1-year follow-up visit, no tumour recurrence was observed by computed tomography (CT). We also performed a literature review.
Conclusions
We should be aware that PMP can rarely be accompanied by rectal cancer, which represents an easily missed diagnosis and increases the difficulty of diagnosis and treatment. Additionally, there are some typical characteristics of PMP with respect to diagnosis and treatment.
Similar content being viewed by others
Background
Pseudomyxoma peritonei (PMP) is a disease involving the peritoneum characterized by the production of large quantities of mucinous ascites, which progressively fill the peritoneal cavity [1]. It has been 176 years since Carl Rokitansky first described an appendiceal mucocele in 1842. However, the origin, pathology, treatment, prognosis, and even the very definition remain controversial [2, 3]. The primary lesion typically originates from adenoma, mucinous appendicular adenocarcinoma or ovarian tumours. Dissemination occurs by the rupture of the lesion, which releases tumour cells into the abdominal cavity. The progressive accumulation of mucinous ascites occasionally produces partial or complete obstructive symptoms [1, 2]. Because PMP lacks specific clinical manifestations, it is difficult to diagnose before surgery [4].
PMP induced by low-grade appendiceal mucinous neoplasm (LAMN) accompanied by rectal cancer is extremely rare. There are a few reports of PMP accompanied by rectal cancer [5,6,7,8]. To the best of our knowledge, no cases of PMP induced by low-grade appendiceal mucinous neoplasm (LAMN) accompanied by rectal cancer have been reported. In this paper, we share our experience with this rare presentation.
Case presentation
A 70-year-old male was admitted to our hospital for “abdominal pain, abdominal distension for 1 month, and no exhaustion or defecation for 4 days” as the chief complaint on April 10, 2017. He had no fever, nausea or vomiting.
The physical examination revealed abdominal distension (Fig. 1a), full abdominal tenderness and weak bowel sounds (1 beat/min). The following laboratory data were observed: WBC: 9.02 × 109/L, NET%: 78.90%, and CEA: > 60.00 μg/L. No obvious electrolyte, coagulation or liver biochemistry abnormalities were noted.
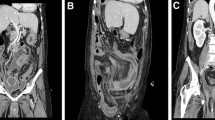
A CT scan of the abdomen revealed peritoneal effusion and bowel dilatation (Fig. 1b). The admitting diagnoses that were investigated were acute intestinal obstruction and abdominal effusion. On the first day, a transabdominal ultrasound-guided biopsy was performed, and a characteristic yellow jelly-like mucus containing microscopic mesothelial cells, fibrous tissue and lymphocytes with mild atypia was extracted (Fig. 2a-c). Therefore, PMP was suspected.
Operation: Because the patient complained of increasing abdominal distension and his abdominal pressure reached 35 mmHg, he underwent an emergency exploratory laparotomy. A significant amount of yellow, jelly-like mucus (approximately 5000 mL) was found during the operation (Fig. 3a). Numerous metastases were noted on the omentum and mesenteric root. After removing the mucus, we identified a hard mass measuring 10 cm × 15 cm with an unclear boundary and an abundant blood supply on the ileocecal junction (Fig. 3c). After carefully separating the appendix, the gangrenous rupture of the ileocecal tumour was observed, and the appendiceal lumen was interlinked with the abdomen. The patient’s small intestine and colon were expanded, but the colon’s expansion was more obvious, corresponding to low intestinal obstruction (Fig. 3b). Considering that explanations other than paralytic intestinal obstruction caused by the significant accumulation of intraperitoneal mucus might be plausible, we further explored the pelvic cavity. A hard mass measuring 4 × 5 cm with an unclear boundary infiltrating the rectal muscle layer was identified in the upper rectum (Fig. 3d). The peritoneal cancer index (PCI) was estimated intraoperatively, and the aggregative score of 13 abdominopelvic regions reached 20. We performed cytoreductive surgery (CRS), enterolysis, intestinal decompression and special tumour treatment to remove the lesions and relieve the obstruction as much as possible. Although some residual cancer remained, there was no nodule larger than 2.5 mm in diameter. Thus, we performed CC1 cytoreduction on the patient. Radical resection of the rectal carcinoma was also performed because the patient had PMP accompanied by rectal cancer.
The postoperative course was uneventful. The patient was discharged on postoperative day 15.
The postoperative histological pathologic diagnoses were appendiceal mucinous neoplasm, rectal cancer and PMP. The rectal cancer was a medium differentiated adenocarcinoma, approximately 50% of which was a mucinous adenocarcinoma. Serosa invasion, intestinal ulcerations and perineural invasion were noted, but vascular invasion was not observed (Fig. 4a). In the appendiceal mass, a crowded glandular epithelium with mild nuclear abnormalities, including the pseudo-layer arrangement, was noted. The tumour was LAMN (Fig. 4b). Moreover, numerous cavities containing mucus were observed in the fibrous tissue (Fig. 4c). The immunohistochemical staining of the rectal tumour revealed the following: PTEN (++), ERRCC1 (++), VEGF (++), TS (−), EGFR (++), HER2 (0), PMS2 (+), MLH1 (++), MSH2 (+++), MSH6 (+++), and MGMT (+) (Fig. 4d).
Postoperative histological pathologic diagnoses were appendiceal mucinous neoplasm, rectal cancer and PMP. The rectal cancer was medium differentiated adenocarcinoma (a). The appendiceal mass was low-grade appendiceal mucinous neoplasm (b). Large quantities of cavities containing mucus were observed in the fibrous tissue (c). Immunohistochemical staining of the rectal tumour revealed the following staining results: PTEN (++), ERRCC1 (++), VEGF (++), TS (−), EGFR (++), HER2 (0), PMS2 (+), MLH1 (++), MSH2 (+++), MSH6 (+++), and MGMT (+) (d)
Hyperthermic intraperitoneal chemotherapy (HIPEC) was not performed during the surgery because of disagreement among the patient’s family members. We strongly recommended that the patient receives chemotherapy or radiotherapy after surgery. However, to date, the patient did not receive these treatments due to economic difficulties. At the 1-year follow-up visit, no tumour recurrence was discovered by CT.
Discussion and conclusion
Incidence
PMP is a rare type of peritoneal secondary tumour. The incidence of PMP was initially proposed to be approximately 1 per million population per year [9]. The incidence of PMP has been estimated to be approximately 2 per million annually based on Smeenk’s research. According to experience at high volume centres, the actual incidence may be 3–4 operable cases per million per year [10]. Because limited data are available, the true incidence of PMP in the population is unknown [10,11,12]. PMP is difficult to diagnose preoperatively, and most diagnoses are confirmed by laparotomy or postoperative pathology [4].
Pathophysiology
Generally, the primary lesion originates from an adenoma, mucinous appendiceal adenocarcinoma or an ovarian tumour. However, primary peritoneal PMP has also been described. Indeed, case reports describing PMP originating from nearly every abdominal organ, including the fallopian tube, pancreas, intestine, urachus, and stomach, have been published, although some origins are unclear [13,14,15,16,17]. With the recent development of molecular diagnostic techniques, the appendix has been confirmed as the main organ affected by PMP, whereas the ovary is generally a secondary site [18]. Mucinous appendicular adenocarcinoma was the origin of PMP in this case.
PMP metastasis mainly occurs via implanted metastasis rather than lymphatic metastasis or haematogenous metastasis. Dissemination occurs by the rupture of the primary lesion, which releases tumour cells into the abdominal cavity. Tumour cells produce mucin and are responsible for the development of the characteristic “jelly belly” [19, 20].
Classification
The PMP histopathology and classification are confusing and challenging. PMP can be histologically divided into the following 3 types according to Ronnett’s classification: disseminated peritoneal adenomucinosis (DPAM), which tends to form benign lesions; peritoneal mucinous carcinomas (PMCA), which tends to form malignant tumours; and an intermediate type, which exhibits hybrid features of DPAM and PMCA [21]. DPAM is characterized by peritoneal lesions composed of abundant extracellular mucin containing scanty focally proliferative mucinous epithelium and small cytological atypia. PMCA is characterized by peritoneal lesions composed of more abundant mucinous epithelium with the architectural and cytologic features of carcinoma [22]. According to the WHO’s classification reported in 2010 evaluating the histogenesis, molecular genetic findings and clinical behaviour of PMP, PMP can be unanimously divided into low-grade and high-grade PMP. Low-grade PMP is characterized by mucin pools with low cellularity (< 10%), unremarkable cytology and a non-stratified cuboidal epithelium. High-grade PMP is characterized by mucin pools with high cellularity, moderate/severe cytologic atypia and a cribriform/signet ring morphology with desmoplastic stroma [23].
Clinical features
PMP is often asymptomatic during the initial stages and classically presents with vague abdominal symptoms when marked disease burden is noted. Patients often do not recall any acute abdominal pain associated with the tumour rupture. With increasing mucous accumulation, PMP can manifest as nausea, vomiting, anaemia, fatigue, loss of appetite, weight loss, ascites and other nonspecific symptoms [24, 25]. Intestinal obstruction is occasionally noted [26]. Upon physical examination, PMP patients exhibit increased abdominal girth, negative shifting dullness, dough kneading sensation upon abdominal palpation, abdominal tenderness and abdominal mass [27,28,29].
Image characteristics
PMP typically appears on ultrasound as a moderate amount of ascites containing septation and echoes, invasive parenchymal nodules and peritoneal masses. Serrated or scalloping changes around the liver, spleen, uterus and other abdominal organs are noted. One of the most specific signs is the presence of hypoechoic areas in the thickened peritoneum, which typically has a cake-like appearance on ultrasound [30,31,32,33,34].
High-frequency ultrasound can clearly show the pressure trace of the abdominal organs and occasionally reveal primary PMP lesions [35]. Colour Doppler ultrasonography can reveal branched or reticulate blood vessels passing through the mass [34, 36]. It is important for patients to undergo a cytological evaluation and ultrasound-guided biopsy, which is considered safe, simple and effective, to diagnose PMP preoperatively. The jelly-like mucinous material can be obtained with ultrasound-guided biopsy [37, 38]. In this case, the initial diagnosis of suspected PMP depended on the characteristic yellow, jelly-like mucus extracted though ultrasound-guided biopsy and exfoliative cytology of the mucus.
CT has become the first choice because it can show the distribution and infiltrated range of the primary PMP lesion. An abnormal density of ascites is noted in CT scans of PMP patients. Other features include a shell-like pressure trace on the surface of the liver and spleen, omentum thickening, peritoneal infiltration and mesentery and grid-like changes [39,40,41]. The calcification in the low-density area is also a specific finding in pseudomyxoma peritonei [39]. When severely invaded by the tumour, the omentum will become a large piece of soft tissue in front of the intestine and typically appears as a density shadow called “omental cake”. The small intestine can exhibit “cloak sign” in the CT reconstruction due to considerable mucus ascites extruding into one side or both sides of the spine [42,43,44]. The above signs represent characteristic CT features of PMP and are diagnostically helpful.
Magnetic resonance imaging (MRI) can show similar characteristics as CT, but MRI identifies the intestinal wall and the tumour’s boundary. Multi-directional MRI shows the relationship between the tumour and organ involvement, representing another advantage of MRI [45,46,47]. PET-CT is most useful for predicting peritoneal dissemination and evaluating the pathologic grade and potential for complete cytoreduction preoperatively [48]. The laparoscopic technology used for selective biopsy during the pathological examination can also collect ascites to identify the tumour cells, thereby improving the diagnostic accuracy. If the tumour widely transplants without surgical indications, HIPEC could be immediately performed through a peritoneal catheter under the guidance of a laparoscope as further treatment [49, 50].
Tumour markers
Currently, PMP has no specific tumour markers. CEA, CA199 and CA125 are useful for the auxiliary diagnosis of PMP and reflect the severity and prognosis of the disease [51, 52]. The postoperative survival time of patients negative for CEA, CA199 and CA125 was 2.6–fold higher than that of patients positive for the three tumour markers. The CA199-negative group not only received sufficient cytoreductive surgery more easily but also exhibited a significantly longer median time to recurrence than the CA199-positive group [48, 49, 53, 54]. Pirjo Nummela et al. found that pseudomyxoma peritonei tumour cells invariably express CEA and EpCAM. These authors propose that CEA and EpCAM could be exploited to develop targeted therapies against this malignancy [55]. Immunohistochemistry can aid in the diagnosis of PMP based on the following features: CK7 (+), CK20 (+), CDX2 (+), MUC2 (+), MUC5AC (+), ER (−) and PR (−) [56,57,58,59]. Next generation sequencing (GNAS) plays a prognostic role, and patients with GNAS mutations exhibited significantly poorer outcomes in terms of progression-free survival [60].
Treatment
Recently, CRS combined with HIPEC has been recommended as a standard treatment for PMP [61,62,63]. The completeness of cytoreduction (CC) is assessed at the end of surgery. By measuring the diameter of the largest remaining tumour lesion, the operation can be categorized as CC0 cytoreduction (complete removal of all visible lesion), CC1 cytoreduction (the largest residual lesion is < 0.25 cm), CC2 cytoreduction (0.25 cm ≤ largest residual lesion < 2.5 cm) and CC3 cytoreduction (the largest residual lesion is ≥2.5 cm). CC0 and CC1 are considered complete cytoreduction (CCRS), which is one of the most important prognostic factors for PMP [64,65,66,67]. The peritoneal carcinomatosis index (PCI) is widely used to assess the extent of disease. The abdomen could be divided into 9 anatomical areas with 4 further areas of the small bowel (upper and lower jejunum and upper and lower ileum) according to this scoring system. The tumour is accurately assessed in each area, and a score of 0–3 is given to each area (0 for no tumour, 1 for nodules < 0.5 cm, 2 for nodules between 0.5–5 cm and 3 for nodules > 5 cm) [68]. Although complete excision is not possible in some cases, maximal tumour debulking still offers significant survival advantages and significant improvements in the quality of life. Once cytoreduction is complete, HIPEC should be delivered. The cytotoxic drugs for HIPEC include 5FU, mitomycin C, doxorubicin, irinotecan, and cisplatin. An ex vivo assessment of drug sensitivity in PMP provides prognostic information. PMCA is slightly more resistant to platinum and 5FU than PMCA intermediate or disseminated peritoneal adenomucinosis. Tumour cells from patients previously treated with chemotherapy were generally less sensitive than those from untreated patients. Among patients with complete CRS, progression-free survival tends to be associated with the sensitivity to mitomycin C and cisplatin. Prior research further suggests that HIPEC could be used as a therapeutic adjunct to CRS, and a pretreatment assessment of drug sensitivity could benefit the individualization of HIPEC [69]. Unfortunately, HIPEC was not performed during the surgery due to disagreement in the patient’s family.
Compared with perioperative systemic chemotherapy (SC), SC after surgery exhibits remarkable effects. SC has minimal significance for LAMN, but a patient with high-grade appendiceal mucinous neoplasm can receive curative treatment [70]. Whole abdominopelvic radiotherapy using intensity-modulated arc therapy should be considered a palliative treatment option for the management of patients with recurrent PMP [71]. A study investigated the life quality of PMP patients who were treated by CRS combined with HIPEC and found that 79% of patients reported that they would accept this combined therapy again because it improved their lives. Moreover, their life quality was not reduced as a result of repeated treatments [72].
Prognosis
Regarding the outcome of patients treated with CRS and HIPEC, Terence C et al. investigated 2298 cases of PMP patients who were treated with CRS + HIPEC. The results revealed that the median overall survival time was 196 months (16.3 years). The median progression-free survival time was 98 months (8.2 years). The 3-year survival rate was 80%. The 5-year survival rate was 74%. The 10-year survival rate was 63%. The 15-year survival rate was 59%. The authors also reported the outcome of 242 patients treated with CRS without HIPEC and showed that the 5-year survival rate was 40%, while the 10-year survival rate was 27%. This research confirms that patients with PMP treated with CRS combined with HIPEC exhibit good long-term therapeutic outcomes and guarded prognoses [73].
PMP accompanied by rectal cancer is highly rare, and only 4 cases have been reported worldwide by 2018 [5,6,7,8]. Khaldi F et al. first reported Pseudomyxoma peritonei complicating cancer of the rectum in 1993 [5]. Saad-Hossne R et al. presented “Peritoneal pseudomyxoma associated with synchronic malignant mucinous neoplasias of the caecum, appendix and rectum. Case report and review of the literature” in 2007 [6]. Newman CM and Moran BJ reported pseudomyxoma peritonei presenting as recurrent rectal cancer in 2010 [7]. Pseudomyxoma anorectum was described by Wang S et al. in 2017 [8]. To the best of our knowledge, PMP induced by low-grade appendiceal mucinous neoplasm (LAMN) accompanied by rectal cancer has never been reported. Rectal cancer was not identified until the signs of low intestinal obstruction and obvious expansion of the colon were noted. Identifying the low intestinal obstruction, which is easily covered by paralytic intestinal obstruction, was key. On the one hand, the analysis of the paralytic intestinal obstruction revealed the accumulation of considerable mucus in the abdominal cavity, which caused severe alterations in neurological, body fluid and metabolic function, potentially causing paralytic ileus. On the other hand, acute complete obstruction caused by rectal cancer could also cause paralytic ileus due to the overexpansion of the intestine for an excessively long time.
This study presents a rare case of PMP induced by LAMN accompanied by rectal cancer. There are some typical characteristics of PMP in imaging features, clinical manifestation and treatments. A CT scan of the abdomen and pelvis and ultrasound-guided biopsy of the abdomen should be performed in patients with suspected PMP as soon as possible. It should also be emphasized that PMP is accompanied by rectal cancer in rare cases. Therefore, it is important to not overlook the possibility of rectal cancer.
Abbreviations
- CC:
-
Completeness of cytoreduction
- CRS:
-
Cytoreductive surgery
- CT:
-
Computed tomography
- DPAM:
-
Disseminated peritoneal adenomucinosis
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- LAMN:
-
Low-grade appendiceal mucinous neoplasm
- MRI:
-
Magnetic resonance imaging
- PMCA:
-
Peritoneal mucinous carcinomas
- PMP:
-
Pseudomyxoma peritonei
- SC:
-
Systemic chemotherapy
References
Sugarbaker PH, Ronnett BM, Archer A, et al. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233–80.
Pai RK, Longacre TA. Appendiceal mucinous tumors and Pseudomyxoma Peritonei: histologic features, diagnostic problems, and proposed classification. Adv Adv Anat Pathol. 2005;12(6):291–311.
Nakakura EK. Pseudomyxoma peritonei: more questions than answers. J Clin Oncol. 2012;30:2429–30.
Smeenk RM, Bruin SC, van Velthuysen ML, Verwaal VJ. Pseudomyxoma peritonei. Curr Probl Surg. 2008;45(8):527–75.
Khaldi F, Ben Chehida F, Mrabet S, et al. Iconographic rubric Pseudomyxoma peritonei complicating cancer of the rectum. Arch Fr Pediatr. 1993;50(1):55–6.
Saad-Hossne R, Prado RG, Bakonyi Neto A, Marchezan MA. Peritoneal pseudomyxoma associated with synchronic malignant mucinous neoplasias of the cecum, appendix and rectum. Case report and review of the literature. Acta Cir Bras. 2007;22(5):407–11.
Newman CM, Moran BJ. Pseudomyxoma peritonei presenting as recurrent rectal cancer: a preventable condition? Tech Coloproctol. 2011;15(1):89–90.
Wang S, Yin WB, Kong LY. Anal Fistulas Due to Pseudomyxoma Anorectum. Am J Med Sci. 2017;354(5):e9.
Sugarbaker (2007 Complete cytoreduction for pseudomyxoma peritonei (Sugarbaker technique). Guidance and guidelines NICE [Internet] https://www.nice.org.uk/guidance/ipg56. Accessed 24 Nov 2016.
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34(2):196–201.
Reel PJ. Cystic dilatation of the vermiform appendix. Ann Surg. 1917;65(6):743–6.
Dayal S, Taflampas P, Riss S, et al. Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum. 2013;56(12):1366–72.
Martínez A, Ferron Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12(3):585–603.
Lee SE, Jang JY, Yang SH, Kim SW. Intraductal papillary mucinous carcinoma with atypical manifestations: report of two cases. World J Gastroenterol. 2007;13(10):1622–5.
Smeenk RM, Bex A, Verwaal VJ, et al. Pseudomyxoma peritonei and the urinary tract: involvement and treatment related complications. J Surg Oncol. 2006;93(1):20–3.
Wang H, Wang X, Ju Y, et al. Clinicopathological features and prognosis of pseudomyxoma peritonei. Exp Ther Med. 2014;7(1):185–90.
Martínez A, Ferron G, Mery E, et al. Peritoneal pseudomyxoma arising from the urachus. Surg Oncol. 2012;21(1):1–5.
Bevan KE, Mohamed F, Moran B. Pseudomyxoma peritonei. World J Gastrointest. 2010; Oncol 2(1):44–50.
Galani E, Marx GM, Steer CB, Culora G, Harper PG. Pseudomyxoma peritonei: the 'controversial' disease. Int J Gynecol Cancer. 2003;13(4):413–8.
Sugarbaker PH. The subpyloric space: an important surgical and radiologic feature in pseudomyxoma peritonei. Eur J Surg Oncol. 2002;28(4):443–6.
Ronnett BM, Yan H, Kurman RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92(1):85–91.
Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous arcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19(12):1390–408.
Panarelli N, Yantiss R. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med. 2011;135(10):1261–8.
Esquivel J, Sugarbaker PH. Clinical presentation of the Pseudomyxoma peritonei syndrome. Br J Surg. 2000;87(10):1414–8.
Zhou Q, Shi F, Zhao HY, Li Y, Song QK. Clinicopathologic characteristics of pseudomyxoma peritonei. Zhonghua Bing Li Xue Za Zhi. 2018;47(3):192–5.
Kshirsagar AY, Kulkarni SH, Wader JV, Satwik AA, Vinchurkar KM. Pseudomyxoma peritonei presenting as sub-acute large bowel obstruction. J Indian Med Assoc. 2004;102(11):649–50.
Leinwand JC, Bates GE, Allendorf JD, et al. Body surface area predicts plasma oxaliplatin and pharmacokinetic advantage in hyperthermic intraoperative intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20(4):1101–4.
Shelekhova KV, Zhuravlev AS, Krylova DD, et al. Pseudomyxoma Peritonei as a first manifestation of KRAS-mutated urachal mucinous cystadenocarcinoma of the bladder: a case report. Int J Surg Pathol. 2017;25(6):563–6.
Nawaz A, Karakurum A, Weltman D, et al. Pseudomyxoma peritonei manifesting as intestinal obstruction. South Med J. 2000;93(9):891–3.
Chira RI, Nistor-Ciurba CC, Mociran A, Mircea PA. Appendicular mucinous adenocarcinoma associated with pseudomyxoma peritonei, a rare and difficult imaging diagnosis. Med Ultrason. 2016;18(2):257–9.
Lee YT, Lau JY, Chan KF, Chung SC, Sung JJ. Endosonographic features of pseudomyxoma peritonei. Gastrointest Endosc. 2000;51(1):85–8.
Khan S, Patel AG, Jurkovic D. Incidental ultrasound diagnosis of pseudomyxoma peritonei in an asymptomatic woman. Ultrasound Obstet Gynecol. 2002;19(4):410–2.
Krause J, Bergman A, Graf W, Nilsson A, Mahteme H. Ultrasonography findings and tumour quantification in patients with pseudomyxoma peritonei. Eur J Radiol. 2012;81(4):648–51.
Li Y, Guo A, Tang J, et al. Role of preoperative sonography in the diagnosis and pathologic staging of pseudomyxoma peritonei. J Ultrasound Med. 2013;32(9):1565–70.
Que Y, Tao C, Wang X, Zhang Y, Chen B. Pseudomyxoma peritonei: some different sonographic findings. Abdom Imaging. 2012;37(5):843–8.
Tsai CJ. Ultrasound features of disseminated adenomucinosis (pseudomyxoma). Br J Radiol. 1998;71(845):564–6.
Chen YN, Lee JJ, Cheng SP, Tsai CH. Transformation of low-grade mucinous neoplasm of the appendix with pseudomyxoma peritonei to high-grade sarcomatoid carcinoma. J Clin Med Res. 2015;7(7):571–4.
Darr U, Renno A, Alkully T, et al. Diagnosis of Pseudomyxoma peritonei via endoscopic ultrasound guided fine needle aspiration: a case report and review of literature. Scand J Gastroenterol. 2017;52(5):609–12.
Takahashi M, Takekawa S, Suzuki K, Takahashi M, Ishiwata J. CT findings of pseudomyxoma peritonei. Rinsho Hoshasen. 1989;34(12):1453–7.
Guilarte López-Mañas J, Cantero Hinojosa J, Mantas Avila JA. Computed tomography and sonographic features of pseudomyxoma peritonei. Rev EspEnferm Dig. 1997;89(10):798–9.
Sulkin TV, O'Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol. 2002;57(7):608–13.
Bouquot M, Dohan A, Gayat E, et al. Prediction of Resectability in Pseudomyxoma Peritonei with a new CT score. Ann Surg Oncol. 2018;25(3):694–701.
Matsuoka Y, Ohtomo K, Itai Y, et al. Pseudomyxoma peritonei with progressive calcifications: CT findings. Gastrointest Radiol. 1992;17(1):16–8.
Diop AD, Fontarensky M, Montoriol PF, Da ID. CT imaging of peritoneal carcinomatosis and its mimics. Diagn Interv Imaging. 2014;95(9):861–72.
Matsuoka Y, Masumoto T, Suzuki K, et al. Pseudomyxoma retroperitonei. J Eur Radiol. 1999;9(3):457–9.
Menassel B, Duclos A, Passot G, et al. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur J Surg Oncol. 2016;42(4):558–66.
Buy JN, Malbec L, Ghossain MA, Guinet C, Ecoiffier J. Magnetic resonance imaging of pseudomyxoma peritonei. Eur J Radiol. 1989;9(2):115–8.
Dubreuil J, Giammarile F, Rousset P, et al. FDG-PET/ceCT is useful to predict recurrence of Pseudomyxoma peritonei. Eur J Nucl Med Mol Imaging. 2016;43(9):1630–7.
Seshadri RA, Hemanth RE. Diagnostic laparoscopy in the pre-operative assessment of patients undergoing Cytoreductive surgery and HIPEC for peritoneal surface malignancies. Indian J Surg Oncol. 2016;7(2):230–5.
Kotani Y, Shiota M, Umemoto M, et al. Laparoscopic mucin removal in patients with pseudomyxoma peritonei. JSLS. 2009;13(2):203–36.
Kusamura S, Baratti D, Hutanu I, et al. The role of baseline inflammatory-based scores and serum tumor markers to risk stratify pseudomyxoma peritonei patients treated with cytoreduction (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol. 2015;41(8):1097–105.
van Ruth S, Hart AA, Bonfrer JM, Verwaal VJ, Zoetmulder FA. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9(10):961–7.
Canbay E, Ishibashi H, Sako S, et al. Preoperative carcinoembryonic antigen level predicts prognosis in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg. 2013;37(6):1271–6.
Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal Pseudomyxoma Peritonei: analysis of 519 patients. Eur J Surg Oncol. 2014;40(5):515–20.
Nummela P, Leinonen H, Järvinen P, et al. Expression of CEA, CA19-9, CA125, and EpCAM in pseudomyxoma peritonei. Hum Pathol. 2016;54:47–54.
Baratti D, Kusamura S, Nonaka D, Cabras AD, Laterza B, Deraco M. Pseudomyxoma peritonei: biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2009;249(2):243–9.
Ferreira CR, Carvalho JP, Soares FA, Siqueira SA, Carvalho FM. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. Int J Gynecol Cancer. 2008;18(1):59–65.
O'Connell JT, Hacker CM, Barsky SH. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol. 2002;15(9):958–72.
Nonaka D, Kusamura S, Baratti D, Casali P, Younan R, Deraco M. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology. 2006;49(4):381–7.
Pietrantonio F, Berenato R, Maggi C, et al. GNAS mutations as prognostic biomarker in patients with relapsed peritoneal pseudomyxoma receiving metronomic capecitabine and bevacizumab: a clinical and translational study. J Transl Med. 2016;14(1):125–31.
Virzì S, Iusco D, Bonomi S, Grassi A. Pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic chemotherapy: a 7-year single-center experience. Tumori. 2012;98(5):588–93.
Tsilimparis N, Bockelmann C, Raue W, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol. 2013;20(1):226–32.
Moran B, Cecil T, Chandrakumaran K, et al. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Color Dis. 2015;17(9):772–8.
Galan A, Rousset P, Mercier F, et al. Overall survival of pseudomyxoma peritonei and peritoneal mesothelioma patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy can be predicted by computed tomography quantified sarcopenia. Eur J Surg Oncol. 2018;44(11):1818–23.
Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007;14(8):2289–99.
Gomez-Portilla A, Sugarbaker PH, Chang D. Second-look surgery after cytoreductive and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg. 1999;23(1):23–9.
Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6(8):727–31.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Bjersand K, Mahteme H, Sundström Poromaa I, et al. Drug Sensitivity Testing in Cytoreductive Surgery and Intraperitoneal Chemotherapy of Pseudomyxoma Peritonei. Ann Surg Oncol. 2015. https://doi.org/10.1245/s10434-015-4675-0.
Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2014;109(7):740–5.
Berkovic P, van de Voorde L, De Meerleer G, et al. Whole abdominopelvic radiotherapy in the palliative treatment of pseudomyxoma peritonei. Strahlenther Onkol. 2014;190(2):223–8.
Kirby R, Liauw W, Zhao J, Morris D. Quality of life study following cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei including redo procedures. Int J Surg Oncol. 2013. https://doi.org/10.1155/2013/461041.
Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–56.
Acknowledgements
We acknowledge the contribution of the Department of General Surgery of the Second Hospital of Dalian Medical University for making all resources available and providing the technical support needed for the realization of this article.
Funding
This work was supported by Provincial Natural Science Foundation of Liaoning (No. 2015020316 to F.L.).
Availability of data and materials
The data supporting the conclusions of this article are included within the manuscript and its supplementary information files.
Author information
Authors and Affiliations
Contributions
SN and YY analysed the data, conducted the literature search and wrote the paper. CW took care of the patient. FL performed the operation and revised the manuscript. SN and YY contributed equally to the work and should be regarded as co-first authors. All the authors listed have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocols were approved by the Ethical Committee of the Second Affiliated Hospital of Dalian Medical University.
Consent for publication
Written informed consent for the publication of relevant medical information was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ning, S., Yang, Y., Wang, C. et al. Pseudomyxoma peritonei induced by low-grade appendiceal mucinous neoplasm accompanied by rectal cancer: a case report and literature review. BMC Surg 19, 42 (2019). https://doi.org/10.1186/s12893-019-0508-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-019-0508-6