Abstract
Background
Despite similar outcomes for surgery and conservative care, the number of surgeries to treat rotator cuff related shoulder pain has increased. Interventions designed to enhance treatment expectations for conservative care have been shown to improve patient expectations, but no studies have yet explored whether such interventions influence patient decisions to pursue surgery. The purpose of this randomized clinical trial is to examine the effect of an intervention designed to improve expectations of conservative care on the decision to have surgery.
Methods
We will test the effectiveness of the Patient Engagement, Education, and Restructuring of Cognitions (PEERC) intervention which is intended to change expectations regarding conservative care. The PEERC intervention will be evaluated in a randomized, pragmatic “add-on” trial, to better understand the effect the intervention has on outcomes. Ninety-four (94) participants with rotator cuff related shoulder pain referred for physical therapy will be randomized to receive either impairment-based care or impairment-based care plus PEERC. Both groups will receive impairment-based conservative treatment created by compiling the evidence associated with established, effective interventions. Participants assigned to the impairment-based care plus PEERC condition will also receive the PEERC intervention. This intervention, informed by principles of cognitive behavioral therapy, involves three components: (1) strategies to enhance engagement, (2) education and (3) cognitive restructuring and behavioral activation. Outcomes will be assessed at multiple points between enrolment and six months after discharge. The primary outcome is patient reported decision to have surgery and the secondary outcomes are pain, function, expectations and satisfaction with conservative care.
Discussion
Rotator cuff related shoulder pain is highly prevalent, and because conservative and surgical treatments have similar outcomes, an intervention that changes expectations about conservative care could alter patient reports of their decision to have surgery and ultimately could lead to lower healthcare costs and decreased risk of surgical complications.
Trial registration
This study is registered as NCT03353272 at ClincialTrials.gov.
Similar content being viewed by others
Background
In comparative trials involving rotator cuff related shoulder pain (RCRSP), conservative interventions have yielded comparable outcomes with surgery [1,2,3,4]. However, despite the greater risks of harms, higher costs, and a high percentage of re-tears associated with a surgical approach, the number of shoulder surgeries for all forms of RCRSP pain continues to escalate [5,6,7]. In patients with RCRSP, pre-treatment expectations of the success of surgical and/or conservative approaches have demonstrated strong relationships with post-treatment outcomes [8,9,10]. The shoulder is not unique in these associations as patient expectations are known to influence treatment outcomes for cervical, low back and lower extremity disorders as well [11,12,13,14].
Patient expectations are beliefs or attitudes that include pre-treatment thoughts and beliefs regarding the need for specific treatment methods and the timing and intensity of these methods. Brief interventions designed to alter and enhance treatment expectations for conservative treatment appear to result in slight improvements in expectations [15, 16], but not outcomes. The few interventions that have been tested have methodological problems including the failure to attend to issues of treatment fidelity and reliance on overly simplistic methods for altering expectations such as a patient handout or a one-time educational program [15, 16].
To date, no studies have explored whether a cognitive-behavioral intervention can influence patient reports of their decision to pursue surgery. We posit that previous approaches to change patient expectations have had only modest effects because they do not include theory-based treatment techniques known to influence patient beliefs. Our study purpose is to test an innovative intervention to alter expectations about conservative care that is informed by principles of cognitive-behavioral theory: Patient Engagement, Education, and Restructuring of Cognitions (PEERC). The cognitive-behavioral therapy (CBT) treatment techniques that form the core of our PEERC intervention are patient-centered and are designed not only to alter expectations but also decisions to pursue surgical treatment.
Primary and Secondary Objectives
The purpose of this randomized clinical trial is to examine the effect of PEERC, an intervention designed to improve expectations of conservative care, on the patient reports of their decision to have versus not have surgery (primary outcome). Our secondary aim is to evaluate the impact of PEERC on pain, function, expectations and satisfaction with conservative care (secondary).
Trial Design
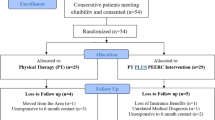
The study design is a randomized, pragmatic, “add-on” clinical trial. Pragmatic trials optimize normal everyday care processes and are designed to show the ‘real-world’ effectiveness of an intervention in broad patient groups. The PRagmatic-Explanatory Continuum Indicator Summary 2 (PRECIS-2) is a tool to gauge if the study design matches the intended purpose by rating nine domains on a continuum from very explanatory (ideal conditions) to very pragmatic (usual care conditions) (ICC > 0.67) [17, 18]. Fig. 1 illustrates the continuum for the PEERC trial based on input from the study team. Add-on trials are appropriate when an experimental intervention is tested on participants with a condition in which an established, effective treatment is present. In “add-on” trials, all participants (in both conditions) receive an established, effective treatment. Add-on designs are especially useful for testing of experimental interventions with mechanisms of action different from that of the established, effective treatment [19]. Add-on trials allow the investigators to better understand the isolated ‘effect’ of the “add-on” intervention [20]. In this study, the PEERC intervention will be the “add-on” component to the established impairment-based physical therapy treatment. Planning for this study was initiated in 2017 and continues with enrolment. This study protocol is described using both the SPIRIT [21, 22] guideline for the minimum content of a clinical trial protocol and the CONSORT Statement and Checklist [21] for reporting in clinical trials to facilitate complete reporting. The TIDieR [22] guidelines were followed when describing the study interventions. Figure 2 illustrates the flow of enrolment, allocation, and follow up. The trial is registered on ClinicalTrials.gov: NCT03353272.
The PRagmatic-Explanatory Continuum Indicator Summary 2 (PRECIS-2) wheel [17]
Methods
Recruitment and Consent
Consecutive patients with RCRSP referred by primary care physicians, orthopaedic surgeons, and physician assistants for physical therapy are recruited for the trial. RCRSP diagnoses include conditions such as subacromial pain (impingement) syndrome, rotator cuff tendinopathy, and symptomatic partial and full thickness rotator cuff tears. The informed consent process is performed by institutionally-trained research personnel. Study activities are not initiated until after the patient provides written consent.
Setting
All patients are treated at Duke Sports Physical Therapy at the James R. Urbaniak, MD Sports Sciences Institute at Duke University located in Durham, North Carolina, United States. This clinic employs over 30 physical therapists, the majority having advanced specialization in musculoskeletal-based disorders and experience participating in clinical trials. Several physicians, both primary care physicians and orthopaedic surgeons, from the James R. Urbaniak, MD, Sports Sciences Institute, who specialize in management of shoulder conditions, refer patients directly to this hospital-based outpatient physical therapy clinic.
Inclusion and Exclusion Criteria
Inclusion criteria for this protocol include: ages 18 to 70; a mobile or land-line telephone; the ability to read, write, and speak English; and an RCRSP diagnosis inclusive of both acute and chronic cases for which the date of onset will be recorded. We exclude patients who have received, or are scheduled for, a surgical intervention for their shoulder condition, demonstrate any evidence of cervicogenic pain and/or radiculopathy from cervical origin, or who demonstrate symptoms consistent with thoracic outlet syndrome; all of which will be identified during the clinical examinations by the attending physician and physical therapist. We also exclude individuals who are undergoing treatment for a serious psychological disorder (e.g., severe depression, psychosis).
Randomization and Blinding
Consented participants are randomized to receive either the (1) impairment based care group or (2) impairment based care plus PEERC group (Fig. 2). Consecutively numbered, sealed, opaque envelopes containing group allocation were prepared by a researcher with no other involvement in the study. Condition allocation involves randomization within random permuted blocks using the random number function in Excel and is stratified according to treating therapist so that all physical therapists will deliver approximately equal numbers of patients in both conditions to control for therapist variation. Participants are blinded to study purpose of improving expectations of conservative care and decisions to pursue surgery. Rather than employ deceit, it is communicated that the investigators wish to improve the patient experience through additional education and interaction.
Interventions
Both groups receive a dedicated impairment-based, physical therapy approach that is performed in the same clinic.
Impairment-based care- The impairment based care is pragmatic, but involves an established, three step phased approach supported by Kuhn [23], Garrison [24], and Stevenson [25]. The three phases include an inflammatory phase, a subacute/early strengthening phase, and an advanced strengthening phase. Patients move from one phase to another based on report of pain and mastery of the activities within the current phase. The home exercise program of the phased approach will be standardized but the dosage of the clinical interventions will be specific to the examination findings. The phased approach allows patient-centred care that is unique to the needs of the patient and his/her progress, but reduces the variability of care that is common in physical therapy settings. Table 1 outlines the staging criteria, goals, and sample exercises of the three phases used in this protocol. Subsequent visit frequency and duration is determined pragmatically by the evaluating physical therapist and may be adjusted in response to progress toward goals. This evaluating therapist is considered the primary therapist, with a secondary physical therapist providing care in the event that the primary therapist is unavailable. No participant is treated by more than two different therapists over the course of his or her care. Participants who have received a corticosteroid injection for rotator cuff related shoulder pain will not be excluded, nor will subsequent concomitant use of injection be cause for withdrawal from the study. Corticosteroid injections, along with the date of the injection, will be recorded in the participant’s study record and in the study database. Oral NSAIDS will also be permitted at the patient’s discretion. In the absence of extenuating circumstances, patients are discharged from care when all goals (see Table 1) of each treatment phase are met.
PEERC Intervention- All patients in this condition receive the care outlined above for the impairment-based care condition. In addition, they receive a telephone-based intervention (PEERC), designed by the authors, to challenge and change underlying thoughts, beliefs, and attitudes related to treatment expectations regarding conservative care. PEERC, based on cognitive-behavioural principles, is delivered by one of two specifically trained physical therapists who conduct six 30-minute telephone sessions with participants over a six-week period beginning the second week of physical therapy participation. Treatment techniques used in PEERC are drawn from CBT to address issues related to thought distortions and irrational beliefs common in patients who have RCRSP. These techniques, summarized in Table 2, are grouped into three domains: engagement, education and cognitive structuring.
Outcome Collection
Outcomes of interest are collected by study personnel at the time of consent, after 3 weeks of intervention, after 6 weeks of intervention, at discharge, and at 6 months following discharge. With the exception of the final time point, these measures are collected in person. Table 3 illustrates the schedule of enrolment, interventions, and assessments.
Primary Outcome
Our primary outcome measure is patient report of the decision to pursue surgery. Since the “add on” intervention of our study included patient decision-making support options, education, and facts that were designed to influence expectations, we were interested in whether this approach truly influenced high-stakes patient decisions, such as pursuance of surgery. Prior surgical decision-making studies have used similar or Likert-based outcomes [27, 28]. We selected a question framed around the patient’s choice among treatment approaches, and included the binary question: “Have you had surgery or are you scheduled for surgery for the shoulder problem that you were treated for in physical therapy?” We will use telephone contact at 6 months after discharge from impairment-based care. Because patients may pursue surgery outside of the study institution, in which case a scheduled procedure would not be documented in the medical record, this direct contact will better serve to capture decision of surgical pursuance.
Secondary Outcomes
Secondary outcome measures include changes pain, function, expectations and satisfaction with conservative care. The construct of pain is assessed through the Shoulder Pain and Disability Index (SPADI) [29,30,31,32] and the Numeric Pain Rating Scale (NPRS) [33,34,35]. Functional constructs are measured with the SPADI, Tegner Activity Scale (TEGNER) [36], Single Assessment Numeric Evaluation (SANE) [37,38,39], and the Global Rating of Change (GRoC) [40,41,42,43,44,45] score. Expectations and satisfaction are measured with the MODEMS-E and MODEMS-S questionnaires respectively. The MODEMS [26, 46, 47] is a set of musculoskeletal assessment instruments created by the American Academy of Orthopaedic Surgeons.
Expectations - The MODEMS expectations scale (MODEMS-E) is a six-item instrument designed to capture patient expectations across a wide range of musculoskeletal conditions. The MODEMS-E patient expectation scale has been used by a number of studies and has shown validity in predicting outcomes in conservative and surgical interventions. The instrument is a Likert-based scoring tool with a mean score of 5 out of 5 (indicating high expectations of positive outcomes) and a mean score of 1 out of 5 (indicating very poor expectations of positive outcomes) [46].
Satisfaction - Patient satisfaction with the conservative care received in our clinic will be measured using the MODEMS-S. The MODEMS –S consists of five similar stated questions from the MODEMS-E, but the questions are written to assess whether one’s expectations were met. The MODEMS-S instrument is also a Likert-based scoring tool with a mean score of 1 out of 5 (indicating expectations were met) and a mean score of 5 out of 5 (indicating expectations were not met) [46]. Table 4 details further description and the psychometric properties of each patient reported outcome included in the study.
Patient Demographics and Characteristics
To describe patient demographics and presentation, we will report age, sex, education level, work status, marital status, referral source (primary care physician or orthopaedist), history of corticosteroid injection for the current episode, and prior participation in physical therapy for same or different diagnosis. Instruments to assess systemic and comorbidities include the Optimal Screening for Prediction of Referral and Outcome- Review of Systems (OSPRO-ROS) [48] and the Pain Catastrophizing Scale (PCS) [50, 51]. The OSPRO-ROS is a review-of-systems screening tool that includes constructs associated with comorbidities and systemic pathologies [55]. The PCS is a 12 item questionnaire ranking types of thoughts and feelings one has while in pain from 0 (not at all) to 4 (all the time) [50]. Table 4 details the psychometric properties of the OSPRO-ROS and PCS.
Sample Size Estimate
We powered the study for proportional differences between conditions on decision to have surgery for up to 6 months. Using projections from previous data, and assuming offset inequity between two independent conditions; we modelled power on the following assumptions. In the absence of prior studies, the authors project 30 % of the impairment-based condition only to pursue surgery versus 5 % from the impairment-based plus PEERC condition. This assumes an allocation ratio of 1/1 and error of probability of 0.05 and projected power of 80 %. With these assumptions, our projected sample size requires 94 participants. We will employ intention to treat in the primary analysis and do not plan for dropouts.
Statistical Analysis
We will evaluate descriptive statistics of the two conditions using appropriate parametric and nonparametric tests for differences, depending on the data (continuous or frequency based). For our primary outcome (patient-reported surgery or intention to have surgery), we will measure condition differences in proportions between the impairment-based care only shoulder treatment and the impairment-based care plus PEERC, using a chi-square analysis (or Fisher Exact).
For our secondary aims, we will use linear mixed effects modelling to compare pain (NPRS and SPADI), function (SPADI, GRoC, TEGNER, and SANE), and follow up expectations and satisfaction (MODEMS) between the two conditions. Linear mixed effects modelling methods are flexible, model individual change, and accommodate for missing data (when present). We will run two analyses, unadjusted and adjusted, in which we will control for all baseline characteristics that are significantly different (if present) and baseline patient expectation, functional outcome, and pain. Pain intensity measures will be evaluated using a negative binomial Poisson, which accounts for count variables with significant skew.
Data Collection and Management
Study data are managed using REDCap (Research Electronic Data Capture) [56] electronic data capture tools hosted by the study institution. REDCap is a secure, web-based platform designed to provide an interface for validated data capture and export of data to statistical packages. De-identified data, both from the secure electronic medical record as well as paper questionnaires, is entered into the REDCap instrument by study personnel. Ownership of the final dataset rests with the institution.
Monitoring
Because this investigation presents less than minimal risk or psychosocial harm, an independent data monitoring committee is not required. Adherence to the impairment based treatment intervention will be monitored via checklist for treatment fidelity by non-treating study personnel. To enhance treatment fidelity, the study therapists underwent a formal training program, use a treatment manual to guide their sessions. Participant retention is promoted through contact between the physical therapist and the patient along with participant honorarium provided at the initial physical therapy evaluation and at the conclusion of ten weeks of active participation.
Ethics and Financial Support
This trial has the approval of the Institutional Review Board of Duke University under protocol identification number Pro00088103. Unanticipated problems involving risks to participants or others; information that indicates an adverse change to the risks or potential benefits of the research; or a protocol departure that harmed participants or others or compromises the integrity of the research data require prompt reporting to the institutional review board. This study is externally funded by the Academy of Orthopaedic Physical Therapy. The role of this funding source is solely financial and not influential or contributory to design or interpretation of results. All results from the study will be submitted for publication in peer-reviewed scientific journals. Prior to publication, the authors expect to present the results at professional conferences. For all forms of publication, the authors must meet the four tenets of authorship set forth by the International Committee of Medical Journal Editors. The authors declare no competing interests.
Discussion
This trial aims to measure effects of a novel PEERC intervention designed to change expectations of conservative care. In the primary analysis, the efficacy of PEERC will be assessed via participant report of their having had or have the intent to have surgery 6 months after finishing impairment-based care. In secondary analyses we will also investigate the effect of PEERC on pain, function, expectations and satisfaction with conservative care. Reliance on patient reported outcome measures, because they often lack evidence of validity, is a recognized limitation of clinical trials. Our primary outcome, pursuance of surgery, is no exception. The medical record can be queried, but this would only serve to verify the presence, not the absence, of planned or performed surgery because the patient may seek care outside of the study institution.
When considering the management decisions for RCRSP, clinicians, policy makers, and patients are faced with an intriguing dilemma. Despite higher risks/harms with surgical treatments, conservative treatments have similar outcomes [1,2,3,4]. The comparable results may be a reflection of a dedicated focus toward a biomedically oriented strategy [57]. Patient expectations (outside the biomedically oriented mechanisms associated with conservative and non-conservative treatment approaches) may also influence functional outcomes and the decision to pursue additional care options. This assumption is supported by studies that have identified the prognostic role of patient expectations across a variety of conservative and non-conservative care options [47, 58].
A patient-centered approach emphasizes patients’ treatment expectations - i.e. their desires, thoughts, and beliefs about treatment and its outcome [59]. Patient-centered models of care allow patients more control in directing their treatment and are a viable alternative method of enhancing treatment outcomes [60]. No studies of RCRSP have investigated the influence of brief, theory-based interventions for changing expectations about what treatment is appropriate/optimal when evidence suggests there is not an obvious choice based on superiority. Our study will focus on addressing patient expectations to understand that a conservative approach will provide a similar outcome to surgery, without unnecessary risks.
Despite recognition that patient expectations has a role in influencing functional outcomes and the decision to pursue surgical care in patients with RCRSP [8,9,10], there have been few studies designed to test interventions designed to change expectations [15, 16]. Research has shown that the best predictor of functional outcomes with a conservative approach are pre-treatment, patient expectations [15, 61]. Expectations (i.e. attitudes, thoughts, and beliefs) are malleable using methods rooted in cognitive-behavioral science that focus on education, engagement and cognitive restructuring.
If this innovative PEERC intervention is successful, the approach may provide the appropriate foundations that could be applied to other musculoskeletal disorders where there are viable conservative options to surgical care.
Trial Status
Recruitment of participants began on September 18, 2018 and currently continues. Completion is anticipated at the conclusion of 2021. The final decision to terminate the trial rests with the primary investigator.
Availability of data and materials
Not applicable.
Abbreviations
- PEERC:
-
Patient Engagement Education and Restructuring of Cognitions
- CBT:
-
Cognitive Behavioral Therapy
- RCRSP:
-
Rotator Cuff Related Shoulder Pain
- MODEMS:
-
Musculoskeletal Outcome Data Evaluation Management System
- SPADI:
-
Shoulder Pain and Dysfunction Index
- OSPRO-ROS:
-
Optimal Screening Prediction of Referral and Outcome
- PCS:
-
Pain Catastrophizing Scale
- SANE:
-
Single Assessment Numeric Evaluation
- TEGNER:
-
Tegner Activity Scale
- NPRS:
-
Numeric Pain Rating Scale
- VAS:
-
Visual Analog Scale
- GRoC:
-
Global Rate of Change
References
Dalton SE. The conservative management of rotator cuff disorders. Br J Rheumatol. 1994;33(7):663–7.
Goldberg BA, Nowinski RJ, Matsen FA. Outcome of nonoperative management of full-thickness rotator cuff tears. Clin Orthop Relat Res. 2001;382:99–107.
Kukkonen J, Joukainen A, Lehtinen J, Mattila KT, Tuominen EKJ, Kauko T, et al. Treatment of non-traumatic rotator cuff tears: A randomised controlled trial with one-year clinical results. Bone Joint J. 2014;96-B(1):75–81.
Moosmayer S, Lund G, Seljom U, Svege I, Hennig T, Tariq R, et al. Comparison between surgery and physiotherapy in the treatment of small and medium-sized tears of the rotator cuff: A randomised controlled study of 103 patients with one-year follow-up. J Bone Joint Surg Br. 2010;92(1):83–91.
Iyengar JJ, Samagh SP, Schairer W, Singh G, Valone FH, Feeley BT. Current trends in rotator cuff repair: surgical technique, setting, and cost. Arthroscopy. 2014;30(3):284–8.
Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227–33.
Fermont AJM, Wolterbeek N, Wessel RN, Baeyens J-P, de Bie RA. Prognostic factors for successful recovery after arthroscopic rotator cuff repair: a systematic literature review. J Orthop Sports Phys Ther. 2014;44(3):153–63.
Henn RF, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913–9.
O’Malley KJ, Roddey TS, Gartsman GM, Cook KF. Outcome expectancies, functional outcomes, and expectancy fulfillment for patients with shoulder problems. Med Care. 2004;42(2):139–46.
Skatteboe S, Røe C, Fagerland MW, Granan L-P. Expectations of pain and functioning in patients with musculoskeletal disorders: a cross-sectional study. BMC Musculoskelet Disord. 2017;18(1):48.
Bishop MD, Mintken PE, Bialosky JE, Cleland JA. Patient expectations of benefit from interventions for neck pain and resulting influence on outcomes. J Orthop Sports Phys Ther. 2013;43(7):457-65.
Cormier S, Lavigne GL, Choinière M, Rainville P. Expectations predict chronic pain treatment outcomes. Pain. 2016;157(2):329–38.
Ellis DJ, Mallozzi SS, Mathews JE, Moss IL, Ouellet JA, Jarzem P, et al. The Relationship between Preoperative Expectations and the Short-Term Postoperative Satisfaction and Functional Outcome in Lumbar Spine Surgery: A Systematic Review. Global Spine J. 2015;5(5):436–52.
Kongsted A, Vach W, Axø M, Bech RN, Hestbaek L. Expectation of recovery from low back pain: a longitudinal cohort study investigating patient characteristics related to expectations and the association between expectations and 3-month outcome. Spine. 2014;39(1):81–90.
Riley SP, Bialosky J, Cote MP, Swanson BT, Tafuto V, Sizer PS, et al. Thoracic spinal manipulation for musculoskeletal shoulder pain: Can an instructional set change patient expectation and outcome? Man Ther. 2015;20(3):469–74.
Martínez-Cervera FV, Olteanu TE, Gil-Martínez A, Díaz-Pulido B, Ferrer-Peña R. Influence of expectations plus mobilization with movement in patient with lateral epicondylalgia: a pilot randomized controlled trial. J Exerc Rehabil. 2017;13(1):101–9.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;8:350:h2147.
Loudon K, Zwarenstein M, Sullivan FM, Donnan PT, Gágyor I, Hobbelen HJSM, et al. The PRECIS-2 tool has good interrater reliability and modest discriminant validity. J Clin Epidemiol. 2017;88:113–21.
Institute of Medicine (US) Committee on Strategies for Small-Number-Participant Clinical Research Trials. Small clinical trials: issues and challenges. Evans CH, Ildstad ST, editors. Washington (DC): National Academies Press (US); 2001.
Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann Intern Med. 2000;133(6):455-63.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–7.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138–60.
Garrison JC, Shanley E, Thigpen C, Hegedus E, Cook C. Between-session changes predict overall perception of improvement but not functional improvement in patients with shoulder impingement syndrome seen for physical therapy: an observational study. Physiother Theory Pract. 2011;27(2):137–45.
Stevenson K, Jackson S, Shufflebotham J, Roddy E, Foster NE. Development and delivery of a physiotherapist-led exercise intervention in a randomised controlled trial for subacromial impingement syndrome (the SUPPORT trial). Physiotherapy. 2017;103(4):379–86.
Zywiel MG, Mahomed A, Gandhi R, Perruccio AV, Mahomed NN. Measuring expectations in orthopaedic surgery: a systematic review. Clin Orthop Relat Res. 2013;471(11):3446–56.
Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370(15):1379–81.
Torrens C, Miquel J, Santana F. Do we really allow patient decision-making in rotator cuff surgery? A prospective randomized study. J Orthop Surg Res. 2019;14(1):116.
Breckenridge JD, McAuley JH. Shoulder pain and disability index (SPADI). J Physiother. 2011;57(3):197.
Roddey TS, Olson SL, Cook KF, Gartsman GM, Hanten W. Comparison of the University of California-Los Angeles Shoulder Scale and the Simple Shoulder Test with the shoulder pain and disability index: single-administration reliability and validity. Phys Ther. 2000;80(8):759–68.
Ekeberg OM, Bautz-Holter E, Tveitå EK, Keller A, Juel NG, Brox JI. Agreement, reliability and validity in 3 shoulder questionnaires in patients with rotator cuff disease. BMC Musculoskelet Disord. 2008;9:68.
Schmitt J, Di Fabio RP. The validity of prospective and retrospective global change criterion measures. Arch Phys Med Rehabil. 2005;86(12):2270–6.
Michener LA, Snyder AR, Leggin BG. Responsiveness of the numeric pain rating scale in patients with shoulder pain and the effect of surgical status. J Sport Rehabil. 2011;20(1):115–28.
Herr KA, Spratt K, Mobily PR, Richardson G. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain. 2004;20(4):207–19.
Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–2.
Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–7.
Williams JW, Holleman DR, Simel DL. Measuring shoulder function with the Shoulder Pain and Disability Index. J Rheumatol. 1995;22(4):727–32.
Wickman JR, Lau BC, Scribani MB, Wittstein JR. Single Assessment Numeric Evaluation (SANE) correlates with American Shoulder and Elbow Surgeons score and Western Ontario Rotator Cuff index in patients undergoing arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2020;29(2):363–9.
Thigpen CA, Shanley E, Momaya AM, Kissenberth MJ, Tolan SJ, Tokish JM, et al. Validity and Responsiveness of the Single Alpha-numeric Evaluation for Shoulder Patients. Am J Sports Med. 2018;46(14):3480–5.
Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–70.
Costa LOP, Maher CG, Latimer J, Ferreira PH, Ferreira ML, Pozzi GC, et al. Clinimetric testing of three self-report outcome measures for low back pain patients in Brazil: which one is the best? Spine. 2008;33(22):2459–63.
Watson CJ, Propps M, Ratner J, Zeigler DL, Horton P, Smith SS. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136–46.
Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;29(12):1157–62. 282(.
van der Windt DA, van der Heijden GJ, de Winter AF, Koes BW, Devillé W, Bouter LM. The responsiveness of the Shoulder Disability Questionnaire. Ann Rheum Dis. 1998;57(2):82–7.
Stewart M, Maher CG, Refshauge KM, Bogduk N, Nicholas M. Responsiveness of pain and disability measures for chronic whiplash. Spine. 2007;32(5)(1):580–5.
Soroceanu A, Ching A, Abdu W, McGuire K. Relationship between preoperative expectations, satisfaction, and functional outcomes in patients undergoing lumbar and cervical spine surgery: a multicenter study. Spine. 2012;37(2):E103-8.
Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752–8.
George SZ, Beneciuk JM, Lentz TA, Wu SS, Dai Y, Bialosky JE, et al. Optimal screening for prediction of referral and outcome (OSPRO) for musculoskeletal pain conditions: results from the validation cohort. J Orthop Sports Phys Ther. 2018;48(6):460–75.
George SZ, Beneciuk JM, Bialosky JE, Lentz TA, Zeppieri G, Pei Q, et al. Development of a Review-of-Systems Screening Tool for Orthopaedic Physical Therapists: Results From the Optimal Screening for Prediction of Referral and Outcome (OSPRO) Cohort. J Orthop Sports Phys Ther. 2015;45(7):512–26.
Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65.
Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7(4):524–32.
Briggs KK, Kocher MS, Rodkey WG, Steadman JR. Reliability, validity, and responsiveness of the Lysholm knee score and Tegner activity scale for patients with meniscal injury of the knee. J Bone Joint Surg Am. 2006;88(4):698–705.
Kamper S. Global Rating of Change scales. Aust J Physiother. 2009;55(4):289.
Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214–21.
George SZ, Beneciuk JM, Lentz TA, Wu SS. The Optimal Screening for Prediction of Referral and Outcome (OSPRO) in patients with musculoskeletal pain conditions: a longitudinal validation cohort from the USA. BMJ Open. 2017;7(6).
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Main CJ, George SZ. Psychologically informed practice for management of low back pain: future directions in practice and research. Phys Ther. 2011;91(5):820–4.
Dunn WR, Kuhn JE, Sanders R, An Q, Baumgarten KM, Bishop JY, et al. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25(8):1303–11.
Zeppieri G, George SZ. Patient-defined desired outcome, success criteria, and expectation in outpatient physical therapy: a longitudinal assessment. Health Qual Life Outcomes. 2017;15(1):29.
Brown JL, Edwards PS, Atchison JW, Lafayette-Lucey A, Wittmer VT, Robinson ME. Defining patient-centered, multidimensional success criteria for treatment of chronic spine pain. Pain Med. 2008;9(7):851–62.
Chester R, Shepstone L, Daniell H, Sweeting D, Lewis J, Jerosch-Herold C. Predicting response to physiotherapy treatment for musculoskeletal shoulder pain: a systematic review. BMC Musculoskelet Disord. 2013;14:203.
Acknowledgements
Not Applicable.
Funding
This study is externally funded by the Academy of Orthopaedic Physical Therapy. The role of this funding source is solely financial and not influential or contributory to design or interpretation of results.
Author information
Authors and Affiliations
Contributions
Conception and design: CC, HM, Critical revision for important intellectual content CC, FK, SZG, Drafting the manuscript HM, CC, Authors’ approval of the manuscript. CC, HM, SZG, FK, CM, JK, ADL, Data collection and analysis CC, HM, CM, JK, ADL.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This trial has the approval of the Institutional Review Board of Duke University under protocol identification number Pro00088103. Study activities are not initiated until the patient provides written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Myers, H., Keefe, F., George, S.Z. et al. The influence of a cognitive behavioural approach on changing patient expectations for conservative care in shoulder pain treatment: a protocol for a pragmatic randomized controlled trial. BMC Musculoskelet Disord 22, 727 (2021). https://doi.org/10.1186/s12891-021-04588-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04588-9