Abstract
Background
In this study, we aimed to illustrate the association between the Healthy Eating Index (HEI) and Dietary Quality Index (DQI) with bone mineral density (BMD) among postmenopausal Iranian women with osteoporosis compared to the healthy control.
Methods
In the current case-control study, 131 postmenopausal women with osteoporosis and 131 healthy postmenopausal women participated. Dual-energy X-ray absorptiometry was used to assess the lumbar vertebrae and femoral neck BMD. The subjects completed a validated food frequency questionnaire (FFQ), and then HEI and DQI were calculated based on the FFQ data. Crude and adjusted multivariable logistic regression was used to assess the relation between HEI and DQI with the odds of the femoral and lumbar BMD.
Results
According to the results, participants in the last tertile of HEI were more likely to have higher femoral and lumbar BMD in the crude model (odds ratio (OR) = 0.38; 95% confidence interval (CI): 0.20–0.71 and OR = 0.20; 95% CI: 0.10–0.40, respectively) and also in the adjusted model (OR = 0.40; 95% CI: 0.20–0.78 and OR = 0.20; 95% CI: 0.10–0.41, respectively). Also, in terms of DQI-I, participants in the last tertile were more likely to have higher femoral and lumbar BMD in the crude model (OR = 0.23; 95% CI: 0.12–0.45 and OR = 0.29; 95% CI: 0.15–0.55, respectively) and also in the adjusted model (OR = 0.29; 95% CI: 0.14–0.58 and OR = 0.34; 95% CI: 0.17–0.67, respectively).
Conclusions
The results of the current study supported the hypothesis that high-quality diets with healthy patterns can be clinically effective in maintaining bone health. Thus, recommendations regarding the consumption of nutrient-rich food groups in a healthy diet can serve as a practical non-pharmacological strategy against osteoporosis.
Similar content being viewed by others
Introduction
Bones change lifelong through the remodeling process to maintain structural integrity and regulate the balance of calcium and phosphorous [1]. This process happens according to osteoblasts’ and osteoclasts’ activities in formation and resorption, respectively. The imbalance between bone formation and resorption can lead to bone diseases such as osteoporosis and osteopenia [2]. Osteopenia is a condition in which a decrease in bone mineral density (BMD) and subsequent fractures due to fragility is seen [3]. The World Health Organization (WHO) has defined osteopenia as a T-score of BMD between − 1 to -2.5, while values less than − 2.5 are considered osteoporosis [4]. The T-score is the difference between the BMD of the patient and the normal young population divided by the standard deviation (SD) of the normal young population [5].
Osteopenia and osteoporosis can influence both genders, but postmenopausal women are more prone. Moreover, a history of bone fracture, older ages, and vitamin D and calcium deficiency are remarkable associated risk factors for osteopenia and osteoporosis [3, 6]. Other critical pathogenic mechanisms comprise unfavorable development and strength, bone loss due to extreme resorption and inappropriate structure, impaired compensatory activities to bone loss, and estrogen deficiency [7].
The Healthy Eating Index (HEI) is a measure to evaluate the nutritional quality of a diet based on the recommendations of the Dietary Guidelines for Americans [8]. This 13-component index considers multidimensional food groups regarding adequacy and moderation [9]. The Diet Quality Index (DQI) is another nutritional assessment that can evaluate diet variety, adequacy, moderation, and balance [10]. The DQI was constructed due to the importance of diet-associated chronic disease and undernutrition problems [11]. These dietary quality indices are inversely related to the risk of chronic diseases, including obesity, cancer, cardiovascular diseases, type 2 diabetes, and all-cause mortality [12,13,14].
Bone extracellular tissue consists of organic matrix and inorganic salts. While inorganic components include calcium, magnesium, phosphorous, sodium, potassium, zinc, and other ions, the organic part is composed of proteins, particularly collagenous proteins [2]. Thus, dietary factors from micronutrients (minerals and vitamins) to macronutrients and varied types of diets can positively or negatively affect bone health through changes in bone structure and metabolism, modification of paracrine and endocrine pathways, alteration in the homeostasis of bone compounds, and suppression of inflammatory processes [15,16,17]. Despite inconsistent observations, it’s claimed that high-quality healthy diets can serve as a protective approach against bone disease, mainly osteopenia, and osteoporosis [18]. To our knowledge, few studies have investigated the correlation between HEI and DQI with BMD. Thus, in the current study, we aimed to illustrate the association between HEI and DQI with BMD among postmenopausal women with osteopenia/osteoporosis compared with the healthy postmenopausal control.
Materials and methods
Study population
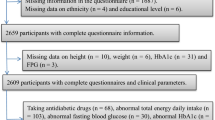
In the current case-control study, 131 postmenopausal women with osteopenia/osteoporosis and 131 healthy postmenopausal women participated. These individuals were chosen from the Isfahan bone density measurement center in Iran from May to December 2021. The lack of a menstrual cycle in 12 months was considered menopause. In this study, the exclusion criteria were taking glucocorticoids (each dose for more than three months was excluded), consuming any kind of alcohol, premenopausal, diabetes, cancer, renal disease, and history of chemotherapy (Fig. 1). The present study’s details have been previously published [19, 20].
A general information questionnaire was used to gather information, such as drug use, smoking, and socio-demographic variables. Stadiometer was used to measure height, and a digital scale was used to measure body weight. Body mass index (BMI) was calculated as weight [21] divided by height squared (m2) [21].
The participant’s physical activity level was evaluated by the International Physical Activity Questionnaire (IPAQ) [22]. Women were divided into three groups based on the metabolic equivalent of task (MET)-minutes less than 600 MET-minutes/week: low activity, between 600 and 3000 MET-minutes/week: moderate activity, and more than 3000 MET-minutes/week: intense activity.
Bone mineral density measurement
The method of dual-energy X-ray absorptiometry (DXA) was used for assessing the BMD of lumbar vertebrae and femoral neck (Horizon Wi (S/N 200,451)). The bone mass status was evaluated with WHO criteria (T-score more than − 1: normal BMD, T-score between − 1 and − 2.5: osteopenia, and T-score equal to or less than − 2.5: osteoporosis) [23].
Dietary assessment and food grouping
A validated food frequency questionnaire (FFQ) was completed by individuals [24]. Also, we used HEI-2015 in our study [8, 25]. So, scores were calculated by 13 food groups. The maximum score was 100. The groups contain four components of moderation (added sugars, refined grains, saturated fats, and sodium) and nine components of adequacy (greens and beans, whole grains, whole fruits, total fruits, vegetables, protein foods, sea foods, dairy, and fatty acids (polyunsaturated fatty acid (PUFA) + monounsaturated fatty acid (MUFA)/saturated fatty acid (SFA)). The score of moderation components was between 0 and 10. The minimum and maximum range of adequacy components were 0 to 5, respectively. The score of every participant was calculated, and they were placed into tertiles.
DQI- International (DQI-I) contains four dietary components. First is food variety, with a score of 0 to 20 points. The food variety includes two elements, a wide variety of food categories (meats and meat products, fish, pulse products, fruits, grains, eggs, vegetables, milk, and milk products) and a within-group variety for protein foods (fish, meat products, milk products, eggs, pulse products). Second is adequacy (protein, grains, fruits, vegetables, fiber, calcium, vitamin C, ferric) and it scores between 0 and 40. The third is moderation (empty calorie foods, sodium, cholesterol, saturated fat, and total fat), with a score from 0 to 30 points. The fourth is overall balance (fatty acid and macronutrient ratios), with a score between 0 and 10. Finally, DQI-I scores from 0 to 100 [11, 26].
Statistical analysis
For statistical analysis, SPSS (version 26) was used. P-value < 0.05 was considered statistically significant. For continuous variables, the mean with SD was used. For categorical variables, we used frequency and percentage. Independent samples T-test and chi-square test were used for continuous and categorical variables, respectively. Analysis of variance (ANOVA) test was used for the association between nutrient and food group intake across HEI and DQI tertiles. Crude and adjusted multivariable logistic regression was used to assess the relation between HEI and DQI with the odds of the femoral and lumbar abnormality. In the adjusted model, we controlled the effects of BMI, age, income, physical activity, education, taking vitamin D, and calcium supplements.
Results
As Table 1 shows, the mean age of the control group was significantly lower than the case group (P = 0.036). The femoral and lumbar BMD was higher in the control group (P < 0.001 for both). Also, physical activity (P = 0.01), education level (P < 0.001), and vitamin D supplement (P = 0.018) were significantly different between the two groups.
Table 2 shows the nutrient intake of participants. Protein and fiber were higher in HEI’s last tertile than in the first tertile (P < 0.001 for both). Also, vitamins A, B6, C, calcium, magnesium, iron, zinc, and copper were higher in the last tertile of HEI in comparison to the first tertile (P < 0.05 for all), but vitamin B12 was more in the second tertile of HEI (P = 0.007). Energy, carbohydrate, protein, and fiber were more in the last tertile of DQI-I (P < 0.001 for all). Moreover, vitamins A, E, B6, C, B9, calcium, magnesium, iron, zinc, and copper were higher in the last tertile of DQI-I compared to the first tertile (P < 0.05 for all). Sodium was more in the first tertile of both HEI and DQI-I (P < 0.05).
According to Table 3, whole grains, fruits, vegetables, nuts, legumes, and dairy were higher in the last tertile of HEI compared to the first tertile (P < 0.05 for all). Refined grains, sweets and sugar beverages, and processed meat were more in the first tertile (P < 0.05 for all). In terms of DQI-I, fruits, vegetables, legumes, dairy, and meats were higher in the last tertile in comparison to the first tertile (P < 0.05 for all) but the whole grains group was higher in the first tertile of DQI-I (P = 0.001).
Based on Table 4, participants in the last tertile of HEI were more likely to have higher femoral and lumbar BMD in the crude model (odds ratio (OR) = 0.38; 95% confidence interval (CI): 0.20–0.71 and OR = 0.20; 95% CI: 0.10–0.40, respectively) and also in adjusted model (OR = 0.40; 95% CI: 0.20–0.78 and OR = 0.20; 95% CI: 0.10–0.41, respectively). In terms of DQI-I, participants in the last tertile were more likely to have higher femoral and lumbar BMD in the crude model (OR = 0.23; 95% CI: 0.12–0.45 and OR = 0.29; 95% CI: 0.15–0.55, respectively) and also in adjusted model for the femoral (OR = 0.29; 95% CI: 0.14–0.58) and lumbar (OR = 0.34; 95% CI: 0.17–0.67).
Discussion
Osteoporosis is an age-related chronic condition that is a concern globally as life expectancy increases. It is agreed that lifestyle modification, mostly following high-quality dietary patterns, is the primary practical strategy to attenuate the risk of osteoporosis. As available evidence shows, few studies have illustrated correlations between HEI and DQI with BMD. The result of this case-control study among 131 postmenopausal women with osteoporosis and 131 healthy postmenopausal control group demonstrated a strong direct associations between HEI and DQI with bone health status.
Controversial results were obtained from previous studies. While some found a significant correlation between healthy eating patterns and bone health, other studies failed to find a clear association. For instance, a similar cross-sectional study among adult Iranian women revealed positive correlations between HEI and BMD at the femoral neck and lumbar spine [27]. Moreover, in a case-control study of patients with hip fracture, diets with higher HEI, DQI, the Alternate HEI (AHEI), and alternate Mediterranean Diet (aMED) score [28] were associated with a reduced risk of hip fracture [28]. Inconsistently, in a prospective cohort study among postmenopausal women, higher aMED index was correlated with a lower risk of hip fracture, and no significant relationship was seen between HEI-2010, AHEI-2010, or Dietary Approaches to Stop Hypertension (DASH) diet and the risk of hip fracture [29]. Furthermore, despite the negative relationship between dairy intake and urinary N-telopeptides/creatinine (uNTx/Cr) -as a marker of bone resorption- HEI-2005 wasn’t associated with uNTx/Cr among postmenopausal women [30]. It can be mentioned that menopausal status exerts detrimental effects on bones and thus might attenuate the protective roles of healthy dietary patterns [31].
From the standpoint of single nutrients’ effect on BMD, components like protein, fiber, vitamins A, B6, B12, C, and minerals, including sodium, calcium, magnesium, iron, zinc, and copper, can partly explicate differences in BMD score across tertiles of HEI through various mechanisms and pathways. Based on the studies, BMD can be affected by micro-and macronutrients. It has been shown that people who consumed less vegetables, fruits, and dairy products had a lower BMD [32]. Also, the occurrence of some diseases such as the outbreak of COVID-19 may have adverse effects on bone health by creating unhealthy dietary patterns [32].
In the current study, increased consumption of vegetables, fruits, legumes, whole grains, and dairy products was observed among study participants across the tertiles of HEI and DQI. It can be hypothesized that a higher intake of mentioned food groups might be the proposed reason for the observed significant linkage between HEI and DQI with BMD. Previous research has presented that adequate consumption of food groups positively influences bone health status, as well [33,34,35]. The beneficial effects of these groups are attributed to their nutrients, such as vitamins, minerals, protein, and fiber. For instance, protein has potential roles in modulating bone metabolism. Despite increasing calciuria, dietary protein promotes osteoblast activity and calcium absorption, which results in bone mineralization and can strengthen muscles as a protection for the skeleton [36,37,38]. In addition, dietary fiber intake is reported to be effective against bone loss, probably through prebiotic properties, which can increase the production of short-chain fatty acids by modulating gut microbiota and, after that, improving calcium absorption [39]. Moreover, high salt diet consumption was reported to interrupt calcium metabolism by increasing calciuria and thus negatively alter bone calcium balance [40]. Plant-based diets, such as HEI, emphasize consuming limited amounts of sodium with less added salt and processed meat, thereby reducing the risk of osteoporosis [41]. Furthermore, another plausible explanation for the protective effect of HEI and DQI against osteoporosis is the antioxidant components of such healthy diets, notably higher vitamin C intake. A U-shape correlation is suggested between vitamin C consumption and BMD. High-dose vitamin C leads to oxidative stress and cell death, and its deficiency increases osteoclast and, subsequently, decreases bone formation [42].
Some limitations of the present study can be discussed. First, due to the case-control design of the study, the causality may not be indicated clearly. Additionally, bone health status is influenced by environmental and dietary factors from birth. On the other hand, assessing dietary intake by FFQ is limited to one year. Thus, evaluating the correlation between BMD and dietary patterns in longer durations is suggested. Third, while a validated FFQ was used to evaluate the score of dietary patterns, it can be influenced by the memory of the participant, so assessment errors might happen. Moreover, since the study was conducted in Isfahan city, the result of the current study cannot be generalized to other populations. Furthermore, measuring serum biomarkers of bone turnover could be helpful in future research. Nevertheless, the present study was the first to demonstrate the association between HEI, DQI, and BMD in Isfahan, Iran. Different confounders were considered to reduce the risk of bias during the assessment. Limiting the study population to out-patient postmenopausal women attenuated the confounding effects of menopausal status and restricted low-quality diets of the care centers.
In conclusion, the result of the current study supported the hypothesis that high-quality diets with healthy patterns can clinically be effective in maintaining bone health. Therefore, recommendations regarding the consumption of nutrient-rich food groups in a healthy diet can serve as a practical non-pharmacological strategy against osteoporosis.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Supplement 3):131–S139.
Florencio-Silva R, Sasso GRdS, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Research International 2015, 2015:421746.
Silva ACV, da Rosa MI, Fernandes B, Lumertz S, Diniz RM, dos Reis Damiani MEF. Factors associated with osteopenia and osteoporosis in women undergoing bone mineral density test. Revista Brasileira de Reumatologia (English Edition). 2015;55(3):223–8.
Porter JL, Varacallo M. Osteoporosis. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
Binkley N, Adler R, Bilezikian JP. Osteoporosis diagnosis in men: the T-score controversy revisited. Curr Osteoporos Rep. 2014;12:403–9.
Karaguzel G, Holick MF. Diagnosis and treatment of osteopenia. Reviews in Endocrine and Metabolic Disorders. 2010;11(4):237–51.
Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–25.
Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI-2015. J Acad Nutr Dietetics. 2018;118(9):1591–602.
Vinyard M, Zimmer M, Herrick KA, Story M, Juan W, Reedy J. Healthy eating Index-2015 scores vary by types of Food Outlets in the United States. Nutrients 2021, 13(8).
Tur JA, Romaguera D, Pons A. The Diet Quality Index-International (DQI-I): is it a useful tool to evaluate the quality of the Mediterranean diet? Br J Nutr. 2005;93(3):369–76.
Kim S, Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133(11):3476–84.
Zarrin R, Ibiebele TI, Marks GC. Development and validity assessment of a diet quality index for Australians. Asia Pac J Clin Nutr. 2013;22(2):177–87.
Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet Quality as assessed by the healthy eating index, alternate healthy eating Index, Dietary Approaches to stop hypertension score, and Health Outcomes: a second update of a systematic review and Meta-analysis of Cohort Studies. J Acad Nutr Diet. 2020;120(12):1998–2031. e1915.
Cho IY, Lee KM, Lee Y, Paek CM, Kim HJ, Kim JY, Lee K, Han JS, Bae WK. Assessment of Dietary Habits using the Diet Quality Index-International in Cerebrovascular and Cardiovascular Disease Patients. Nutrients 2021, 13(2).
Cashman KD. Diet, Nutrition, and Bone Health. J Nutr. 2007;137(11):2507S–12.
Remacle C, Reusens B. Functional foods, ageing and degenerative disease. Woodhead Publishing; 2004.
Miggiano GA, Gagliardi L. [Diet, nutrition and bone health]. Clin Ter. 2005;156(1–2):47–56.
Movassagh EZ, Vatanparast H. Current evidence on the association of dietary patterns and Bone Health: a scoping review. Adv Nutr. 2017;8(1):1–16.
Nouri M, Mahmoodi M, Shateri Z, Ghadiri M, Rajabzadeh-Dehkordi M, Vali M, Gargari BP. How do carbohydrate quality indices influence on bone mass density in postmenopausal women? A case–control study. BMC Womens Health. 2023;23(1):42.
Ghadiri M, Cheshmazar E, Shateri Z, Gerami S, Nouri M, Gargari BP. Healthy plant-based diet index as a determinant of bone mineral density in osteoporotic postmenopausal women: a case-control study. Front Nutr 2022, 9.
Waterland RA, Travisano M, Tahiliani K, Rached M, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32(9):1373–9.
Collaborators GO. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, Fiore CE, Iolascon G, Maggi S, Michieli R. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. 2019;14(1):85–102.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–62.
Leilami K, Zareie A, Nouri M, Bagheri M, Shirani M. The association between healthy eating index score with semen parameters in infertile men: a cross-sectional study. Int J Reproductive Biomed (IJRM) 2022:931–40.
Foroumandi E, Alizadeh M, Kheirouri S. Dietary quality index is negatively associated with serum advanced glycation end products in healthy adults. Clin Nutr ESPEN. 2020;36:111–5.
Babazadeh-Anvigh B, Abedi V, Heydari S, Karamati D, Babajafari S, Najafi A, Rashidkhani B, Shariati-Bafghi SE, Karamati M. Healthy eating index-2015 and bone mineral density among adult iranian women. Arch Osteoporos. 2020;15(1):151.
Zeng FF, Xue WQ, Cao WT, Wu BH, Xie HL, Fan F, Zhu HL, Chen YM. Diet-quality scores and risk of hip fractures in elderly urban chinese in Guangdong, China: a case-control study. Osteoporos Int. 2014;25(8):2131–41.
Haring B, Crandall CJ, Wu C, LeBlanc ES, Shikany JM, Carbone L, Orchard T, Thomas F, Wactawaski-Wende J, Li W, et al. Dietary patterns and fractures in Postmenopausal Women: results from the women’s Health Initiative. JAMA Intern Med. 2016;176(5):645–52.
Hamidi M, Tarasuk V, Corey P, Cheung AM. Association between the healthy eating index and bone turnover markers in US postmenopausal women aged ≥ 45 y. Am J Clin Nutr. 2011;94(1):199–208.
Ji MX, Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med. 2015;1(1):9–13.
Moretti A, Liguori S, Paoletta M, Migliaccio S, Toro G, Gimigliano F, Iolascon G. Bone fragility during the COVID-19 pandemic: the role of macro-and micronutrients. Therapeutic Adv Musculoskelet Disease. 2023;15:1759720X231158200.
Mangels AR. Bone nutrients for vegetarians. Am J Clin Nutr. 2014;100(suppl1):469S–75.
Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, Feriche B, Lorenzo ML, Olea F. Mediterranean diet and bone mineral density in two age groups of women. Int J Food Sci Nutr. 2013;64(2):155–61.
Choi E, Park Y. The Association between the consumption of Fish/Shellfish and the risk of osteoporosis in men and postmenopausal women aged 50 years or older. Nutrients. 2016;8(3):113.
Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90(6):1674–92.
Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90(1):26–31.
Genaro Pde S, Martini LA. Effect of protein intake on bone and muscle mass in the elderly. Nutr Rev. 2010;68(10):616–23.
Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between Dietary Fiber Intake and Bone loss in the Framingham offspring study. J Bone Miner Res. 2018;33(2):241–9.
Teucher B, Dainty JR, Spinks CA, Majsak-Newman G, Berry DJ, Hoogewerff JA, Foxall RJ, Jakobsen J, Cashman KD, Flynn A, et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23(9):1477–85.
Pasdar Y, Hamzeh B, Moradi S, Mohammadi E, Cheshmeh S, Darbandi M, Faramani RS, Najafi F. Healthy eating index 2015 and major dietary patterns in relation to incident hypertension; a prospective cohort study. BMC Public Health. 2022;22(1):734.
Chin KY, Ima-Nirwana S. Vitamin C and bone health: evidence from cell, animal and human studies. Curr Drug Targets. 2018;19(5):439–50.
Acknowledgements
The researchers appreciate Tabriz University of Medical Sciences for its financial aid and all the participants and those who helped us in this study.
Funding
The sponsor of this study was the Nutrition Research Center at Tabriz University of Medical Sciences (grant number Pazhoohan Code: 66934).
Author information
Authors and Affiliations
Contributions
M.S, M.R, M.G, S.G, and Z.S; Contributed to data collection, writing, and editing of the draft. M.N; Contributed to all data, statistical analysis, and interpretation of data. B.P.G contributed to the design of the work, interpretation of data, funding acquisition, and project administration. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (IR.TBZMED.REC.1400.114) and the informed consents were completed by all participants. The present study was performed based on the amended Helsinki Declaration.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghadiri, M., Soltani, M., Rajabzadeh-Dehkordi, M. et al. The relation between dietary quality and healthy eating index with bone mineral density in osteoporosis: a case-control study. BMC Musculoskelet Disord 24, 584 (2023). https://doi.org/10.1186/s12891-023-06704-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06704-3