Abstract
Background
Development of valid and feasible quality indicators (QIs) is needed to track quality initiatives for osteoarthritis pain management in primary care settings.
Methods
Literature search identified published guidelines that were reviewed for QI extraction. A panel of 14 experts was assembled, including primary care physicians, rheumatologists, orthopedic surgeons, pain specialists, and outcomes research pharmacists. A screening survey excluded QIs that cannot be reliably extracted from the electronic health record or that are irrelevant for osteoarthritis in primary care settings. A validity screening survey used a 9-point Likert scale to rate the validity of each QI based on predefined criteria. During expert panel discussions, stakeholders revised QI wording, added new QIs, and voted to include or exclude each QI. A priority survey used a 9-point Likert scale to prioritize the included QIs.
Results
Literature search identified 520 references published from January 2015 to March 2021 and 4 additional guidelines from professional/governmental websites. The study included 41 guidelines. Extraction of 741 recommendations yielded 115 candidate QIs. Feasibility screening excluded 28 QIs. Validity screening and expert panel discussion excluded 73 QIs and added 1 QI. The final set of 15 prioritized QIs focused on pain management safety, education, weight-management, psychological wellbeing, optimizing first-line medications, referral, and imaging.
Conclusion
This multi-disciplinary expert panel established consensus on QIs for osteoarthritis pain management in primary care settings by combining scientific evidence with expert opinion. The resulting list of 15 prioritized, valid, and feasible QIs can be used to track quality initiatives for osteoarthritis pain management.
Similar content being viewed by others
Background
More than 32 million adults in the United States suffer from osteoarthritis (OA), accounting for $373 billion in annual direct medical costs [1,2,3]. Persistent pain caused by OA impairs patients’ ability to perform activities of daily living, and pain management is an integral component of maintaining a good quality of life for patients with OA [2, 4]. Despite guideline recommendations to avoid or minimize opioid use for OA, opioids are prescribed in 26% of outpatient encounters for OA [5]. Approximately 35% of hip or knee OA patients who are treated with opioids will fail opioid therapy, defined as the need for higher opioid doses, addition of non-opioid analgesics, surgery, or opioid misuse [6]. An estimated 5% of knee OA patients are chronic users of strong opioids, which generates $14 billion in societal costs driven by medical care, lost productivity, diversion, and criminal justice [7]. Most patients with OA initially seek pain management at primary care clinics. Since primary care physicians (PCPs) are common prescribers of opioids for OA [8], they need to take an active role in optimizing the safety and efficacy of pain regimens for OA to reduce reliance on opioids and combat the opioid crisis that currently plagues the United States [9, 10].
Numerous national and international guidelines provide an abundance of recommendations regarding evidence-based strategies to optimize pain management while minimizing the risk of adverse events from pain medications. However, primary care practice does not always correlate with guideline recommendations, potentially due to lack of incentives for PCPs, lack of PCP buy-in, lack of integration between researchers and PCPs, and low perceived prioritization of OA among PCPs and patients [11,12,13,14].
A potential solution to improve adherence to guideline recommendations is to develop a research program that is embedded in a health-system and established through adequate buy-in from PCPs and related physician specialists. The initial step for this program is to develop a set of quality indicators (QIs) that are deemed to be meaningful and relevant among stakeholders. The objective of this consensus project was to develop a set of prioritized, valid, and feasible QIs that can be used to track quality initiatives for OA pain management in the primary care setting.
Methods
Setting
The Houston Methodist health system consists of 148 PCPs that practice at 39 locations in the greater urban area of Houston, Texas, USA [15]. In 2018, our Opioid Stewardship Program at Houston Methodist successfully established consensus on QIs for opioid stewardship and pain management in the hospital and emergency department settings [16]. This study applied a similar pragmatic modification to the Research and Development Corporation/University of California Los Angeles (RAND/UCLA) method to establish consensus on valid and feasible QIs for OA pain management in the primary care setting [17, 18]. Consensus was established using a 5-step mixed-methods approach: (i) literature search, (ii) feasibility screen, (iii) face validity screen, (iv) expert panel discussions, and (v) priority ranking. The Houston Methodist Research Institute’s Institutional Review Board approved this study with a waiver of informed consent.
Composition of expert panel
Our OA expert panel consisted of healthcare leaders (medical director of primary care, medical director of pain, and the chief medical information officer), clinicians, and health services researchers from the Opioid Stewardship Program. This 14-member multidisciplinary team included 5 PCPs, 3 pharmacist researchers, 2 rheumatologists, 2 orthopedic surgeons, and 2 pain specialists.
Literature search
A literature search identified practice guidelines for OA management that were published from January 2015 to March 2021 and indexed in MEDLINE (accessed via PubMed) or SCOPUS (Supplementary Methods 1 section of Additional File 1). Our search strategy was limited to recently published practice guidelines to represent the period of the publicly acknowledged opioid crisis in the United States that was declared in 2016–2017 [9, 19, 20]. Additionally, federal agency websites and professional society websites were reviewed for relevant position statements and guidelines that were not otherwise published and indexed. Only guidelines that contained evidence-based recommendations for management of OA in adults and were associated with a professional organization or government were included. Guidelines that exclusively focused on diagnosis or surgical management of OA were excluded. All abstracts identified were independently screened by two investigators for inclusion; discrepancies were settled by a third investigator.
Using a standardized electronic data collection tool, investigators extracted evidence-based recommendations from each included reference. For each recommendation, investigators also extracted information on applicable joints, comorbidities, and strength of recommendation. To focus on pain management in the primary care setting, recommendations related to OA diagnosis, intraoperative management, postoperative recovery, and assessment of postoperative outcomes were not extracted. Recommendations were consolidated into brief, commonly worded proposed QIs and organized into 11 domains: topical medications, intraarticular injections, biologics, systemic medications, support devices, supplements, alternative therapies, education, behavior and psychosocial interventions, procedures, exercise, and other. Proposed QIs were advanced to feasibility and validity screening processes.
Feasibility screen survey
Feasibility was evaluated using an electronic survey to score each proposed QI as not feasible (score = 0) or feasible/unsure (score = 1) based on 2 feasibility screening criteria that were modeled after antimicrobial stewardship and opioid stewardship consensus methodology [16, 21]: (i) Assuming healthcare documentation is compliant with hospital policy and expectations, this QI can be reliably extracted from structured data fields within the electronic health record (EHR) (current state or future state); (ii) This QI is relevant to management of OA pain in a primary care clinic (family medicine or internal medicine). Five experts scored the feasibility of each proposed QI as 0 or 1, and proposed QIs with a total score of 3 to 5 were considered feasible and were retained as candidate QIs.
Face validity screen survey
Face validity was evaluated using an electronic survey to score each candidate QI using a 9-point Likert scale (with 1 indicating lowest validity and 9 indicating highest validity) based on 3 face validity criteria that were modeled after antimicrobial stewardship and opioid stewardship consensus methodology [16, 21, 22]: (i) This QI is associated with improved OA pain management in primary care clinics (family medicine or internal medicine); (ii) This QI is associated with improved quality of care and patient safety; and (iii) This QI can be influenced by electronic medical record enhancements. Survey scores were used to preliminarily categorize candidate QIs as appropriate (median score > 6, without disagreement), inappropriate (median score < 4, without disagreement), or uncertain (disagreement or median score 4–6). Disagreement was defined as ≥ 5 ratings of 1–3 with ≥ 5 ratings of 7–9 for the same candidate QI for a panel size of 14 in accordance with RAND/UCLA methodology [18]. Experts were asked to provide free-text comments to list additional QIs that should be considered at future expert panel discussions. To standardize their familiarity with evidence-based recommendations, expert panel members were provided with a supplemental literature review report that displayed all candidate QIs, organized by domain, along with their associated guidelines recommendations, strength of recommendation, joints, and comorbidities (Additional File 2).
Expert panel discussion
Expert panel members convened via the health system’s tele-conferencing platform to discuss survey results, preliminary categories, and the supplemental literature review report. Experts discussed their interpretation of the literature, their insights from clinical practice, and compared the merits of a QI versus other QIs under consideration. After discussing an individual QI during a meeting, all experts in attendance voted to include or exclude that QI. Quality indicators that received ≥ 8 include votes (58% of 14 voting members) were considered valid and feasible and were advanced to priority ranking.
Priority ranking
Priority was evaluated using an electronic survey to score each valid and feasible QI using a 9-point Likert scale (with 1 indicating lowest priority and 9 indicating highest priority).
Data management and analysis
Extraction of guideline recommendations from literature search and distribution of electronic surveys for feasibility screening, validity screening, and priority ranking were managed using Research Electronic Data Capture (REDCap) electronic data capture tools [23]. All statistical analyses were performed using STATA (version 16, StataCorp LLC, College Station, Texas, United States).
Results
Literature search
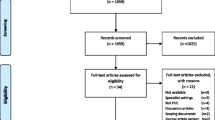
Of 520 unique references identified from literature search and 4 identified from government/society websites, we included 41 evidence-based guidelines (Fig. 1) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. We then extracted 741 recommendations. If the guideline presented recommendations in table format, we formulated recommendation sentences using the intervention, strength of recommendation, applicable joints, and applicable comorbidities. Two investigators (Rizk and Swan) collapsed these 741 recommendations into 115 proposed QIs (Supplemental Results 1 section of Additional File 1).
Feasibility
A subgroup of 5 expert panel members (Fink, Flores, Rizk, Swan, Tajchman) completed the feasibility survey of 115 proposed QIs. The feasibility screen excluded 28 proposed QIs. If the subgroup was uncertain about the feasibility of a proposed QI, the item was retained and advanced to validity screening for further evaluation.
Face validity
All 14 members of the expert panel completed the face validity survey of 87 candidate QIs that were retained after feasibility screening. Scores from survey responses were used to preliminarily categorize candidate QIs as appropriate (n = 22), uncertain (n = 40), or inappropriate (n = 25).
Expert panel discussion
The expert panel convened 13 times over a 2-month period (08/2021 to 10/2021). All 14 members participated in expert panel discussions. Of 87 candidate QIs that were discussed, 73 were excluded. The expert panel split one QI into two QIs, both of which were included. This consensus process yielded a total of 15 valid and feasible QIs (Fig. 2). Although candidate QIs were originally worded as non-directional statements (e.g., “proportion of patients on combinations of non-steroidal anti-inflammatory drugs [NSAIDs]”), the panel was instructed to indicate a direction when appropriate (e.g., “avoid combinations of NSAIDs”). The expert panel carefully reviewed clusters of candidate QIs that were similar and looked for opportunities to consolidate key concepts into a unique and non-overlapping set of quality indicators. Additionally, the expert panel reworded QIs to focus on the tasks that are explicitly managed by PCPs (Supplemental Results 2 section of Additional File 1).
Prioritization
All 14 members of the expert panel completed the prioritization survey of the 15 valid and feasible QIs. Table S-1 (Additional File 1) shows all the ranks and scores used to calculate the final priority ranking. The prioritized list of valid and feasible QIs is shown in Table 1.
Discussion
This study established a set of 15 prioritized, valid, and feasible QIs by systematically combining recent scientific evidence from published guidelines with expert opinion from clinical stakeholders.
Over 700 recommendations were extracted from 41 guidelines that were published during the era of acknowledgement of an opioid crisis in the United States. A panel of 14 experts completed surveys and participated in in-depth discussions to evaluate potential merits and limitations of QIs. To ensure buy-in from PCPs, the expert panel considered pragmatic factors of clinic workflow, referral systems, payor models, perceived impact, and patient acceptance/engagement that are relevant for primary care in the United States.
QIs for safety of OA pain medications
Since the relative effectiveness of medications commonly used to manage OA pain is unclear and may be dose dependent [65], the expert panel prioritized six QIs to optimize safe use of pain medications: add proton pump inhibitor (PPI) to NSAID for patients with gastrointestinal risk (#1), avoid oral NSAIDs in chronic kidney disease (CKD) (#2), minimize opioids (#3), select naproxen if an NSAID is used among patients with cardiovascular risk (#9), avoid combinations of NSAIDs (#10), and minimize tramadol (#12).
Guideline recommendations to reduce the risk of gastrointestinal injury from NSAIDs among at risk patients include use of cyclooxygenase-2 (COX2) selective inhibitors [25, 27], concurrent PPI [24, 25, 27, 30], or use of COX2 selective inhibitors plus concurrent PPI [24, 31]. Although OA guidelines do not explicitly define gastrointestinal risk, the 2009 American College of Gastroenterology guidelines provide a pragmatic definition: age > 65, history of peptic ulcer disease, or concomitant use of aspirin, antiplatelets, anticoagulants, or steroids [66]. The expert panel believed that the addition of the PPI was more important than focusing on selection of an NSAID based on COX1 vs COX2 selectivity. Among patients with elevated risk for cardiovascular side effects from NSAIDs, guidelines suggest that naproxen is allowable, whereas COX2 inhibitors or non-selective NSAIDs may increase risk for cardiovascular adverse events [27, 30]. Although short courses and low doses of NSAIDs may be reasonable for some patients with stage 3 CKD, the expert panel recommended that NSAIDs be avoided for CKD stages 4 and 5 [24]. Because patients with OA may receive NSAID prescriptions from multiple providers (PCPs, rheumatologists, and orthopedic surgeons) and may take over-the-counter NSAIDs, they are at risk for using multiple of NSAIDs simultaneously. Therefore, comprehensive medication reconciliation should be conducted during primary care visits to identify and remove combinations of NSAIDs.
The panel included two QIs focused on opioid use. Several guidelines provide specific recommendations for tramadol [26, 30, 32]. Historically, tramadol was not scheduled by the Drug Enforcement Agency but is currently listed as schedule IV. The expert panel believed that many primary care clinicians may be more comfortable prescribing tramadol compared with other opioids although tramadol 50 mg has a morphine milligram equivalent of 5, which is equivalent to other commonly used opioids (e.g., hydrocodone 5 mg and codeine 30 mg). Therefore, the expert panel believed it was necessary to specifically track and minimize tramadol use. The expert panel chose the phrase “track and minimize” rather than “avoid” for QIs related to opioids as short courses of opioids are appropriate for some patients with advanced OA that is refractory to conventional therapy. The expert panel believed that tracking this QI and reporting this information back to PCPs would facilitate opioid stewardship. Some patients with OA will be taking opioids for other comorbid conditions and matching the opioid prescription to the specific indication through automated alerting or reporting may be challenging. Therefore, it is unreasonable to target 0% for these two QIs.
QIs for education, weight-management, and psychological wellbeing
Experts included three QIs for providing general OA education (#4), referring patients with BMI > 40 kg/m2 and lower extremity OA to weight management (#8), and screening for depression and anxiety (#14). Many guidelines recommend education alone or in combination with other interventions (e.g., exercise or weight management) as a safe and cost-effective intervention. Experts acknowledged that providers may have different styles for delivering education (e.g., verbal counseling, written material, or videos) and documenting in the EHR that this education was provided. Therefore, the overall prioritization of this QI was slightly reduced due to resources that would be needed to standardize workflow among PCPs for documenting that education was provided. Experts envisioned that an educational packet could be loaded into the EHR, printed in the after-visit summary, and posted in the patient-facing medical record portal. Although experts excluded QIs related to exercise, transcutaneous electrical nerve stimulation, and weight management if BMI > 25 kg/m2, these could be included in educational material provided to patients.
Weight management is an evidence-based strategy to reduce stress on joints in the lower extremities among obese OA patients. The expert panel believed that BMI thresholds of > 25 kg/m2 (overweight) or > 30 kg/m2 (obese) from guidelines would include a large volume of OA patients due to high prevalence of obesity in our community [28, 29, 31, 41, 53, 55], and that a higher threshold > 40 kg/m2 would be more appropriate to trigger referral. Although some PCPs provide weight management services for patients with a BMI < 40 kg/m2 as part of their practice, the expert panel expected PCPs to refer patients with a BMI > 40 kg/m2 for weight management interventions.
Primary care physicians are strategically positioned to screen for depression and anxiety. This is feasible at our health system since a quick and simple Patient Health Questionnaire 2 depression screening tool is already available within ambulatory visits in our EHR [67].
QIs for optimizing first-line medications
The expert panel included QIs that promote use of topical NSAIDs for superficial joints (#6), systemic/oral NSAIDs (#7), and acetaminophen (#13). Given their relatively low cost, ease of access, and acceptable safety profiles, these medications are commonly considered as first-line pharmacological therapies for OA pain management in OA guidelines. Although some OA guidelines [24, 32, 36] and previous lists of QIs [68,69,70] emphasized the use of these medications as first-line medication therapy, the expert panel believe that it would not always be feasible for quality analysts to establish the sequence of medications previously trialed when calculating these QIs, especially for patients with long histories of OA or multiple prescribing providers. Even though these therapies may not be effective monotherapies for all patients, they can be used in combination across the continuum of OA severity. Therefore, our QIs focus on the proportion of patients (or clinic visits) that are receiving these therapies at a given time (point prevalence). Although the goal is not to achieve 100% due to contraindications and treatment failure, these QIs can be used to track changes in prescribing patterns over time and evaluate the impact of targeted quality initiatives. Another potential use of these QIs is to compare the relative exposure of these therapies against exposure to opioids in a population over time. One limitation identified by the expert panel is that use of over-the-counter formulations of acetaminophen, topical NSAIDs, and oral NSAIDs may be underreported in the EHR since prescriptions are not required.
QIs for referral
The expert panel believed that most patients with OA can benefit from and should be referred to physical therapy/occupational therapy (#5). The expert panel believed that PCPs should refer patients who fail conservative therapy to a specialist (#11). Conservative therapy could be pharmacological or non-pharmacological and can be provided by a PCP. Candidate QIs regarding choice of intraarticular injection or surgical approach (e.g., arthroscopic procedure) are not made by the PCP at the time of referral and were therefore excluded from the list of final QIs.
QIs for imaging
The expert panel recommended that PCPs avoid unnecessary imaging for OA management (#15). Specifically, imaging for evaluating the effect of pharmacological or non-pharmacological interventions would be unnecessary. At our health-system, physician orders for imaging are associated with diagnosis codes which allow for feasible evaluation of this QI. Experts believed that streamlined coordination (e.g., consensus order panels) between PCPs and the surgeons could reduce unnecessary imaging. This QI does not include imaging related to the original diagnosis of OA or a substantial change in clinical status.
Limitations
Although the multi-disciplinary expert panel represents clinicians with a variety of backgrounds who received training at diverse institutions, all experts were recruited from a single health-system. The experts were asked to evaluate feasibility regarding the current status of care at our health-system, and it is possible that some QIs that are not feasible at our health-system may be feasible at other health-systems. As a pragmatic modification of the RAND/UCLA approach, our expert discussions occurred over multiple 60/90-min meetings rather than a focused workshop on 1–2 days. Some experts were not available for some discussions. Relevant clinical guidelines may have been published before or after the time frame of January 2015 to March 2021 that was used for the literature search. Patients with osteoarthritis were not invited to participate in the expert panel for this study.
Conclusion
A multi-professional expert panel engaged in a consensus strategy that was guided by literature to develop a set of 15 prioritized, valid, and feasible QIs that can be used to track quality initiatives for OA pain management in the primary care setting. Future research is needed to develop operational definitions to measure and track each QI using structured data in the EHR. Additionally, future studies should evaluate associations between these QIs and important health outcomes.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- CV:
-
Cardiovascular
- EHR:
-
Electronic health record
- GI:
-
Gastrointestinal
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PPI:
-
Proton pump inhibitor
- PT/OT:
-
Physical therapy/occupational therapy
- PCPs:
-
Primary care physicians
- QI:
-
Quality indicator
- RAND/UCLA:
-
Research and Development Corporation/University of California Los Angeles
- REDCap:
-
Research Electronic Data Capture
References
Centers for Disease Control and Prevention. Osteoarthritis (OA). 2020.
Centers for Disease Control and Prevention. A national public health agenda for osteoarthritis: 2020 update. 2020.
US Bone and Joint Initiative. The burden of musculoskeletal diseases in the United States. https://www.boneandjointburden.org/fourth-edition/iiib10/osteoarthritis. Accessed 27 June 2023.
Centers for Disease Control and Prevention. Joint Pain and Arthritis. 2020.
Alamanda VK, Wally MK, Seymour RB, Springer BD, Hsu JR, Prescription Reporting With Immediate Medication Utilization Mapping G. Prevalence of opioid and benzodiazepine prescriptions for osteoarthritis. Arthritis Care Res. 2020;72(8):1081–6.
Gandhi K, Wei W, Huang A, Wang L, Iyer R, Katz NP. A real-world study using claims data to evaluate possible failure of opioid treatment regimens among patients with hip and/or knee osteoarthritis in the US. Clinicoecon Outcomes Res. 2020;12:285–97.
Huizinga JL, Stanley EE, Sullivan JK, Song S, Hunter DJ, Paltiel AD, Neogi T, Edwards RR, Katz JN, Losina E. Societal cost of opioid use in symptomatic knee osteoarthritis patients in the United States. Arthritis Care Res. 2021;74(8):1349-58.
Johnson CA, Goodloe JB, Durante EC, Barfield WR, Gross CE. Non-orthopedic encounters increase opioid exposure in joint osteoarthritis: a single-institution analysis. J Arthroplasty. 2020;35(9):2386–91.
U.S. Department of Health and Human Services. What is the U.S. opioid epidemic? 2021.
U.S. Department of Health and Human Services. Overdose prevention strategy. 2021.
Healey EL, Afolabi EK, Lewis M, Edwards JJ, Jordan KP, Finney A, Jinks C, Hay EM, Dziedzic KS. Uptake of the NICE osteoarthritis guidelines in primary care: a survey of older adults with joint pain. BMC Musculoskelet Disord. 2018;19(1):295.
Swaithes L, Paskins Z, Dziedzic K, Finney A. Factors influencing the implementation of evidence-based guidelines for osteoarthritis in primary care: a systematic review and thematic synthesis. Musculoskeletal Care. 2020;18(2):101–10.
Basedow M, Esterman A. Assessing appropriateness of osteoarthritis care using quality indicators: a systematic review. J Eval Clin Pract. 2015;21(5):782–9.
Hagen KB, Smedslund G, Østerås N, Jamtvedt G. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res. 2016;68(10):1443–52.
Houston Methodist. Facts and statistics. 2020.
Rizk E, Swan JT, Cheon O, Colavecchia AC, Bui LN, Kash BA, Chokshi SP, Chen H, Johnson ML, Liebl MG, Fink E. Quality indicators to measure the effect of opioid stewardship interventions in hospital and emergency department settings. Am J Health Syst Pharm. 2019;76(4):225–35.
Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2(1):53–63.
Fitch K, Berstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lázaro P. The RAND/UCLA appropriateness method user’s manual. Santa Monica: RAND Corporation; 2001.
Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49.
U.S. Department of Health and Human Services. Determination that a public health emergency exists. 2017.
Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds Ashley ES, Structured Taskforce of Experts Working at Reliable Standards for Stewardship Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis. 2017;64(3):377–83.
van den Bosch CM, Geerlings SE, Natsch S, Prins JM, Hulscher ME. Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin Infect Dis. 2015;60(2):281–91.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Bruyere O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G, Martel-Pelletier J, Pelletier JP, Rannou F, Rizzoli R, Roth R, Uebelhart D, Cooper C, Reginster JY. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–50.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–62.
Ariani A, Manara M, Fioravanti A, Iannone F, Salaffi F, Ughi N, Prevete I, Bortoluzzi A, Parisi S, Scire CA. The Italian Society for rheumatology clinical practice guidelines for the diagnosis and management of knee, hip and hand osteoarthritis. Reumatismo. 2019;71(S1):5–21.
Tuncer T, Cay FH, Altan L, Gurer G, Kacar C, Ozcakir S, Atik S, Ayhan F, Durmaz B, Eskiyurt N, Genc H, GokceKutsal Y, Gunaydin R, Hepguler S, Hizmetli S, Kaya T, Kurtais Y, Saridogan M, Sindel D, Sutbeyaz S, Sendur OF, Ugurlu H, Unlu Z. 2017 update of the Turkish League Against Rheumatism (TLAR) evidence-based recommendations for the management of knee osteoarthritis. Rheumatol Int. 2018;38(8):1315–31.
Ruiz Iban MA, Macule F, Torner P, Gil Garay E, Oteo-Alvaro A, Lopez Millan JM, Diaz Heredia J, Loza E. SECOT-GEDOS consensus on pre-surgical pain management in knee and hip arthrosis. Rev Esp Cir Ortop Traumatol. 2015;59(3):186–99.
Rillo O, Riera H, Acosta C, Liendo V, Bolanos J, Monterola L, Nieto E, Arape R, Franco LM, Vera M, Papasidero S, Espinosa R, Esquivel JA, Souto R, Rossi C, Molina JF, Salas J, Ballesteros F, Radrigan F, Guibert M, Reyes G, Chico A, Camacho W, Urioste L, Garcia A, Iraheta I, Gutierrez CE, Aragon R, Duarte M, Gonzalez M, Castaneda O, Angulo J, Coimbra I, Munoz-Louis R, Saenz R, Vallejo C, Briceno J, Acuna RP, De Leon A, Reginato AM, Moller I, Caballero CV, Quintero M. PANLAR consensus recommendations for the management in osteoarthritis of hand, hip, and knee. J Clin Rheumatol. 2016;22(7):345–54.
Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y, Liu S, Chen Y, Mei Y, Ding C, Chen M, Gu X, Xing D, Gao M, He L, Ye Z, Wu L, Xu J, Yang P, Zhang X, Zhang Y, Chen J, Lin J, Zhao L, Li M, Yang W, Zhou Y, Jiang Q, Chu CQ, Chen Y, Zhang W, Tsai WC, Lei G, He D, Liu W, Fang Y, Wu D, Lin J, Wei CC, Lin HY, Zeng X. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann Transl Med. 2020;8(19):1213.
Bruyere O, Cooper C, Pelletier JP, Maheu E, Rannou F, Branco J, Luisa Brandi M, Kanis JA, Altman RD, Hochberg MC, Martel-Pelletier J, Reginster JY. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3-11.
Brazilian Medical A, Silvinato A, Bernardo WM. Inflammatory arthritis or osteoarthritis of the knee - Efficacy of intra-joint infiltration of methylprednisolone acetate versus triamcinolone acetonide or triamcinolone hexacetonide. Rev Assoc Med Bras (1992). 2017;63(10):827–36.
Petzke F, Bock F, Huppe M, Nothacker M, Norda H, Radbruch L, Schiltenwolf M, Schuler M, Tolle T, Viniol A, Hauser W. Long-term opioid therapy for chronic noncancer pain: second update of the German guidelines. Pain Rep. 2020;5(5):e840.
Manchikanti L, Centeno CJ, Atluri S, Albers SL, Shapiro S, Malanga GA, Abd-Elsayed A, Jerome M, Hirsch JA, Kaye AD, Aydin SM, Beall D, Buford D, Borg-Stein J, Buenaventura RM, Cabaret JA, Calodney AK, Candido KD, Cartier C, Latchaw R, Diwan S, Dodson E, Fausel Z, Fredericson M, Gharibo CG, Gupta M, Kaye AM, Knezevic NN, Kosanovic R, Lucas M, Manchikanti MV, Mason RA, Mautner K, Murala S, Navani A, Pampati V, Pastoriza S, Pasupuleti R, Philip C, Sanapati MR, Sand T, Shah RV, Soin A, Stemper I, Wargo BW, Hernigou P. Bone Marrow Concentrate (BMC) therapy in musculoskeletal disorders: evidence-based policy position statement of American Society of Interventional Pain Physicians (ASIPP). Pain Physician. 2020;23(2):E85–131.
Arthroscopy Association of C, Wong I, Hiemstra L, Ayeni OR, Getgood A, Beavis C, Volesky M, Outerbridge R, Sheehan B, McCormack R, Litchfield R, Whelan D, Mohtadi N, Coady C, MacDonald PB. Position statement of the Arthroscopy Association of Canada (AAC) concerning arthroscopy of the knee joint-September 2017. Orthop J Sports Med. 2018;6(2):2325967118756597.
Arthroscopy Association of C, Kopka M, Sheehan B, Degen R, Wong I, Hiemstra L, Ayeni O, Getgood A, Beavis C, Volesky M, Outerbridge R, Matache B. Arthroscopy Association of Canada position statement on intra-articular injections for knee osteoarthritis. Orthop J Sports Med. 2019;7(7):2325967119860110.
van Doormaal MCM, Meerhoff GA, Vliet Vlieland TPM, Peter WF. A clinical practice guideline for physical therapy in patients with hip or knee osteoarthritis. Musculoskeletal Care. 2020;18(4):575–95.
Eymard F, Ornetti P, Maillet J, Noel E, Adam P, Legre-Boyer V, et al. Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: a consensus statement from French-speaking experts. Knee Surg Sports Traumatol Arthrosc. 2021;29(10):3195–210.
Sellam J, Courties A, Eymard F, Ferrero S, Latourte A, Ornetti P, Bannwarth B, Baumann L, Berenbaum F, Chevalier X, Ea HK, Fabre MC, Forestier R, Grange L, Lellouche H, Maillet J, Mainard D, Perrot S, Rannou F, Rat AC, Roux CH, Senbel E, Richette P, French Society of R. Recommendations of the French Society of Rheumatology on pharmacological treatment of knee osteoarthritis. Joint Bone Spine. 2020;87(6):548–55.
Cibulka MT, Bloom NJ, Enseki KR, Macdonald CW, Woehrle J, McDonough CM. Hip pain and mobility deficits-hip osteoarthritis: revision 2017. J Orthop Sports Phys Ther. 2017;47(6):A1–37.
Browne JA, Nho SJ, Goodman SB, Della Valle CJ. American association of hip and knee surgeons, hip society, and knee society position statement on biologics for advanced hip and knee arthritis. J Arthroplasty. 2019;34(6):1051–2.
Khazzam M, Gee AO, Pearl M. Management of glenohumeral joint osteoarthritis. J Am Acad Orthop Surg. 2020;28(19):781–9.
Hawk C, Whalen W, Farabaugh RJ, Daniels CJ, Minkalis AL, Taylor DN, Anderson D, Anderson K, Crivelli LS, Cark M, Barlow E, Paris D, Sarnat R, Weeks J. Best practices for chiropractic management of patients with chronic musculoskeletal pain: a clinical practice guideline. J Altern Complement Med. 2020;26(10):884–901.
Iolascon G, Ruggiero C, Fiore P, Mauro GL, Moretti B, Tarantino U. Multidisciplinary integrated approach for older adults with symptomatic osteoarthritis: SIMFER and SI-GUIDA joint position statement. Eur J Phys Rehabil Med. 2020;56(1):112–9.
Brosseau L, Wells GA, Pugh AG, Smith CA, Rahman P, Alvarez Gallardo IC, Toupin-April K, Loew L, De Angelis G, Cavallo S, Taki J, Marcotte R, Fransen M, Hernandez-Molina G, Kenny GP, Regnaux JP, Lefevre-Colau MM, Brooks S, Laferriere L, McLean L, Longchamp G. Ottawa panel evidence-based clinical practice guidelines for therapeutic exercise in the management of hip osteoarthritis. Clin Rehabil. 2016;30(10):935–46.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Alvarez Gallardo IC, Gifford W, Laferriere L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil. 2017;31(5):612–24.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Alvarez Gallardo IC, Gifford W, Laferriere L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31(5):596–611.
Chen WH, Liu XX, Tong PJ, Zhan HS, Orthopaedic Professional Committee CAoR, Advancement of Chinese Traditional Medicine C, Joint Professional Committee BoOoCAoIMC. Diagnosis and management of knee osteoarthritis: Chinese medicine expert consensus (2015). Chin J Integr Med. 2016;22(2):150–3.
Siemieniuk RAC, Harris IA, Agoritsas T, Poolman RW, Brignardello-Petersen R, Van de Velde S, Buchbinder R, Englund M, Lytvyn L, Quinlan C, Helsingen L, Knutsen G, Olsen NR, Macdonald H, Hailey L, Wilson HM, Lydiatt A, Kristiansen A. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;357:j1982.
Ayhan FF, Sunar I, Umay E, KeskIn D, Altan L, DİnÇer F, DuruOz T, Karalezl IN, Kuran B, Tuncer T. The Turkish league against rheumatism recommendations for the management of hand osteoarthritis under guidance of the current literature and 2018 European league against rheumatism recommendations. Arch Rheumatol. 2020;35(3):309–20.
Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, Haugen IK, Herrero-Beaumont G, Jonsson H, Kjeken I, Maheu E, Ramonda R, Ritt MJ, Smeets W, Smolen JS, Stamm TA, Szekanecz Z, Wittoek R, Carmona L. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78(1):16–24.
Royal Australian College of General Practitioners. Guideline for the management of knee and hip osteoarthritis. 2nd ed. 2018.
Sakellariou G, Conaghan PG, Zhang W, Bijlsma JWJ, Boyesen P, D’Agostino MA, Doherty M, Fodor D, Kloppenburg M, Miese F, Naredo E, Porcheret M, Iagnocco A. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann Rheum Dis. 2017;76(9):1484–94.
National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management. 2014.
Geenen R, Overman CL, Christensen R, Asenlof P, Capela S, Huisinga KL, Husebo MEP, Koke AJA, Paskins Z, Pitsillidou IA, Savel C, Austin J, Hassett AL, Severijns G, Stoffer-Marx M, Vlaeyen JWS, Fernandez-de-Las-Penas C, Ryan SJ, Bergman S. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797–807.
Department of Veterans Affairs. VA/DoD clinical practice guideline for the non-surgical management of hip and knee osteoarthritis. 2020.
American Academy of Orthopaedic Surgeons. Management of osteoarthritis of the hip evidence-based clinical practice guideline. 2017.
Brosseau L, Thevenot O, MacKiddie O, Taki J, Wells GA, Guitard P, Leonard G, Paquet N, Aydin SZ, Toupin-April K, Cavallo S, Moe RH, Shaikh K, Gifford W, Loew L, De Angelis G, Shallwani SM, Aburub AS, Mizusaki Imoto A, Rahman P, Alvarez Gallardo IC, Cosic MB, Osteras N, Lue S, Hamasaki T, Gaudreault N, Towheed TE, Koppikar S, Kjeken I, Mahendira D, Kenny GP, Paterson G, Westby M, Laferriere L, Longchamp G. The Ottawa panel guidelines on programmes involving therapeutic exercise for the management of hand osteoarthritis. Clin Rehabil. 2018;32(11):1449–71.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Imoto AM, Toupin-April K, Westby M, Gallardo ICA, Gifford W, Laferriere L, Rahman P, Loew L, Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part one: introduction, and mind-body exercise programs. Clin Rehabil. 2017;31(5):582–95.
Crossley KM, Stefanik JJ, Selfe J, Collins NJ, Davis IS, Powers CM, McConnell J, Vicenzino B, Bazett-Jones DM, Esculier JF, Morrissey D, Callaghan MJ. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50(14):839–43.
Conrozier T, Monfort J, Chevalier X, Raman R, Richette P, Diracoglu D, Bard H, Baron D, Jerosch J, Migliore A, Henrotin Y. EUROVISCO recommendations for optimizing the clinical results of viscosupplementation in osteoarthritis. Cartilage. 2020;11(1):47–59.
Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Gunther KP, Hurkmans E, Juhl CB, Kennedy N, Kiltz U, Knittle K, Nurmohamed M, Pais S, Severijns G, Swinnen TW, Pitsillidou IA, Warburton L, Yankov Z, Vliet Vlieland TPM. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(9):1251–60.
Polido-Pereira J, Serra S, Teixeira F, Ponte C, Cerqueira M, Cruz M, Araujo F, Barros R, Costa T, Santos-Faria D, Lopes C, Madruga-Dias J, Oliveira M, Teixeira R, Vilar A, Falcao S, Saraiva F, Figueiredo G. Portuguese recommendations for the use of ultrasound in rheumatology. Acta Reumatol Port. 2019;44(1):7–28.
da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, Trelle S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–33.
Lanza FL, Chan FK, Quigley EM, Practice Parameters Committee of the American College of G. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–38.
Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92.
Pencharz JN, MacLean CH. Measuring quality in arthritis care: the arthritis foundation’s quality indicator set for osteoarthritis. Arthritis Rheum. 2004;51(4):538–48.
Arslan IG, Rozendaal RM, van Middelkoop M, Stitzinger SAG, Van de Kerkhove MP, Voorbrood VMI, Bindels PJE, Bierma-Zeinstra SMA, Schiphof D. Quality indicators for knee and hip osteoarthritis care: a systematic review. RMD Open. 2021;7(2):e001590.
Petrosyan Y, Sahakyan Y, Barnsley JM, Kuluski K, Liu B, Wodchis WP. Quality indicators for care of osteoarthritis in primary care settings: a systematic literature review. Fam Pract. 2018;35(2):151–9.
Acknowledgements
Authors would like to acknowledge Amy Taylor, MLS (Medical Librarian, Houston Methodist Hospital) for assistance with constructing a literature search strategy.
Prior presentation
This work was not previously presented before submission to the journal.
Funding
This study was sponsored by Pfizer and Eli Lilly & Company. Joshua Swan is an employee of Houston Methodist Hospital, which received funding from Pfizer and Eli Lilly & Company in connection with this study and the development of this manuscript.
Author information
Authors and Affiliations
Contributions
JTS and ST designed the study and obtained study funding. ER, TI, DKA, and JTS designed and executed the literature search and extraction of evidence-based recommendations. ER and JTS consolidated and organized recommendations into proposed quality indicators. E Fink, E Flores, ER, ST, and JTS completed the feasibility survey. ER, ST, E Fink, E Flores, AEB, SPC, SND, AKD, SAK, MG, SN, CPR, VV, and JTS completed the validity survey, participated in expert panel discussions, and completed the priority survey. JTS moderated expert panel discussions. ER and JTS analyzed study data and drafted the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Houston Methodist Research Institute’s Institutional Review Board approved this study with a waiver of informed consent (approval number PRO00024493). All study methods were carried out in accordance with the relevant guidelines and regulations including the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
This study was sponsored by Pfizer and Eli Lilly & Company. Joshua Swan is an employee of Houston Methodist Hospital, which received funding from Pfizer and Eli Lilly & Company in connection with this study and the development of this manuscript. Dr. Swan’s employer (Houston Methodist) has received funding on his behalf for analgesia research from Pacira Pharmaceuticals, Inc and Heron Therapeutics. Co-author Sharla Tajchman, who is an employee at Pfizer with stock and/or stock options, participated in study design, interpretation of study data, and writing of the manuscript. All remaining authors declare no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rizk, E., Tajchman, S., Fink, E. et al. Quality indicators for osteoarthritis pain management in the primary care setting. BMC Musculoskelet Disord 24, 538 (2023). https://doi.org/10.1186/s12891-023-06637-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06637-x