Abstract
Background
Adolescent idiopathic scoliosis (AIS) is a three-dimensional spinal deformity that predominantly occurs in girls. While skeletal growth and maturation influence the development of AIS, accurate prediction of curve progression remains difficult because the prognosis for deformity differs among individuals. The purpose of this study is to develop a new diagnostic platform using a deep convolutional neural network (DCNN) that can predict the risk of scoliosis progression in patients with AIS.
Methods
Fifty-eight patients with AIS (49 females and 9 males; mean age: 12.5 ± 1.4 years) and a Cobb angle between 10 and 25 degrees (mean angle: 18.7 ± 4.5) were divided into two groups: those whose Cobb angle increased by more than 10 degrees within two years (progression group, 28 patients) and those whose Cobb angle changed by less than 5 degrees (non-progression group, 30 patients). The X-ray images of three regions of interest (ROIs) (lung [ROI1], abdomen [ROI2], and total spine [ROI3]), were used as the source data for learning and prediction. Five spine surgeons also predicted the progression of scoliosis by reading the X-rays in a blinded manner.
Results
The prediction performance of the DCNN for AIS curve progression showed an accuracy of 69% and an area under the receiver-operating characteristic curve of 0.70 using ROI3 images, whereas the diagnostic performance of the spine surgeons showed inferior at 47%. Transfer learning with a pretrained DCNN contributed to improved prediction accuracy.
Conclusion
Our developed method to predict the risk of scoliosis progression in AIS by using a DCNN could be a valuable tool in decision-making for therapeutic interventions for AIS.
Similar content being viewed by others
Background
Adolescent idiopathic scoliosis (AIS) is a three-dimensional spinal deformity that predominantly occurs in girls aged between 10 and 13 years that is diagnosed by a coronal plane angle (measured by the Cobb method) of more than 10 degrees [1], without congenital, neuromuscular, or symptomatic etiology. Approximately 2–4% of children under the age of 16 years develop scoliosis, among which only 0.3–0.5% have a progressive curve that requires treatment [2,3,4]. Severe scoliosis is associated with low back pain, functional disability, cardiopulmonary disorders [5], early degenerative changes in the spine, and mental impairment [6]. In general, the risk of curve progression is most likely to occur during the prepubertal period of peak height velocity (PHV). Therefore, skeletally immature patients with AIS should be carefully monitored using serial examinations and radiographic studies. Conservative treatment with rigid bracing is indicated for patients with residual skeletal growth [2] and a curve size of more than 20–25 degrees [4, 7, 8] until skeletal maturation is achieved to control the progression of scoliosis. Surgical treatment with spinal fusion is generally performed for patients with a curve of more than 40–50 degrees. As the curve progresses, highly invasive spinal fusion surgery is required with the risk of complications. Therefore, early diagnosis and appropriate intervention are important to prevent curve progression.
Regular follow-up with radiography is necessary for patients with relatively mild scoliosis between 10 and 25 degrees. It is widely accepted that skeletal growth and maturation influence the progression of AIS, and the risk of curve progression was found to be significantly correlated with time to PHV [9]. Maturity of the skeleton and PHV can be evaluated by menarche in girls, Tanner staging of sexual maturation, and various radiographic parameters, including the Risser sign [10], Tanner-Whitehouse scores [11], distal radius and ulna stages [12], digital skeletal age scores [9], and Sanders simplified the skeletal maturity system [13]. However, accurate prediction of curve progression remains elusive because the prognosis of the deformity varies in each individual [14, 15]. To prevent future curve progression, determining the risk of scoliosis prognosis and the potential benefit of treatment with orthosis or brace is the responsibility of the primary clinician [16]. Therefore, it may be clinically valuable to accurately predict the risk of scoliosis progression as early as possible.
Rapidly developing machine-learning technology can be applied to automatically determine the risk of scoliosis progression. Artificial neural networks are a set of techniques and algorithms for discovering complex patterns in large datasets, known as deep learning [17]. These models are being widely applied to form state-of-the-art approaches in various areas, such as speech recognition [18], visual object recognition [19], neuronal cell biology [20], and genomics [21, 22]. In particular, the deep convolutional neural network (DCNN) [23], a type of hierarchical neural network, has emerged as a powerful solution that excels in learning the features of objects from large amounts of training images and has successfully improved decision making in several clinical settings, such as diagnosis in radiation oncology [24] and diabetic retinopathy [25], detecting gastric cancer with endoscopic images [26], and the prediction of prognosis [27]. A large number of training images are essential for the successful development of the DCNN. However, owing to the limited number of patients, especially for medical images of rare diseases, it is difficult to collect sufficient datasets to train the network. Transfer learning, which utilizes pretrained models, is adequate for solving this problem. Pre-training with a vast majority of natural images, such as ImageNet [28], can improve the learning accuracy of the DCNN and accelerate its processing.
In this study, we sought to build a diagnostic platform to predict scoliosis curve progression in patients with AIS using a pre-trained DCNN. To achieve this goal, X-rays of patients with AIS were used as training images to learn the progression or non-progression of scoliosis. Our deep learning model achieved a diagnostic accuracy superior to that of spine surgeons. This diagnostic platform will be beneficial for specialists and non-expert clinicians in decision-making for therapeutic interventions for scoliosis.
Methods
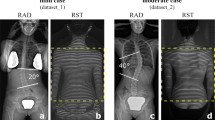
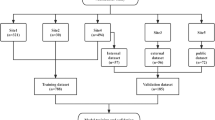
This study was approved by the ethics committee of Toyama University Hospital. We retrospectively reviewed the data of patients who visited or were referred to our university hospital for AIS screening between 2012 and 2021. All patients received total spine radiograms and standing frontal and sagittal views were used for analysis. The study included a total of fifty-eight patients (49 females and 9 males; mean age: 12.5 ± 1.4 years) with a Cobb angle between 10 and 25 degrees (mean angle: 18.7 ± 4.5). The patients were divided into two groups: those whose Cobb angle increased more than 10 degrees within two years (progression group: 28 patients, 23 females and 5 males; mean age: 12.2 ± 1.6 years) and those whose Cobb angle changed by less than 5 degrees within two years (non-progression group: 30 patients, 26 females and 4 males; mean age: 12.8 ± 1.0 years) (Figs. 1 and 2, Table 1). Because most of the patients who enrolled in this study had non-constructive scoliotic curvature, the Cobb angle could vary with posture and examination time. Therefore, patients with Cobb angle changes between 5 and 10 degrees were excluded from the study to clarify the predictive criteria. Bracing therapy was initiated if a patient developed a Cobb angle of greater than 25 degrees and a Risser sign of less than two or if the curve progressed more than 5 degrees at the semiannual follow-up. Contrarily, bracing therapy was terminated for patients with a Risser sign of 3 or greater and no worsening of the Cobb angle at the semiannual follow-up. The average follow-up duration in the non-progression group was 3.9 ± 1.2 years, and that in the progression group was 2.0 ± 0.9 years.
The frontal view of the total spine radiographs was used as the source data for learning and prediction by the DCNN. Images were cropped using a combination of three horizontal lines and two vertical lines passing through the acromion on both sides, and images using three regions of interest (ROI) were prepared: the C7 vertebra and diaphragm (ROI1, lung); diaphragm and ilium (ROI2, abdomen); and C7 vertebra and ilium (ROI3, total spine) (Fig. 3). Each cropped image was resized while maintaining the aspect ratio such that the long side was 299 pixels according to the input size of the pre-trained DCNN (Xception) with ImageNet [29]. Black pixels were inserted on the short side to obtain a resized image with 299 × 299 pixels. Computational processing was performed using a CPU: Intel Corei7—10,700, memory: 128 GB, GPU: NVIDIA Quadro RTX 8000, and MATLAB 2020a. In the learning by Xception, fine-tuning was conducted using the weights by pre-training on ImageNet, and leave-one-out cross-validation was used for evaluation. In some experiments, the accuracy of scoliosis progression prediction was assessed with or without transfer learning to evaluate the effect of the pre-trained DCNN and fine-tuning. The training conditions were as follows: learning rate, 0.001; batch size, 8; number of epochs, 50; and optimizer, stochastic gradient descent with momentum (SGDM). The gradient-weighted class activation mapping (Grad-CAM) method was used to visualize the regions recognized by the DCNN [30]. First, ROI3 was divided into six regions: the upper to middle thoracic spine, lower thoracic spine, lumbar spine, lung, abdomen, iliac, and others. Next, the regions in which the heat map gave the strongest signal were compared between the successfully and unsuccessfully classified groups (Fig. 6). Five spine surgeons also predicted the progression of scoliosis by reading the X-rays in a blinded manner. Frontal radiographs of ROI3 images from all 58 patients with AIS were used for the prediction and personal information such as patient gender, age, or presence or absence of menarche was not provided.
Frontal view of total spine radiographs for prediction of curve progression by DCNN. Images were cropped into three different regions of interest (ROI); C7 vertebra to diaphragm (ROI1, lung), diaphragm to iliac (ROI2, abdomen), and C7 vertebra to iliac (ROI3, total spine). DCNN, deep convolutional neural network
Data are presented as mean and standard deviation. Significant differences between means were analyzed using the t-test (two-sided) and the chi-square test. In comparing AUC (area under the curve) among the three types of ROI, we used analysis of variance (ANOVA) followed by Tukey–Kramer post hoc test. In addition, the Pearson correlation test was used to analyze the correlation between the curve progression and several parameters. Statistical analysis was performed using Excel statistical software (Statcel4; OMS, Tokorozawa, Japan) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). Classification accuracy was evaluated using receiver operating characteristic (ROC) analysis. A computer program (LABMRMC, The University of Chicago, Chicago, IL) was used to obtain ROC curves and evaluate statistically significant differences [31, 32]. Statistical significance was set at P < 0.05.
Result
In the progression group, the Cobb angle increased by an average of 13.6 degrees within two years, whereas in the non-progression group, the Cobb angle only increased by 0.5 degrees about four years (Table 1). The two groups demonstrated no significant difference in sagittal radiographic alignment, such as lumbar lordosis and thoracic kyphosis. In contrast, the percentage of pre-menarche patients was significantly higher in the progression group than in the non-progression group (Table 1). This cohort included 76% skeletally immature patients with Risser signs of 3 or less, and 34% of the females were premenarcheal. The correlation between the patient profiles at the first visit and curve progression was also evaluated. There was a weak negative correlation between scoliosis progression and age (r = -0.23, p = 0.07) at the first examination; however, there was no correlation between scoliosis progression and Cobb angle (r = 0.003, p = 0.98), lumbar lordosis (r = 0.199, p = 0.13), thoracic kyphosis (r = -0.01, p = 0.91), or Risser sign (r = 0.01, p = 0.88) (Fig. 4, Table 2).
The performance of the DCNN for the curve progression of AIS using ROI3 achieved a 61% sensitivity, 77% specificity, and 69% accuracy compared to ROI1 (53% sensitivity, 57% specificity, and 55% accuracy) and ROI2 (50% sensitivity, 50% specificity, and 50% accuracy) (Table 3). ROI3 also showed significantly higher AUC (0.7 ± 0.03) than that of ROI1 (0.53 ± 0.04) and ROI2 (0.44 ± 0.04) (Fig. 5). In addition, the results of prediction without transfer learning and fine-tuning showed inferior prediction results for ROI3 (57% sensitivity, 70% specificity, and 64% accuracy) compared to the analysis with pre-trained DCNN and fine-tuning (Table 3). The Grad-CAM heatmap showed the regions that the DCNN paid the most attention to in predicting scoliosis progression using ROI3. In the successfully classified group, Grad-CAM predicted scoliosis progression by focusing on the spinal column in 80% of the cases (33% upper-middle thoracic, 25% lower thoracic, and 22% lumbar). In contrast, non-spinal regions, such as the chest and abdomen, were recognized in the unsuccessful group in 45% of the cases (Fig. 6). Finally, the average accuracy of the five surgeons (47%) was inferior to that predicted by the DCNN (69%) (Table 4).
Comparison of AUC for three different ROIs (A) and ROC curves (B). Error bars denote the means ± SD. Statistical analysis was performed using one-way ANOVA. AUC, area under the curve; ROI, region of interest; ROC, receiver operating characteristic; SD, standard deviation ; TPF, true positive fraction; FPF, false positive fraction
Discussion
The deep learning system developed in this study could predict the progression of scoliosis with the highest accuracy of 69% and an AUC of 0.7. In addition, the DCNN could predict the progression of scoliosis with higher accuracy than that of spine surgeons. Transfer learning with a pretrained DCNN contributed to improved prediction accuracy.
Over the past decades, various indicators, including demographic, radiographic, physiological, biochemical, and their combinations, have been reported to be significantly associated with the progression of spinal deformity in AIS. Patients younger than 12–13 years old at the time of diagnosis have a significantly higher risk of developing spinal deformities [33, 34]. The risk of progression increases with a curve of more than 25 degrees [35], the flexibility of less than 28% [36], and patients with an immature skeleton (based on radiographic criteria) had a significantly higher risk of curve progression [8]. In addition, the progression of spinal deformity is also related to the wedging of the intervertebral discs and adjacent vertebral bodies, as well as longitudinal overgrowth of the vertebral bodies due to disproportionate endochondral-membranous bone growth [37,38,39]. However, the progression of scoliosis does not always coincide with skeletal growth. It is challenging for specialists as well as clinicians to adequately predict which type of scoliosis will progress and require therapeutic intervention, especially in mild scoliosis with a Cobb angle of less than 25 degrees. In fact, the ability of spine surgeon predict prognosis based solely on total spine radiographs at the initial visit was less than 50% in this study.
Skeletally immature patients with mild scoliosis of less than 25 degrees should undergo regular X-ray examinations to monitor the progression of scoliosis [40]. Orthosis or bracing therapy is indicated if the curve progresses more than 5 degrees during follow-up or reaches more than 20–25 degrees [8]. In general, early intervention in mild scoliosis is considered overtreatment because of the chaotic nature of the scoliosis progression [8, 41]. Because scoliosis may progress, stabilize, or disappear, observation is the primary treatment option for skeletally immature patients with mild scoliosis. However, this passive approach may result in missed treatment opportunities in patients with progressive scoliosis and does not address the patient and parents’ anxiety or stress about the progression of the curvature [41, 42]. Therefore, predicting the curve trajectory of a patient with early scoliosis has significant clinical implications. Deep-learning-based prognostic methods can help develop a preemptive treatment strategy that allows for more intensive treatment, such as early bracing or prolonged daily wearing, and early surgical intervention in cases with a high risk of progression, rather than conventional observation.
In this study, the DCNN predicted curve progression with the highest accuracy and AUC using ROI3. Grad-CAM showed that 25% of the successfully classified cases recognized the lower thoracic spine in ROI3. Because the lower thoracic spine is the transition point between the thoracic and lumbar spine, information missing from the chest to the abdomen in ROI1 and ROI2 may have contributed to the low prediction results. Grad-CAM computes gradients by back-propagating the prediction scores to the target layer of the network, and combines the forward feature maps by fitting them as weights. However, these weights do not correctly evaluate the importance of the feature map, and the feature map itself contains noise and includes unimportant parts [43]. Therefore, building an “explainable” predictive model that can adequately interpret the decisions made by the DCNN is critical to improving accuracy.
Only a few studies have investigated the use of deep learning to predict scoliosis progression. Wang et al. built a very accurate scoliosis prediction model using CapsNet [44]. However, their study limited the input images to only the major thoracic curves and did not examine the effect of double or triple major curves on the risk of progression. Although the prediction accuracy of the present study is inferior to that of their study, the present study targeted the entire spine and included more imaging information, such as trunk and rib rotation, pelvic tilt, and spinal deformity. Previous reports have shown that apical vertebral rotation and rib vertebra angle are possible parameters to predict the prognosis of trunk imbalance [45, 46]. Therefore, DCNN, which excels in image recognition, would favor full spine X-rays that include the thorax, ribs, clavicles, and spine in predicting progression. However, the sensitivity, specificity, and accuracy of this pilot study have not reached sufficient levels for clinical application. As we increase the number of cases, we hope to improve the accuracy of our model in identifying the characteristics of scoliosis progression, which will allow us to build a more accurate prediction model based on simple X-rays during the early stages of scoliosis. In addition, our model does not incorporate additional clinical parameters, such as age, sex, growth rate, or measures of skeletal maturity (Risser scoring, Saunders stage, and distal radius and ulna stage). The prediction accuracy can be improved by inputting X-ray images and patient profiles during the initial examination. Further clinical validation will facilitate the application of predictive models to support objective clinical decisions using the DCNN.
When developing deep learning frameworks for medical images, both the volume and quality of the data must be considered. Particularly in the case of rare diseases, it may be impossible to collect enough patients or samples. Transfer learning and fine-tuning can be adapted even when the dataset is small. Transfer learning is a powerful and promising technique that uses pretrained DCNN models to handle tasks with different patterns. For example, transfer learning has been successfully used for brain tumor recognition [47], pneumonia diagnosis [48], and seizure classification [49]. Pre-trained DCNN uses the parameters of the network on which DCNN was trained as the initial values. On the other hand, fine-tuning can adjust the parameters of a portion or all the layers of a trained model. Pre-training and fine-tuning are known to provide better results. In this study, the combination of both methods predicted scoliosis progression better than analysis without pre-training and fine-tuning.
The present study had several limitations that should be addressed in future studies. We only considered whole spine X-rays of 58 patients with AIS in this pilot study; integrating and analyzing the results in a larger study population would allow us to develop a more accurate system. Furthermore, although only Japanese patients with AIS were analyzed in this study, it is essential to establish the feasibility of this study among different racial groups. Furthermore, the accuracy of this prediction model must be verified through prospective follow-up studies. Finally, the ensemble method integrates multiple learning algorithms and obtains better prediction performance than using a single learning algorithm.
Conclusions
Successful treatment of AIS remains a challenging and complex issue for orthopedic surgeons. Therefore, we developed a clinically accessible predictive model for the risk of disease progression that can facilitate the planning of timely interventions to treat AIS during early clinical visits. In addition, predicting the prognosis of skeletally immature patients with mild scoliosis can help establish new evidence for early therapeutic intervention.
Availability of data and materials
The datasets analyzed in this study are not available to the public for privacy reasons. However, the data supporting the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Adolescent Idiopathic Scoliosis
- DCNN:
-
Deep Convolutional Neural Network
- ROI:
-
Regions Of Interest
- PHV:
-
Peak Height Velocity
- Grad-CAM:
-
Gradient-weighted Class Activation Mapping
- SGDM:
-
Stochastic Gradient Descent with Momentum
- AUC:
-
Area Under the Curve
- ANOVA:
-
Analysis Of Variance
- ROC:
-
Receiver Operating Characteristic
References
Kane WJ. Scoliosis prevalence: a call for a statement of terms. Clin Orthop Relat Res. 1977;126:43–6.
Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512–21.
Reamy BV, Slakey JB. Adolescent idiopathic scoliosis: review and current concepts. Am Fam Phys. 2001;64:111–6.
Noshchenko A, Hoffecker L, Lindley EM, Burger EL, Cain CM, Patel VV, et al. Predictors of spine deformity progression in adolescent idiopathic scoliosis: a systematic review with meta-analysis. World J Orthop. 2015;6:537–58.
Liu L, Xiu P, Li Q, Song Y, Chen R, Zhou C. Prevalence of cardiac dysfunction and abnormalities in patients with adolescent idiopathic scoliosis requiring surgery. Orthopedics. 2010;33:882.
Bengtsson G, Fällström K, Jansson B, Nachemson A. A psychological and psychiatric investigation of the adjustment of female scoliosis patients. Acta Psychiatr Scand. 1974;50:50–9.
Richards BS, Bernstein RM, D’Amato CR, Thompson GH. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS committee on bracing and nonoperative management. Spine. 2005;30:2068–75 discussion 76–7.
Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC, et al. SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2016;13(3):2018.
Sanders JO. Maturity indicators in spinal deformity. J Bone Joint Surg Am. 2007;89(Suppl 1):14–20.
Risser JC. The iliac apophysis: an invaluable sign in the management of scoliosis. Clinical orthopaedics and related Research®. Clin Orthop Relat Res. 1958;11:111–9.
Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–29.
Luk KD, Saw LB, Grozman S, Cheung KM, Samartzis D. Assessment of skeletal maturity in scoliosis patients to determine clinical management: a new classification scheme using distal radius and ulna radiographs. Spine J. 2014;14:315–25.
Sanders JO, Khoury JG, Kishan S, Browne RH, Mooney JF 3rd, Arnold KD, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90:540–53.
Busscher I, Wapstra FH, Veldhuizen AG. Predicting growth and curve progression in the individual patient with adolescent idiopathic scoliosis: design of a prospective longitudinal cohort study. BMC Musculoskelet Disord. 2010;11:93.
Elattar EA, Saber NZ, Farrag DA. Predictive factors for progression of adolescent idiopathic scoliosis: a 1-year study. Egypt Rheumatol Rehabil. 2015;42:111–9.
Cheung JPY, Cheung PWH, Samartzis D, Luk KD. APSS-ASJ best clinical Research award: predictability of curve progression in adolescent idiopathic scoliosis using the distal radius and ulna classification. Asian Spine J. 2018;12:202–13.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Hinton G, Deng L, Yu D, Dahl GE, Mohamed A, Jaitly N, et al. Deep neural networks for acoustic modeling in speech recognition: the shared views of four research groups. IEEE Signal Process Mag. 2012;29:82–97.
Farabet C, Couprie C, Najman L, Lecun Y. Learning hierarchical features for scene labeling. IEEE Trans Pattern Anal Mach Intell. 2013;35:1915–29.
Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–74.
Xiong HY, Alipanahi B, Lee LJ, Bretschneider H, Merico D, Yuen RK, et al. RNA splicing. the human splicing code reveals new insights into the genetic determinants of disease. Science. 2015;347:1254806.
Leung MK, Xiong HY, Lee LJ, Frey BJ. Deep learning of the tissue-regulated splicing code. Bioinformatics. 2014;30:i121–9.
Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Commun ACM. 2017;60:84–90.
Bibault JE, Giraud P, Burgun A. Big data and machine learning in radiation oncology: state of the art and future prospects. Cancer Lett. 2016;382:110–7.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10.
Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653–60.
Zhang Y, Lobo-Mueller EM, Karanicolas P, Gallinger S, Haider MA, Khalvati F. CNN-based survival model for pancreatic ductal adenocarcinoma in medical imaging. BMC Med Imaging. 2020;20:11.
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, et al. Imagenet large scale visual recognition challenge. Int J Comput Vis. 2015;115:211–52.
Chollet F. Xception: Deep Learning with Depthwise Separable Convolutions. 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 2017. p. 1251–8.
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D, et al. Grad-CAM: Visual explanations from deep networks via gradient-based localization. Int J Comput Vis. 2020;128:336–59.
Dorfman DD, Berbaum KS, Metz CE. Receiver operating characteristic rating analysis: generalization to the population of readers and patients with the jackknife method. Invest Radiol. 1992;27:723–31.
Roe CA, Metz CE. Dorfman-Berbaum-Metz method for statistical analysis of multireader, multimodality receiver operating characteristic data: validation with computer simulation. Acad Radiol. 1997;4:298–303.
Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, De Mauroy JC, SOSORT guidelines., et al. SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2016;2018(13):3.
Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–6.
Tan KJ, Moe MM, Vaithinathan R, Wong HK. Curve progression in idiopathic scoliosis: follow-up study to skeletal maturity. Spine. 2009;34:697–700.
Cheung JPY, Cheung PWH. Supine flexibility predicts curve progression for patients with adolescent idiopathic scoliosis undergoing underarm bracing. Bone Joint J. 2020;102(B):254–60.
Grivas TB, Vasiliadis E, Malakasis M, Mouzakis V, Segos D. Intervertebral disc biomechanics in the pathogenesis of idiopathic scoliosis. Stud Health Technol Inform. 2006;123:80–3.
Stokes IA, Burwell RG, Dangerfield PH. Biomechanical spinal growth modulation and progressive adolescent scoliosis a test of the “vicious cycle” pathogenetic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis. 2006;1:16.
Will RE, Stokes IA, Qiu X, Walker MR, Sanders JO. Cobb angle progression in adolescent scoliosis begins at the intervertebral disc. Spine. 2009;34:2782–6.
Shannon B, Mackenzie WS, Hariharan A, Shah S. Update in nonoperative management of adolescent idiopathic scoliosis to prevent progression. JPOSNA®. 2021;3(4).
Shu Yan NG, Yln KPC, Chan Yan W., Ho Tsz Ki. Intervention versus observation in mild idiopathic scoliosis in skeletally immature patients. Open Orthop J. 2020;14:186–97.
Sanders AE, Andras LM, Iantorno SE, Hamilton A, Choi PD, Skaggs DL. Clinically significant psychological and emotional distress in 32% of adolescent idiopathic scoliosis patients. Spine Deform. 2018;6:435–40.
Zhang Q, Rao L, Yang Y. Group-cam: Group score-weighted visual explanations for deep convolutional networks. arXiv preprint arXiv preprint arXiv:2103.13859.
Wang H, Zhang T, Cheung KM-C, Shea GK-H. Application of deep learning upon spinal radiographs to predict progression in adolescent idiopathic scoliosis at first clinic visit. EClinicalmedicine. 2021;42:101220.
Hwang CJ, Lee CS, Lee DH, Cho JH. Progression of trunk imbalance in adolescent idiopathic scoliosis with a thoracolumbar/lumbar curve: is it predictable at the initial visit? J Neurosurg Pediatr. 2017;20:450–5.
Sun X, Ding Q, Sha S, Mao S, Zhu F, Zhu Z, et al. Rib-vertebral angle measurements predict brace treatment outcome in Risser grade 0 and premenarchal girls with adolescent idiopathic scoliosis. Eur Spine J. 2016;25:3088–94.
Rehman A, Naz S, Razzak MI, Akram F, Imran M. A deep learning-based framework for automatic brain tumors classification using transfer learning. Circuits Syst Signal Process. 2020;39:757–75.
Varshni D, Thakral K, Agarwal L, Nijhawan R, Mittal A, editors. Pneumonia detection using CNN based feature extraction IEEE international conference on electrical, computer and communication technologies (ICECCT). 2019. p. 1–7.
Raghu S, Sriraam N, Temel Y, Rao SV, Kubben PL. EEG based multi-class seizure type classification using convolutional neural network and transfer learning. Neural Netw. 2020;124:202–12.
Acknowledgements
Not applicable
Funding
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 20K18023. The funding body played no role in the study design, collection, analysis, interpretation of the data, or manuscript writing.
Author information
Authors and Affiliations
Contributions
Conceptualization: YY, SS, YK; Methodology: YY, SS, YK; Formal analysis: YY, MT; Investigation: YY, MT, HM, KW, KK, HF; Writing—original draft: YY, YK; Writing, review & editing: SS, YK; Funding acquisition: YY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Toyama University Hospital (Clinical Research Number R2019122). Patients and their legal guardians provided written informed consent for participation in the analysis. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors (s) declare no potential conflicts of interest for this article, authorship, or publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yahara, Y., Tamura, M., Seki, S. et al. A deep convolutional neural network to predict the curve progression of adolescent idiopathic scoliosis: a pilot study. BMC Musculoskelet Disord 23, 610 (2022). https://doi.org/10.1186/s12891-022-05565-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05565-6