Abstract
Background
The significance of pneumatosis intestinalis (PI) and portal venous gas (PVG) is controversial. This retrospective study evaluated the risk factors for bowel necrosis in patients with PI and/or PVG.
Methods
Between 2002 and 2015, 52 patients were diagnosed with PI and/or PVG and were included in this study. The patients were classified according to the presence or absence of bowel necrosis in surgical findings or at autopsy. Patient characteristics and clinical findings related to bowel necrosis were investigated.
Results
Bowel necrosis was diagnosed in 17 (32.7 %) patients. Amongst these 17, 10 patients received salvage surgical intervention, and seven of those diagnosed with bowel necrosis survived after the operation. The remaining 35 patients received conservative treatment with or without exploratory laparotomy. Between patients with and without bowel necrosis, laboratory data revealed significant differences in the levels of C-reactive protein (P = 0.0038), creatinine (P = 0.0054), and lactate (P = 0.045); clinical findings showed differences in abdominal pain (P = 0.019) and peritoneal irritation signs (P = 0.016); computed tomography detected ascites (P = 0.011) and changes of bowel wall enhancement (P = 0.03) that were significantly higher in patients with bowel necrosis. The rate of PI and/or PVG detected in patients postoperatively was significantly higher in patients with bowel necrosis (P < 0.0001). Multivariate analysis showed that bowel necrosis was significantly more likely when PI or PVG was detected in postoperative patients than in patients who had not had surgery (P = 0.003).
Conclusions
PI and/or PVG, alone, are not automatically indicative of bowel necrosis. However, when these conditions occur postoperatively, they indicate bowel necrosis requiring reoperation.
Similar content being viewed by others
Background
Pneumatosis intestinalis (PI) and the presence of hepatic portal venous gas (PVG) have long been thought to predict bowel necrosis and an associated poor prognosis [1–3]. However, widespread use of computed tomography (CT) has allowed better and more frequent detection of these conditions [4, 5]. PI and PVG are suggested to have various sequelae, including bowel necrosis, which are associated with poor patient outcomes. However, the diagnosis of bowel necrosis is often difficult in patients with PI and/or PVG. Accordingly, we investigated patients with PI/PVG to identify the predictors of bowel necrosis.
Methods
Study groups
Between January 2002 and January 2014, abdominal CT scan was used to diagnose PI/PVG in 57 patients at the National Center for Global Health and Medicine (Tokyo, Japan). Five patients were excluded from the study due to 1) disturbed consciousness or cardiopulmonary arrest upon arrival, 2) post-mortem CT, or 3) patient age < 18 years. The remaining 52 patients were classified as having or not having bowel necrosis. Bowel necrosis was diagnosed based on surgical findings and/or postoperative pathologic examinations or autopsies. The clinical characteristics, laboratory/imaging findings, and condition severity of the patients in the two groups were compared to identify the indicators of bowel necrosis.
CT image interpretation
Study group information was extracted from a database containing interpretations of imaging studies performed by experienced radiologists, using the key words: “pneumatosis intestinalis (PI)” and “portal venous gas (PVG).” Gas within 2 cm of the liver border in the contrast-enhanced images was defined as PVG [1]. If a contrast-enhanced CT image showed a decrease in bowel wall enhancement, bowel ischemia was suspected.
PVG evaluation and PI distribution
Patients with PVG were evaluated to determine whether the amount of gas was a predictor of outcome. PVG generally expands from the left lobe of the liver to the right anterior lobe and then to the right posterior lobe as the amount of gas increases [6]. Therefore, we compared patients with gas confined to the left lobe to those with gas in the right lobe or in both lobes. PI severity is reported to be related to its distribution [7], so patients with PI were classified according to whether they had a bubble type or linear gas pattern.
Severity evaluation
Disease severity was evaluated using the following three scoring systems: (1) Acute Physiology and Chronic Health Evaluation II (APACHE II) score; (2) Sequential Organ Failure Assessment (SOFA) score; and (3) Systemic Inflammatory Response Syndrome (SIRS) score. These scores were calculated using clinical findings and laboratory parameters at the time PI/PVG was diagnosed.
Statistical analysis
Statistical analyses were performed using JMP 10 (SAS Institute, Cary, NC, USA). Univariate analyses were performed using chi-square and Fisher’s exact tests; multivariate analyses were performed using logistic regression analysis. A probability (P) value < 0.05 indicated statistical significance.
Results
Patient characteristics
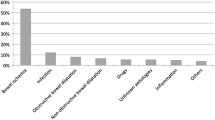
The 52 patients comprised 32 (61.5 %) men and 20 (38.5 %) women, with a mean age of 70.4 years (range, 19–97 years; median, 74 years) (Table 1). Their underlying diseases included diabetes, respiratory disease, oncologic disease, collagen disease, cardiovascular disease, renal disease, hematological disorder, and inflammatory bowel disease; 16 patients (30.7 %) were taking steroids. Of the included patients, 17 were diagnosed with bowel necrosis, and 35 patients were determined not to have bowel necrosis. In patients with bowel necrosis, diagnoses included ileus (n = 6), non-occlusive mesenteric ischemia (NOMI; n = 3), superior mesenteric artery thromboembolism (n = 2), bowel perforation (n = 2), ischemic enteritis (n = 2), fatal toxic megacolon (n = 1), and postoperative abscess (n = 1). In patients without necrosis, diagnoses ranged from infectious enteritis to simple obstruction; the cause of symptoms was unknown in 11 patients. Of the 17 patients with bowel necrosis, 7 (41.2 %) survived, but required surgical intervention (Table 1). Ten of the 17 patients died during their hospitalization. Of the 35 patients without necrosis, 2 died due to pneumonia. The incidence of postoperative PI/PVG detection was significantly higher in patients with bowel necrosis (P < 0.0001); none of the other patient characteristics was associated with the incidence of detection.
Clinical, laboratory, and imaging findings
Clinical findings included abdominal pain, abdominal distension, nausea and vomiting, peritoneal irritation signs, and melena (Table 1). A significant difference was observed between the two groups with respect to abdominal pain (P = 0.019) and peritoneal irritation signs (P = 0.016). Conversely, there were no significant differences in vital signs. There were also significant differences in the levels of C-reactive protein (CRP) (P = 0.0038), creatinine (P = 0.0054), and lactate (P = 0.045) between the two groups (Table 1). In patients with bowel necrosis, levels of CRP (10.56 ± 4.01 mg/dL), creatinine (1.7 ± 0.4 mg/dL), and lactate (4.54 ± 2.03 mmol/L) were higher than the levels in patients without bowel necrosis. With respect to patient condition severity, statistically significant differences were found between patients with and without bowel necrosis in the APACHE II, SOFA, and SIRS scores. The APACHE II (12 ± 2.9), SOFA (2.8 ± 0.9), and SIRS (1.1 ± 0.5) scores were higher in patients with bowel necrosis. Among all patients, CT images revealed abdominal ascites in 18 (34.6 %) and free air in 20 (38.5 %) (Table 1); bowel wall enhancement changes were observed in 17 patients (32.7 %).
PI was detected in 45 patients (86.5 %), and PVG was found in 20 (38.5 %), with 13 patients (25 %) exhibiting both; the presence of PI and/or PVG was not significantly related to bowel ischemia, however. Among the 20 patients with PVG, gas was confined to the left lobe in 12 patients and was seen in the right or in both lobes in 8. No gas location was significantly associated with obowel ischemia (P = 0.71). Bowel necrosis was more frequent in patients with a linear gas pattern, but the difference was not significant (P = 0.082). However, changes in bowel wall enhancement were significantly more frequent in patients with bowel necrosis (P = 0.03).
Treatment and outcomes
Surgery was performed on 11 (21.2 %) patients, and 41 (78.8 %) were treated conservatively (Table 2). Operations included right hemicolectomy with massive bowel resection (n = 4), alleviation of ileus and partial small bowel resection (n = 2), intraperitoneal irrigation and drainage (n = 2), Hartmann’s operation (n = 1), and exploratory laparotomy (n = 2). One patient who underwent exploratory laparotomy had a preoperative diagnosis of sigmoid colon necrosis, but the laparotomy did not reveal bowel necrosis. The other patient undergoing exploratory laparotomy had massive necrosis extending from the stomach to the large bowel, and definitive treatment was considered impossible. This patient died. Conservative treatment included bowel rest only (n = 13); observation plus antibiotic therapy (n = 17); decompression via a gastric/ileus tube (n = 6); oxygen therapy, including hyperbaric oxygen therapy (n = 3); and other treatments (n = 5). Bowel necrosis was suspected in 8 patients receiving conservative treatment, but laparotomies were not performed for various reasons. Seven of these 8 patients died from deterioration of their general condition due to bowel necrosis or the underlying disease. One patient, who probably had transient bowel ischemia, demonstrated an improved clinical state and achieved a good outcome.
Univariate/multivariate analyses
In univariate analyses, abdominal pain, peritoneal irritation, higher CRP, creatinine, and lactate, CT findings of ascites or reduced bowel wall enhancement, and linear PI pattern were associated with the presence of bowel necrosis. Postoperative patients with PI or PVG were more likely to have bowel necrosis than patients who had not had surgery (P = 0.003) (Table 3).
Characteristics of patients with postoperatively detected PI/PVG
Postoperative PI/PVG was detected in 9 (17.3 %) patients (Table 4). Eight had PI, 4 had PVG, and 3 had both. Eight of these patients had bowel necrosis; 5 died, and 3 were successfully treated with further surgical intervention. One patient developed PI/PVG and NOMI was suspected after aortic replacement for an abdominal aortic aneurysm; this individual was treated conservatively.
Discussion
PI is the presence of gas within the submucosa or subserosa of the intestine. A previous study found that the mean age of PI patients is 56.6 ± 19.4 years and that 57 % of patients were male [8]. PI is a rare disease and is still poorly understood. Four main theories exist, positing that PI is caused by: (1) increased intraluminal pressure derived from intestinal obstruction, colonoscopy, or gastroenteric tumor (mechanical theory); (2) pulmonary alveolar rupture resulting from pulmonary diseases, such as chronic obstructive pulmonary disease or asthma (pulmonary theory); (3) gas produced by gas-forming bacteria that enter the mucosal barrier (bacterial theory); or (4) α-glucosidase inhibitors (chemical theory) [9]. However, each theory alone also fails to account for the cause of PI [2, 3].
PVG is the presence of air in the portal venous system. The mechanism for the appearance of gas in the portal vein is also not well understood. There are two major theories: (1) gas dissects into the intestinal wall from the intestinal lumen, or lung and intestinal mucosal damage allows intraluminal gas to enter the venous system (Mechanical theory); and (2) gas-forming bacteria enter the mucosal barrier and produce gas within the intestinal wall, which then enters into the portal vein system (bacterial theory) [4, 10].
Previous reports have suggested that PI and PVG usually originate from bowel necrosis, via similar mechanisms, and are often detected concomitantly in clinical situations. However, PI and PVG have been studied and treated as independent symptoms in many publications. In the current report, we treated patients with PI and/or PVG as having one condition [5].
PI was previously thought to predict poor outcomes due to the development of bowel necrosis, but DuBose et al. evaluated 500 patients with PI and reported that it was not necessarily associated with bowel necrosis [8]. Similarly, PVG was also thought to be indicative of a poor prognosis due to the development of bowel necrosis. This is supported by the findings of Kinoshita et al., who reported that the overall mortality rate for patients with PVG was 39 %, increasing to 75 % in patients with bowel necrosis and only 11.7 % in patients without necrosis [4]. Furthermore, 97 % of PVG cases were reported to be related to bowel necrosis [11]. In recent years, the widespread use of CT has enabled increased detection of PI/PVG, showing that these clinical signs are not always predictive of a poor outcome. However, this is because bowel necrosis is not always found in some patients with PI and/or PVG. The increased sensitivity of CT scans has increased the detection of PI and PVG, increasing the difficulty of determining whether or not a patient with PI/PVG has bowel necrosis. Some authors have reported the development of therapeutic algorithms for treating PI/PVG [5, 12], but the wide variety of potential clinical features makes the development of an algorithm that is applicable to all patients with PI/PVG difficult.
Some clinical findings have also been suggested to predict bowel necrosis in patients with PI/PVG. Some reports suggested that bowel necrosis was associated with the distribution of PVG and bowel gas morphology, though conflicting reports have made this suggestion controversial [7, 13–15]. Bowel necrosis could not be predicted from the distribution or extent of PVG/PI. The distribution of PVG was entirely unrelated to bowel necrosis, whereas linear PI had a nonsignificant association with bowel necrosis. Patient characteristics and past medical histories have been reported to influence the occurrence of PI/PVG [15, 16], but we found no such relationship. Many laboratory tests have also been reported to be useful for the diagnosis of PI/PVG. Some reports have suggested that blood lactate levels and acidosis, indicators of tissue necrosis, are predictive of outcomes [8, 17, 18]. Our univariate analysis showed that elevated levels of lactate, C-reactive protein, and creatinine were significantly associated with bowel necrosis. These laboratory parameters are related to tissue necrosis, inflammation, and dehydration, suggesting that our findings are reasonable. Furthermore, abdominal physical examination findings are important predictors of bowel necrosis. In particular, signs of peritoneal irritation have been thought to be useful for diagnosing peritonitis due to bowel necrosis [19]. Our study supported this contention, suggesting that both abdominal pain and signs of peritoneal irritation predict bowel necrosis. However, the assessment of physical findings is physician-dependent, making an objective or numerical expression of such findings difficult. Several scoring systems that evaluate patient condition severity have been put forth as useful prognostic indicators for patients with bowel necrosis [20, 21]. In this study, the SIRS, APACHE II, and SOFA scores were used to assess severity. All three scores were related to bowel necrosis, indicating that these scores can be useful for evaluating patients undergoing surgery or conservative treatment. Recently, procalcitonin and presepsin may indicate useful prognostic information for patients with severe sepsis or septic shock. These parameters are expected to be useful for evaluating the severity of patients’ condition in the future [22].
PI/PVG is more likely to indicate bowel necrosis in postoperative patients than in patients who have not had surgery. There are several reasons that postoperative PI/PVG may be an indicator of bowel necrosis. First, the proliferation of intestinal bacteria and the production of gas in the bowel increase the intraluminal pressure, particularly in patients with postoperative ileus; this may lead to bowel ischemia and PI/PVG. Second, inflammatory cytokines increase vascular permeability, and the resulting intravascular dehydration may cause bowel ischemia [23]. In our study, the decrease in intravascular fluid, secondary to severe operative stress and perioperative catecholamine use, may have caused bowel ischemia in patients who underwent highly invasive surgeries, such as esophagectomies or pancreaticoduodenectomies. Third, the intestine can be damaged during abdominal surgery, and suture failure sometimes occurs after bowel resection and anastomosis. If there is massive leakage of bowel contents into the abdominal cavity, re-operation may be required due to bowel necrosis or peritonitis. Moreover, vasoconstrictors or vasopressors (e.g., catecholamines) are used if the patient’s blood pressure is unstable. Although vasoconstrictors increase blood pressure, they may also compromise blood flow to the peripheral intestinal vessels. Excessive vasoconstriction or repeated alternation of vasoconstriction and vasodilatation may also induce mesenteric artery spasms, causing ischemic enteritis or NOMI [24, 25]. Diagnosis of PI/PVG in postoperative patients is thus a simple indicator of potential bowel necrosis and may be useful for determining treatment.
In many cases, PI or PVG occurs in patients who have not undergone surgery, and it appears to resolve spontaneously in many of these patients. This is because PI or PVG can occur due to transient and reversible intestinal ischemia under certain conditions. For example, gas-forming bacteria can enter the mucosal barrier, through mucosal rents or increased mucosal permeability, and produce gas within the bowel wall; alternatively, the intestinal wall may be injured, or increased intraluminal pressure may serve as the driving force in PI that causes the intramural gas, due to conditions such as intestinal obstruction, inflammatory bowel disease, bowel preparation, or colonoscopy.
Our study had several limitations, including being a retrospective, single-center investigation involving a small sample size. The PI/PVG and bowel ischemia diagnoses were dependent upon the subjective judgment of the attending physicians, leading to potential selection bias. Furthermore, this study did not evaluate patients with bowel necrosis who did not have PI and/or PVG, so the association between PI/PVG and bowel necrosis cannot be fully assessed. The pathogenesis and clinical significance of PI/PVG should thus be further investigated in future multicenter prospective studies.
Conclusion
In conclusion, postoperative detection of PI/PVG is indicative of bowel necrosis and is a useful indicator for surgeons deciding between further surgical intervention and conservative therapy.
Abbreviations
APACHE II, Acute Physiology and Chronic Health Evaluation II; CRP, C-reactive protein; CT, computed tomography (CT); NOMI, non-occlusive mesenteric ischemia; PI, pneumatosis intestinalis; PVG, portal venous gas; SOFA, Sequential Organ Failure Assessment score; SIRS, Systemic Inflammatory Response Syndrome score
References
Sisk PB. Gas in the portal venous system. Radiology. 1961;77:103–7.
Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. Am J Roentgenol. 2007;188:1604–13.
St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68–75.
Kinosita H, Shinozaki M, Tanimura H, Umemoto Y, Sakaguchi S, Takifuji K, et al. Clinical features and management of hepatic portal venous gas. Arch Surg. 2001;136:1410–4.
Wayne E, Ough M, Wu A, Laio J, Andresen KJ, Kuehn D, et al. Management algorithm for pneumatosis intestinalis and portal venous gas: treatment and outcome of 88 consecutive cases. J Gastrointest Surg. 2010;14:437–48.
Shiotani S, Kohno M, Ohashi N, Yamazaki K, Nakayama H, Watanabe K. Postmortem computed tomographic (PMCT) demonstration of the relation between gastrointestinal (GI) distension and hepatic portal venous gas (HPVG). Radiat Med. 2004;22:25–9.
Soyer P, Martin-Grivaud S, Boudiaf M, Malzy P, Duchat F, Hamzi L, et al. Linear or bubbly: a pictorial review of CT features of intestinal pneumatosis in adults. J Radiol. 2008;89:1907–20. Article in French.
Dubose JJ, Lissauer M, Maung AA, Piper GL, O’Callaghan TA, Luo-Owen X, et al. Pneumatosis Intestinalis Predictive Evaluation Study (PIPES): a multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2013;75:15–23.
Tsujimoto T, Shioyama E, Moriya K, Kawaratani H, Shirai Y, Toyohara M, Mitoro A, Yamao J, Fujii H, Fukui H. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: a case report and review of the literature. World J Gastroenterol. 2008;14:6087–92.
Wiot JF, Felson B. Gas in the portal venous system. Am J Roentgenol Radium Ther Nucl Med. 1961;86:920–9.
Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic-portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg. 1978;87:281–7.
Nelson AL, Millington TM, Sahani D, Chung RT, Bauer C, Hertl M, et al. Hepatic portal venous gas: the ABCs of management. Arch Surg. 2009;144:575–81.
Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. Am J Roentgenol. 2001;177:1319–23.
Peloponissios N, Halkic N, Pugnale M, Jornot P, Nordback P, Meyer A, et al. Hepatic portal gas in adults: review of the literature and presentation of a consecutive series of 11 cases. Arch Surg. 2003;138:1367–70.
Gangliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11:111–8.
Hayakawa T, Yoneshima M, Abe T, Nomura G. Pneumatosis cystoides intestinalis after treatment with a glucosidase inhibitor. Diabetes Care. 1999;22:366–7.
Hawn MT, Canon CL, Lockhart ME, Gonzalez QH, Shore G, Bondora A, et al. Serum lactic acid determines the outcomes of CT diagnosis of pneumatosis of the gastrointestinal tract. Am Surg. 2004;70:19–21.
Hou SK, Chern CH, How CK, Chen JD, Wang LM, Lee CH. Hepatic portal venous gas: clinical significance of computed tomography findings. Am J Emerg Med. 2004;22:214–8.
Bani Hani M, Kamangar F, Goldberg S, Greenspon J, Shah P, Volpe C, et al. Pneumatosis and portal venous gas: do CT findings reassure? J Surg Res. 2013;185:581–6.
Wu JM, Tsai MS, Lin MT, Tien YW, Lin TH. High APACHE II score and long length of bowel resection impair the outcomes in patients with necrotic bowel induced hepatic portal venous gas. BMC Gastroenterol. 2011;11:18–21.
Hsu HP, Shan YS, Hsieh YH, Sy ED, Lin PW. Impact of etiologic factors and APACHE II and POSSUM scores in management and clinical outcome of acute intestinal ischemic disorders after surgical treatment. World J Surg. 2006;30:2152–62.
Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care. 2014;18:R6.
Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet. 1963;116:53–60.
Lock G. Acute intestinal ischaemia. Best Pract Res Clin Gastroenterol. 2001;15:83–98.
Habboushe F, Wallace HW, Nusbaum M, Baum S, Dratch P, Blakemore W. Nonocclusive mesenteric vascular insufficiency. Ann Surg. 1974;180:819–22.
Acknowledgements
The authors would like to thank the nursing staff at the Department of Surgery, National Center for Global Health and Medicine.
Funding
None.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions
HK was responsible for the conception and design of the study. MO and YK drafted the manuscript and conducted the literature search. YH conducted the analysis and interpretation of data and contributed to drafting the manuscript. YH and HM provided the final approval for publication. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the National Center for Global Health and Medicine review board. The approval number is H-039-06f, and it was approved on April 21st, 2006. Informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Higashizono, K., Yano, H., Miyake, O. et al. Postoperative pneumatosis intestinalis (PI) and portal venous gas (PVG) may indicate bowel necrosis: a 52-case study. BMC Surg 16, 42 (2016). https://doi.org/10.1186/s12893-016-0158-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-016-0158-x