Abstract
Background
Official guidelines recommend palliative treatments for patients with liver metastases from gastric cancer. However, many case series reported that hepatectomy for such cases is safe and effective. This systematic review compares the overall survival between hepatectomy and palliative therapy in patients with liver metastases from gastric cancer.
Methods
Two independent reviewers performed a systematic search of literature in EMBASE and PubMed, updated until 26 October 2016. The Newcastle-Ottawa score for cohort studies was used for quality assessment of included studies.
Results
A total of eight cohort studies involving 196 patients in the hepatectomy arm and 481 in the palliative arm were included. Median overall survival of patients in the two arms was 23.7 (range, 13.0 to 48.0) and 7.6 (range, 5.5 to 15.2), respectively. Median rates of overall survival of the two arms were 69, 40, 33 and 27, 8, 4% at 1, 2, and 3 years, respectively. Comparing with palliative therapy, hepatectomy was associated with significantly lower mortality at 1 year (odds ratio 0.17, P < 0.001) and 2 years (odds ratio 0.15, P < 0.001). Among the patients who underwent hepatectomy, Asian cohorts showed higher median rates of overall survival than Western cohorts at 1 year (76 vs. 60%), 2 years (47 vs. 30%) and 3 years (39 vs. 23%).
Conclusions
Hepatectomy in the management of liver metastases from gastric cancer can be considered effective. In the elective setting, hepatectomy provides a potential alternative to palliative therapy.
Similar content being viewed by others
Background
As the sixth highest incidence and the second leading cause of cancer deaths worldwide, gastric cancer is the most common form of cancer, with more than 951,000 new cases worldwide diagnosed in 2012 [1]. Due to late onset and nonspecific symptoms, the majority of gastric cancer cases present in advanced stage, with less than 30% of patients eligible for curative resection [2]. In recent decade, treatment of gastric cancer has been significantly improved, and the 5-year overall survival of patients with T1 tumors is up to 95% [3]. However, the prognoses of patients represented by peritoneal or liver metastases is extremely poor, with a 3-year overall survival lower than 10% after chemotherapy [4, 5].
Gastrectomy is more used in Eastern centers than Western centers. And Eastern patients’ prognoses after gastrectomy are better than those in Western [6]. According to the guideline of the Japanese Classification of Gastric Carcinoma [7], liver metastases from gastric cancer is categorized as stage IV disease. This guideline [7] and the National Comprehensive Cancer Network guideline [8, 9] do not recommend surgery for stage IV gastric cancer, which lead to most patients with liver metastases of gastric cancer receive palliative treatment (such as chemotherapy). Though the necessity of hepatectomy for liver metastases of gastric cancer is still controversial, the Guidelines Committee of the Japan Gastric Cancer Association reconsidered the treatment of potentially resectable M1 disease [10].
Recently, many case-control or case series studies of liver metastases from gastric cancer have been reported. However, most of these studies are presented from a single center, have a small sample size, and/or include old cases from the 1960s and 1980s [11–20]. Therefore, it is important to thoroughly analyze the significance of hepatectomy for liver metastases from gastric cancer, and compare the efficacy of hepatectomy with palliative therapy for such patients in recent two decades (1990–2016) with a systematic review.
Methods
Two independent authors performed a systematic review (Y.-Y.L, D.L) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. A study protocol was followed which defined the study objectives, eligibility criteria, outcome measures, search strategy and methodology of analysis. The quality of included studies was assessed using the Newcastle-Ottawa score for cohort studies [22]. This tool was chosen because of the unavailability of randomized controlled trials and large heterogeneity between studies.
Search strategy
A systematic search of literature, updated until 26 October 2016, was performed by two independent reviewers (Y.-Y.L, D.L). The EMBASE and PubMed databases were searched using MeSH and free text words. MeSH and free text words concerning gastric cancer and metastasis were used. No language or time period restrictions were applied. Titles and abstracts retrieved from the search were screened for relevance and selected studies. Disagreements during the search and selection process were resolved by discussion and, if needed, a third reviewer (N.-F.P) was consulted to reach consensus. Reference lists of all included articles were screened for additional eligible papers.
The following search strategy was used in PubMed (Medline):
((“cancer” [Mesh] AND “gastric Neoplasms” [Mesh]) OR “neoplasm, stomach” [Mesh] OR stomach neoplasm*[tw] OR gastric neoplasm*[tw] OR cancer of stomach*[tw] OR stomach cancer*[tw] OR gastric cancer*[tw]) AND (“Metastases, Neoplasm” [Mesh] OR metastasis*[tw] OR metastases*[tw]) AND (surgery*[tw] OR resection*[tw] OR hepatectomy*[tw]) AND (hepatic*[tw] OR liver*[tw]).
Eligibility criteria and data extraction
Criteria for final study inclusion were: (1) study population formed by patients with liver metastases from gastric cancer in the absence of peritoneal metastasis or extractable from studies in which hepatectomy was performed; (2) sufficient description of the study population; (3) description of patient survival rates for at least 1 year after hepatectomy; (4) the study had a randomized control, cohort, or case-control design. In order to reduce the bias of diagnosis, original treatments, neoadjuvant and adjvuant treatments, only cases after 1990 may be included. In cases in which a study was followed by a more complete study or studies that included the original data set, the most recent and complete report was chosen. Such linked studies were identified on the grounds of authorship, institutions, design, length of follow-up, and study populations. If additional data or results were needed, the corresponding author of each report was contacted by e-mail.
Data were extracted by two authors (Y.-Y.L, D.L) using standardized forms. The following data were collected: author details, country, recruitment period, study design, median follow-up, sample size, gender, positive and negative findings, and methodological quality. A third author (N.-F.P) checked the extracted data against the original studies. Survival data were taken directly from tables or the text whenever possible; if such data were presented only in graphs, they were extracted by manual interpolation. P values associated with inter-group differences in mortality were extracted directly from survival curves, text, or tables wherever possible.
Statistical analysis
Meta-analysis was performed on an intention-to-treat basis. To assess attrition bias, we calculated mortality using a ‘worst-case’ approach in which patients with missing data were counted as treatment failures (death). For patients with missing data, we ’carried forward’ data from the most recent measurement.
Outcomes are displayed as they were reported in the original article. All statistical tests for this meta-analysis were performed using Stata 11.0 softwares. Due to the high likelihood of mortality, odds ratio (ORs) with corresponding 95% confidence intervals (95% CIs) were calculated for dichotomous outcomes using the Mantel-Haenszel method. Point estimates of RR were considered statistically significant when P < 0.05. Statistical heterogeneity was explored by inconsistency (I 2) statistics; in particular, I 2 values of <25% was interpreted as low heterogeneity, between 25 and 50% as medium, between 50 and 75% as substantial and above 75% as considerable [23].
Results
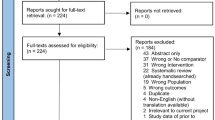
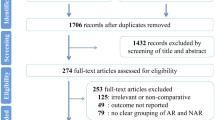
A total of 3629 records were identified. Following screening for duplications, 2108 articles remained. After screening by title and abstract, a further 2057 records were excluded. Of the remaining 52 articles which need full-text assessment, 35 were studies with single arm investigating the role of hepatectomy for liver metastases from gastric cancer [11–20, 24–48]. In the end, only 8 studies compared the efficacy of hepatectomy to that of other palliative treatments met the eligibility criteria and were included in a quantitative synthesis [49–56]. Details are listed in Fig. 1.
Baseline characteristics
Five of the studies based on Eastern population [49, 50, 52, 53, 56] while the other three based on Western population [51, 54, 55]. These eight studies included 196 patients in the hepatectomy arm and 481 in the palliative arm. Most patients in the hepatectomy arm (70%) underwent minor hepatectomy. No study described complications during or after hepatectomy. Patient demographics and characteristics of liver metastases from gastric cancer are summarized in Table 1. The median follow-up was 13.2 months.
Efficacy
Median overall survival of patients in the hepatectomy arm and those in the palliative arm was 23.7 (range, 13.0 to 48.0) and 7.6 (range, 5.5 to 15.2), respectively. Median rates of overall survival of the two arms were 69, 40, 33 and 27, 8, 4% at 1, 2, and 3 years, respectively (Table 2).
Comparing with palliative therapy, hepatectomy was associated with significantly lower mortality at 1 year (OR 0.17, 95% CI 0.11–0.26, P < 0.001; Fig. 2) and 2 years (OR 0.15, 95% CI 0.09–0.25, P < 0.001; Fig. 3).
Among the patients who underwent hepatectomy, comparison of studies performed in Asian countries (5 studies, 117 patients) and Western countries (3 studies, 79 patients) indicated that Asian cohorts showed higher median rates of overall survival at 1 year (76 vs. 60%), 2 years (47 vs. 30%) and 3 years (39 vs. 23%).
Quality assessment
Details on individual quality of studies are displayed in Table 3. Among the 8 cohort studies, only one had eight stars [50], one had seven stars [52], and the remaining 6 studies had six stars [49, 51, 53–56].
Discussion
Among all included studies in this systematic review, a total of 196 patients were treated with hepatectomy for liver metastases from gastric cancer. Hepatectomy was associated significantly better overall survival than palliative therapy for such patients. Our results were consistent with previous reviews [57–60].
The liver is a common site of distant metastasis from gastrointestinal tract cancer, including gastric cancer. Hepatectomy is now widely accepted as a potentially curative treatment for hepatocellular carcinoma [61, 62] and colorectal liver metastases [63], with reported 5-year overall survival of 40–50% [61–63]. Moreover, indications of hepatectomy for hepatocellular carcinoma and colorectal liver metastases have been expanded by progress in surgical procedures, perioperative care and/or chemotherapy. Liver metastases from gastric cancer not only show more aggressive oncological behavior and heterogeneous characteristics, but also are with frequently other metastatic extrahepatic lesions, such as peritoneal seeding or extensive lymph node metastases, leading to a dismal prognosis and debatable benefits of hepatectomy. Therefore, chemotherapy is regarded as the first-choice treatment in most guidelines. However, a lot of case reports and case series supported the role of hepatectomy for liver metastases from gastric cancer [11–20, 24–48], which lead to Japanese guideline reconsidered the role of hepatecotmy for such patients [10].
Previous systematic reviews [57–60] were mainly based on some part of the case series [11–20, 24–48]. Many of the quoted and included studies of these systematic reviews are of small studies stretching back in some cases to the 1960’s. This leads to many concerns over the diagnosis, original treatments, neoadjuvant and adjuvant treatments in such a big time span. Assessment of metastatic disease in the pre multilevel computer tomography and positron emission tomography era is also doubtful when trying to compare outcomes. In addition, those systematic reviews are not comparing like with like. Those undergoing hepatectomy are a highly selected group with resectable liver disease in the absence of other disease. It is not reasonable to compare this with all comers with widespread metastatic disease. It would be more reasonable to cone in on those studies where comparative outcomes were considered. Therefore, we only focus on cohort studies which comparing hepatectomy and palliative therapy and patients who were included after 1990. Such results may be more reliable than previous.
Our systematic review revealed that median overall survival after hepatectomy was 23.7 (range, 13.0 to 48.0). Median overall survival was ranges from 24 to 40.8 months after hepatectomy among other large scale case series (>60 cases) [40, 46–48]. Hepatectomy for liver metastases from gastric cancer is not performed frequently at present. It is notable that none of the patients who underwent hepatectomy in this systematic review or these case series [40, 46–48] was with peritoneal metastasis. Careful patient selection is likely to be important for ensuring good prognosis after hepatectomy. The number of hepatic tumors (≥3), tumor diameter (≥5), and serosal invasion of the primary tumor were identified as prognostic factors associated with a poor overall survival [19, 37, 40, 46]. Moreover, patients with more risk factors have much poor survival rates at 3 or 5 years after hepatectomy [46]. Therefore, palliative therapy should be considered when any of these factors is recognized at diagnosis. Other indications for hepatectomy included adequate physical condition, preserved liver function, and feasibility of complete tumor resection. In addition, indications for hepatectomy may also need to consider response rate to neoadjuvant chemotherapy in those patients who receive it, since prognosis of non-responders is generally worse than that of responders [64, 65].
The role of neoadjuvant or adjuvant therapy for liver metastases from gastric cancer is still controversial. Of the eight included studies, 16.4% patients received neoadjuvant therapy, while 55.2% received adjuvant therapy. If all case series [11–20, 24–48] and these eight cohort studies [49–56] were included into analysis, the percentage of patients who received neoadjuvant therapy for the primary gastric cancer was 11.3%, and the percentage of patients receiving adjuvant therapy was 55.1%. For patients with gastric cancer with extensive lymph node metastasis, Tsuburaya and coworkers found neoadjuvant chemotherapy with 4-weekly S-1 and cisplatin followed by D2 gastrectomy is safe and effective for some patients [66]. Further trials should be performed to investigate the role of neoadjuvant therapy as a biological trial.
We found Asian cohorts showed higher median rates of overall survival than Western cohorts. Western cohorts usually show more advanced stage of disease than Asian cohorts [19, 24, 28, 41, 43, 50, 51, 54, 55]. Such presentation may lead to more Western cases receive chemotherapy while more Asian cases receive curative resection. Even so, there needs to be consideration of the role of chemotherapy versus surgery in the chemotherapy naïve patients. In this systematic review, hepatectomy associated with a median overall survival of 23.7 months. However, median overall survival was only 11.3 to 13.8 months after combination therapy with or without trastuzumb for advanced gastric cancer [4, 67]. Therefore, our results suggest that hepatectomy is associated with substantially longer median overall survival than combination chemotherapy with or without targeted therapy. In selected patients, hepatectomy may be preferable to chemotherapy.
A limitation of our study is the low availability of high level evidence studies in literature. Since all studies were retrospective cohort studies, a potential bias is that the positive effects of hepatectomy are overestimated, as cases of unsuccessful resection are less likely to be reported or published. Moreover, the types and frequency of postoperative complications remain unclear because all of the studies failed to report such data. Most patients underwent minor rather than major hepatectomy. Heterogeneity in surgical technique and skill may also affect patient prognosis.
Conclusions
This systematic review provides comprehensive evidence that hepatectomy is associated with longer median overall survival than palliative treatments for selected patients with liver metastases from gastric cancer. If our findings can be verified and extended in a completion of a randomized controlled trial on hepatectomy versus chemotherapy or a high-quality prospective study with long-term follow-up, they make a strong argument for changing current clinical practices and official guidelines to bring them into line with the evidence base.
Abbreviations
- CI:
-
Confidence interval
- OR:
-
Odds ratio
References
http://www.who.int/mediacentre/factsheets/fs297/en/[Internet]. Accessed 19 Sept 2016.
National Oesophago-gastric Cancer Audit—2013, Annual report [Internet]. 2013. http://www.hscic.gov.uk/catalogue/PUB11093. Accessed 19 Sept 2016.
Yoshikawa T, Sasako M. Gastrointestinal cancer: adjuvant chemotherapy after D2 gastrectomy for gastric cancer. Nat Rev Clin Oncol. 2012;9(4):192–4.
Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):481–9.
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–21.
Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20(7):2328–38.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer. 2011;14(2):101–12.
Strong VE, D'Amico TA, Kleinberg L, Ajani J. Impact of the 7th edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw. 2013;11(1):60–6.
Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):531–46.
Kodera Y, Fujitani K, Fukushima N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014;17(2):206–12.
Ambiru S, Miyazaki M, Ito H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181(3):279–83.
Fujii K, Fujioka S, Kato K, et al. Resection of liver metastasis from gastric adenocarcinoma. Hepato-Gastroenterology. 2001;48(38):368–71.
Miyazaki M, Itoh H, Nakagawa K, et al. Hepatic resection of liver metastases from gastric carcinoma. Am J Gastroenterol. 1997;92(3):490–3.
Ochiai T, Sasako M, Mizuno S, et al. Hepatic resection for metastatic tumours from gastric cancer: analysis of prognostic factors. Br J Surg. 1994;81(8):1175–8.
Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235(1):86–91.
Saito A, Korenaga D, Sakaguchi Y, Ohno S, Ichiyoshi Y, Sugimachi K. Surgical treatment for gastric carcinomas with concomitant hepatic metastasis. Hepato-Gastroenterology. 1996;43(9):560–4.
Saiura A, Umekita N, Inoue S, et al. Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepato-Gastroenterology. 2002;49(46):1062–5.
Sakamoto Y, Ohyama S, Yamamoto J, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133(5):507–11.
Schildberg CW, Croner R, Merkel S, et al. Outcome of operative therapy of hepatic metastatic stomach carcinoma: a retrospective analysis. World J Surg. 2012;36(4):872–8.
Zacherl J, Zacherl M, Scheuba C, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg. 2002;6(5):682–9.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206.
Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244(4):524–35.
Aizawa M, Nashimoto A, Yabusaki H, Nakagawa S, Matsuki A. Clinical benefit of surgical management for gastric cancer with synchronous liver metastasis. Hepato-Gastroenterology. 2014;61(133):1439–45.
Baek HU, Kim SB, Cho EH, et al. Hepatic resection for hepatic metastases from gastric adenocarcinoma. J Gastric Cancer. 2013;13(2):86–92.
Choi SB, Song J, Kang CM, et al. Surgical outcome of metachronous hepatic metastases secondary to gastric cancer. Hepato-Gastroenterology. 2010;57(97):29–34.
Garancini M, Uggeri F, Degrate L, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB. 2012;14(3):209–15.
Hirai I, Kimura W, Fuse A, et al. Surgical management for metastatic liver tumors. Hepato-Gastroenterology. 2006;53(71):757–63.
Imamura H, Matsuyama Y, Shimada R, et al. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96(11):3178–84.
Komeda K, Hayashi M, Kubo S, et al. High survival in patients operated for small isolated liver metastases from gastric cancer: a multi-institutional study. World J Surg. 2014;38(10):2692–7.
Liu J, Li JH, Zhai RJ, Wei B, Shao MZ, Chen L. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J. 2012;125(2):165–71.
Morise Z, Sugioka A, Hoshimoto S, et al. The role of hepatectomy for patients with liver metastases of gastric cancer. Hepato-Gastroenterology. 2008;55(85):1238–41.
Nomura T, Kamio Y, Takasu N, et al. Intrahepatic micrometastases around liver metastases from gastric cancer. J Hepato-Biliary-Pancreat Surg. 2009;16(4):493–501.
Qiu JL, Deng MG, Li W, et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013;39(7):694–700.
Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU. Outcome of hepatic resection for metastatic gastric cancer. Am Surg. 2005;71(2):95–9.
Sakamoto Y, Sano T, Shimada K, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95(7):534–9.
Schmidt B, Look-Hong N, Maduekwe UN, et al. Noncurative gastrectomy for gastric adenocarcinoma should only be performed in highly selected patients. Ann Surg Oncol. 2013;20(11):3512–8.
Shirabe K, Shimada M, Matsumata T, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepato-Gastroenterology. 2003;50(53):1560–3.
Takemura N, Saiura A, Koga R, et al. Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg. 2012;397(6):951–7.
Thelen A, Jonas S, Benckert C, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol. 2008;34(12):1328–34.
Tsujimoto H, Ichikura T, Ono S, et al. Outcomes for patients following hepatic resection of metastatic tumors from gastric cancer. Hepatol Int. 2010;4(1):406–13.
Vigano L, Vellone M, Ferrero A, Giuliante F, Nuzzo G, Capussotti L. Liver resection for gastric cancer metastases. Hepato-Gastroenterology. 2013;60(123):557–62.
Wang W, Liang H, Zhang H, Wang X, Xue Q, Zhang R. Prognostic significance of radical surgical treatment for gastric cancer patients with synchronous liver metastases. Medical oncology (Northwood, London, England). 2014;31(11).
Wang YN, Shen KT, Ling JQ, et al. Prognostic analysis of combined curative resection of the stomach and liver lesions in 30 gastric cancer patients with synchronous liver metastases. BMC Surg. 2012;12:20.
Kinoshita T, Saiura A, Esaki M, Sakamoto H, Yamanaka T. Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br J Surg. 2015;102(1):102–7.
Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric cancer. 2016. doi:10.1007/s10120-016-0604-6. [Epub ahead of print].
Oki E, Tokunaga S, Emi Y, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer. 2016;19(3):968–76.
Chen L, Song MQ, Lin HZ, et al. Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol. 2013;19(13):2097–103.
Cheon SH, Rha SY, Jeung HC, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19(6):1146–53.
Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, et al. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer. 2012;15(2):131–6.
Makino H, Kunisaki C, Izumisawa Y, et al. Indication for hepatic resection in the treatment of liver metastasis from gastric cancer. Anticancer Res. 2010;30(6):2367–76.
Miki Y, Fujitani K, Hirao M, et al. Significance of surgical treatment of liver metastases from gastric cancer. Anticancer Res. 2012;32(2):665–70.
Tiberio GA, Coniglio A, Marchet A, et al. Metachronous hepatic metastases from gastric carcinoma: a multicentric survey. Eur J Surg Oncol. 2009;35(5):486–91.
Tiberio GA, Baiocchi GL, Morgagni P, et al. Gastric cancer and synchronous hepatic metastases: is it possible to recognize candidates to R0 resection? Ann Surg Oncol. 2015;22(2):589–96.
Ueda K, Iwahashi M, Nakamori M, et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394(4):647–53.
Petrelli F, Coinu A, Cabiddu M, et al. Hepatic resection for gastric cancer liver metastases: a systematic review and meta-analysis. J Surg Oncol. 2015;111(8):1021–7.
Grimes N, Devlin J, Dunne DF, Poston G, Fenwick S, Malik H. The role of hepatectomy in the management of metastatic gastric adenocarcinoma: a systematic review. Surg Oncol. 2014;23(4):177–85.
Martella L, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for liver metastases from gastric cancer: a meta-analysis of observational studies. Medicine (Baltimore). 2015;94(31):e1113.
Markar SR, Mikhail S, Malietzis G, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg. 2016;263(6):1092–101.
Zhong JH, You XM, Lu SD, et al. Historical comparison of overall survival after hepatic resection for patients with large and/or multinodular hepatocellular carcinoma. Medicine (Baltimore). 2015;94(35):e1426.
Zhong JH, Rodriguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore). 2015;94(3):e396.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Kurokawa Y, Shibata T, Sasako M, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17(3):514–21.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101(6):653–60.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010;376(9742):687–97.
Acknowledgements
None.
Funding
This work was supported by the Guangxi University of Science and Technology Research Projects (KY2015LX056), the Self-Raised Scientific Research Fund of the Ministry of Health of Guangxi Province (Z2015621, GZZC15-34, Z2016512), the Innovation Project of Guangxi Graduate Education (YCBZ2015030), and the National Natural Science Foundation of China (81560460/H1602, 81260088/H0322).
Availability of data and materials
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Authors’ contributions
J-HZ conceived and designed the study. Y-YL, DL, P-CY, and SZ searched the literature and extracted data. NFP and L-QL performed statistical analyses. All authors wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors who have taken part in this study declare that they do not have anything to disclose regarding funding or conflicts of interest with respect to this manuscript.
Consent for publication
“Not applicable” in this section.
Ethics approval and consent to participate
“Not applicable” in this section.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liao, YY., Peng, NF., Long, D. et al. Hepatectomy for liver metastases from gastric cancer: a systematic review. BMC Surg 17, 14 (2017). https://doi.org/10.1186/s12893-017-0215-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-017-0215-0