Abstract
Background
The prognosis of lung metastasis (LM) in patients with chondrosarcoma was poor. The aim of this study was to construct a prognostic nomogram to predict the risk of LM, which was imperative and helpful for clinical diagnosis and treatment.
Methods
Data of all chondrosarcoma patients diagnosed between 2010 and 2016 was queried from the Surveillance, Epidemiology, and End Results (SEER) database. In this retrospective study, a total of 944 patients were enrolled and randomly splitting into training sets (n = 644) and validation cohorts(n = 280) at a ratio of 7:3. Univariate and multivariable logistic regression analyses were performed to identify the prognostic nomogram. The predictive ability of the nomogram model was assessed by calibration plots and receiver operating characteristics (ROCs) curve, while decision curve analysis (DCA) and clinical impact curve (CIC) were applied to measure predictive accuracy and clinical practice. Moreover, the nomogram was validated by the internal cohort.
Results
Five independent risk factors including age, sex, marital, tumor size, and lymph node involvement were identified by univariate and multivariable logistic regression. Calibration plots indicated great discrimination power of nomogram, while DCA and CIC presented that the nomogram had great clinical utility. In addition, receiver operating characteristics (ROCs) curve provided a predictive ability in the training sets (AUC = 0.789, 95% confidence interval [CI] 0.789–0.808) and the validation cohorts (AUC = 0.796, 95% confidence interval [CI] 0.744–0.841).
Conclusion
In our study, the nomogram accurately predicted risk factors of LM in patients with chondrosarcoma, which may guide surgeons and oncologists to optimize individual treatment and make a better clinical decisions.
Trial registration
JOSR-D-20-02045, 29 Dec 2020.
Similar content being viewed by others
Background
Chondrosarcoma is the third most common primary malignant bone tumor after myeloma and osteosarcoma and the second most common bone malignant neoplasms accounting for 30% of all primary malignant bone tumors. At present, surgical resection is the mainstay therapeutic option for chondrosarcoma, while the radiotherapy is only applied to patients with unresectable lesions or inoperable extensive marginal resection and the chemotherapy only recommended for young patients with good tolerance. However, the effects of treatment are insignificant according to the recent studies [1, 2].
According to previous research, approximately 8 to 38% of the patients with chondrosarcoma developed distant metastasis and lung was the preferred site of metastasis [3,4,5,6]. Furthermore, the 10-year survival rate and the metastasis rate of chondrosarcoma patients with lung metastasis were 17 and 9.6%, respectively. The occurrence of LM had a strong predictor of poor prognosis [7, 8]. In addition, up to 13% of recurrent chondrosarcomas got a higher grade of malignancy than the original neoplasm [9]. Due to the effects of metastasis, complete removal of the tumor became extremely difficult. Therefore, it was imperative to identify the risk factors of chondrosarcoma patients with lung metastasis [10].
Chondrosarcoma is a rare tumor, accounting for 20% of primary malignant bone tumor, with an estimated incidence rate of 1 per 200,000. The lack of clinical research in chondrosarcoma is subjected to the low and sporadic incidence of chondrosarcoma. The Surveillance, Epidemiology, and End Results (SEER) database that sponsored by the National Cancer Institute records cancer incidence and survival data from 18 population-based cancer registries. The database includes approximately 27.8% of the U.S. population and is publicly available [11].
With the advantage of visualization and accurate prediction, nomogram has been widely applied to predict the risk factors of metastasis and oncological outcomes [12]. With Nomogram, clinicians can assess the risk of clinical events, offer individual treatment plans, optimize treatment regimens, and be more active at follow-up. The purpose of this study is to construct a nomogram to evaluate high-risk group of chondrosarcoma patients with LM due to the importance of LM in the prognosis of chondrosarcoma patients,.
Materials and methods
Data collection
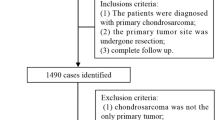
Data were extracted from the SEER database, using the SEER * Stat software 8.3.6 version. And the third edition of the International Taxonomy of Oncology (ICDO-3), morphological code(9220) was used to identify chondrosarcoma. Data in this study consisted of patients diagnosed with chondrosarcoma from 2010 to 2016. The exclusion criteria were as follows: (1) patients with no positive pathology; (2) patients with unknown survival time; (3) not the first-occurrence tumor; (4) more than one primary tumor; (5) lung metastasis information was unknown. (6) incomplete information on regional lymph node metastasis.
Demographic and clinical variables (including age, gender, race, marriage, main location, single or multiple tumors, tumor size and lymphatic metastasis) were considered in this study. These data were determined by the variable “CS site-specific factor 6”. Patients were classified as married and unmarried (including single, divorced, separated, and widowed). Besides, patients with less than 20 tumor sites were classified as “other”.
Construction, validation and clinical utility of a nomogram
To investigate risk factors for LM at the initial diagnosis of chondrosarcoma, we extracted the data of patients diagnosed after 2010 year from SEER database [13], then randomly divided patients into the training sets(n = 644) and validation cohorts (n = 280).
Then, the following variables were chosen for research: age, race, gender, marriage, main location, single or multiple tumors, tumor size, and lymphatic metastasis. All the variables with a significance level P < 0.05 in univariate logistic regression analysis were included in the multivariable logistic regression analysis. The nomogram was constructed based on the results of the univariate and multivariable logistics regression analysis. The calibration plot of clinical prediction model and receiver operating characteristic (ROC) curves were used to estimate the prediction performance of the nomogram. The higher the area under the curves (AUC) of ROC, the better the model was. In addition, the decision curve analysis (DCA) was used to evaluate the clinical utility of nomograms in decision-making. The DCA is a kind of chart that can show net benefits under a series of reasonable risk thresholds in practice. CIC was developed based on DCA to visual display the estimated number of high-risk patients for each risk threshold.
Statistic analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequency (proportions). Hypothesis test was utilized to compare the difference between metastatic and non-metastatic group. T-test and Chi-square were applied to continuous variables and categorical variables respectively by IBM SPSS Statistics version 26.0(SPSS Inc., Chicago, Illinois, USA). R software version 3.6.2 (http://www.r-project.org) including multiple R packages (Including regplot, rms, rmda and pROC) was employed to draw graphics, such as Nomogram, Calibration plot, DCA graph, ROC curve and KM curve. The P < 0.05 was considered as statistically significant, and confidence intervals (CIs) were expressed as 95% confidence levels.
Results
Demographic baseline characteristics
A total of 944 patients were engaged in this study. The baseline information categorized as training group and validation group. There was no statistically significant difference between the training and validation groups (P > 0.05) (Table 1).
Univariate and multivariable logistic regression results
Based on the univariate logistics regression analysis, we identified five significant prognostic factors including age, sex, marital, tumor size and lymph metastasis in the training set (P < 0.05) (Table 2). Then, applying the multivariable logistics regression analysis, we figured out those independent prognostic factors including four protective factors containing gender (female: odds ratio (OR) 0.435, 95%CI 0.212-0.891, P < 0.05), age (OR = 1.026, 1.005-1.048, P < 0.05), tumor size (OR = 1.003, 1.003-1.006, P < 0.05) and lymph metastasis (Yes:OR = 27.164, 6.267-117.741, P < 0.0001; Unknown:8.027, 2.643-24.379, P < 0.0001) (Table 2).
Construction and validation of nomogram for chondrosarcoma patients with pulmonary metastasis
The results of univariate and multivariable logistics regression were used to construct the Nomogram of LM (Fig. 1a). As shown in the Fig. 1, lymph contributed most to prognosis followed by tumor size, marital status, age and sex. The calibration chart of the Nomogram showed a good consistency in the training cohorts and the validation cohorts (Fig. 1b, c). The AUC values of Nomogram were 0.789 (95% CI 0.762-0.851) and 0.796 (95% CI 0.744-0.841) respectively in the training cohorts and the validation cohorts (Fig. 2a, b). Furthermore, the ROC curve displayed that the value of Nomogram was more important than other variables, including age (AUC = 0.674, 95%CI 0.644 to 0.704), lymph node metastasis (0.610, 0.578 to 0.641), and marital status (AUC = 0.600, 95%) CI 0.568 to 0.632), sex (AUC = 0.588, 95%CI 0.568 to 0.632) and tumor size (0.710, 95%CI 0.680 to 0.739) on training sets. The results of the validation cohorts indicated that the value of Nomogram was also higher than that of single factor as shown in Table 3.
Clinical applicability of the nomogram
The Kaplan-Meier survival curves of the overall survival (OS) of 944 patients were plotted (Fig. 3a). The results revealed that the survival significantly decreased in chondrosarcoma patients with LM comparing with the other group(P < 0.001). Moreover, the threshold about 0.1 to 0.8 had the maximum benefit range of the model as shown in the DCA curve (Fig. 3b). In addition, the clinical impact curve of the training cohorts revealed that within the most favorable threshold probability range, the number of predicted high-risk patients were always more than the actual patients with LM, accompanied by an acceptable cost-benefit ratio (Fig. 3c).
a The Kaplan-Meier survival analysis of lung metastasis in patients with chondrosarcoma. b, c Nomogram decision curve (DCA) and clinical implications (CIC) for the risk of the lung metastasis. The red curve (Number of high risk) indicates the Number of people classified as positive (high risk) by Nomogram for each threshold probability. The blue curve (number of high risk with outcome) represents the number of true positives under each threshold probability
Discussion
This study, we found that the following five independent risk factors including gender, age at the diagnosis, marital status, tumor size, and the lymph node involvement,were identified to be associated with LM applying univariate and multivariable logistic regression analysis on the data collected from the SEER database. All these factors were involved into the nomogram. The calibration plots showed the nomogram demonstrated good discrimination (Fig. 1b). Furthermore, as shown in the ROC curves (Table 3), the diagnostic efficiencies of the nomogram were better than any single predictors, which demonstrated the significance of a comprehensive predictive model. The DCA curve showed that the probability threshold for LM patients to obtain the maximum benefit is 0.1-0.8 (Fig. 3b). In addition, the CIC (Fig. 3c) shows that there is an acceptable cost-benefit ratio of the threshold range of. This nomogram could be used as a reliable graphic tool to help surgeons and oncologists distinguish and estimate the risk of lung metastasis and guide personalized treatment for chondrosarcoma patients.
According to a large population-based study, approximately 8% of patients with chondrosarcoma developed distant metastases [14]. The poor prognosis of patients with chondrosarcoma might be associated with the lung metastasis [15]. Therefore, we intended to identify risk factors of chondrosarcoma patients with lung metastasis [8, 16].
The visual nomogram is well known for its predictive accuracy and has made remarkable contributions to modern medical decision-making [17, 18]. Zhang et al. [19] and Song et al. [4, 20] constructed and validated nomograms to predict the overall survival (OS) and cancer-specific survival (CSS) of chondrosarcoma patients. However, it was an innovation that establishing Nomogram based on the SEER database to analyze the high risk of lung metastases in chondrosarcoma.
Generally speaking, regional lymph node metastasis developed quite rarely in chondrosarcoma, and the incidence was approximately 1.3% across all chondrosarcoma [21]. The low prevalence of lymph node involvement might be related to the scarce lymphatic vessels in normal bones, benign tumors and malignant neoplasms. In addition, the research indicated that lymphatic vessels existed in the connective tissue covering the periosteum, and lymphatic metastasis occurred only when the tumor broke through the periosteum and invaded adjacent connective tissue [22]. Patients with this clinicopathological behavior might be diagnosed with a more aggressive chondrosarcoma.
According to the results of logistic regression analysis, taking patients without lymph node metastasis as the baseline, the risk ratios of lung metastasis for patients with lymph node involvement (OR = 27.164) and patients with unknown lymph node metastasis (OR = 8.027) were 27.164 and 8.027 respectively (Table 2). Considering regional lymph node involvement can result in a strong adverse prognostic impact and relatively higher risk of lung metastasis, we recommend biopsy for suspicious patients. Studies verified that it was essential to determine the presence of lymph node metastasis, which corroborated our conclusion [23]. Further research might be focused on applying this association to improve patient long-term survival.
The tumor size was associated with the risk for developing lung metastasis according to the statistical results acquired from univariate and multivariable logistic regression. In particular, the calculated OR value was 1.003, which represented that for each 1 mm increase in tumor size, the risk of LM increased by 1.003 times. Relevant study revealed that larger tumors represent a longer period of time for tumors growth, increasing the likelihood of metastasis [24]. Consistent with our findings, recent studies reported that tumor size was a significant independent predictor of LM and mortality [16, 25, 26], especially when the tumor size exceeding 10 cm [27].
Besides, the older patients with chondrosarcoma had a higher tendency developed with LM and had more negative prognosis conforming to the result of logistic regression. Previous studies identified the same results [28]. The increase in the risk of lung metastasis was 1.026 for each additional 1-year increase in the age of diagnosis with a reference of 60 years (Table 2). Moreover, a retrospective study found that age over 60 was an independent risk factor for LM and revealed the reason of poor prognosis in elderly patients [26]. The main reason why chondrosarcoma is not easily diagnosed and treated early is the late production of obvious symptoms such as swelling, pressure, and pain, especially for older patients, resulting in many patients presenting to the clinic with tumors that have progressed to a more advanced stage, leading to a higher risk of LM and affecting patient prognosis. Therefore, clinicians need to be more alert to the presence of LM in patients with advanced chondrosarcoma.
In addition, the result of logistic regression revealed males had a higher risk than females developing LM. As shown in OR value, the ratio of males and females with LM was 1:0.4. Therefore, we reasonably speculate that males had a more adverse survival expectancy. The standpoint was supported by several studies that indicated sex was an independent risk factor affecting the long-term prognosis of chondrosarcoma patients [24, 29, 30]. One possible reason for the worse prognosis of male patients compared to female patients is that male patients possess a higher risk of LM. Researchers also need to consider that male patients have a higher proportion of adverse habits, such as smoking and alcohol abuse, as well as poorer medical vigilance. These habits may cause men to come to the doctor at a worse level of cancer progression compared to women. This also leads to a higher risk of LM in males.
In addition, it was worth noting that this study considered the marital status as a risk factor on LM. We determined that comparing to unmarried patients, married chondrosarcoma patients with LM had significant survival benefits, which revealed that marital status was associated with a better prognosis [31, 32]. Favorable financial conditions and emotional support from spouses contributed to cultivate the married patients’ treatment adherence and regular follow-ups. Meanwhile, cancer-caused survival was also found to be relatively poor in widowed chondrosarcoma patients. This may be related to the greater psychological stress suffered by widowed patients as well as the greater stress associated with adjusting to a new social role. Unfortunately, the detailed economic status of patients was unavailable in the SEER database, so the impact of finance on LM cannot be further studied.
Finally, several limitations in our study need to be treated with caution. First, the information about asymptomatic LM patients and metastasis during follow-up is not recorded in the SEER database. Second, our retrospective study inevitably leads to a bias by the lack of lack systematic and prospective data. In our single-center study, the method of dividing patients into training and validation cohorts cannot be completely equivalent to external validation at other institutions, which might appear an overfitting LM nomogram.
Conclusion
In summary, we constructed a novel nomogram to predict risk factors for chondrosarcoma patients developing LM, including sex, age, tumor size, marital status and tumor size based on epidemiological characteristics obtained from the SEER database. By combining DCA curve, clinical impact curve and internal validation, our nomogram provided an accurate assessment for individualized risk of LM which guided clinicians to optimize personalized treatment and make superior clinical-related decisions.
Availability of data and materials
The dataset supporting the conclusions of this paper can be obtained from the SEER database.
Abbreviations
- LM:
-
Lung Metastasis
- SEER:
-
The Surveillance, Epidemiology, and End Results database
- ROC:
-
Receiver Operating Characteristic
- DCA:
-
Decision Curve Analysis
- CIC:
-
Clinical Impact Curve
- CI:
-
Confidence Interval
- AUC:
-
Area Under the Curve
- ICD:
-
International Classification of Diseases
- SD:
-
Standard deviation
- KM curves:
-
Kaplan Meier curves
- OR:
-
Odds ratio
References
Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma [J]. Oncologist. 2008;13(3):320–9.
Kamal AF, Husodo K, Prabowo Y, et al. Correlation between survival and tumour characteristics in patients with chondrosarcoma [J]. J Orthop Surg (Hong Kong). 2015;23(3):365–9.
Thorkildsen J, Taksdal I, Bjerkehagen B, et al. Chondrosarcoma in Norway 1990-2013; an epidemiological and prognostic observational study of a complete national cohort [J]. Acta oncologica (Stockholm, Sweden). 2019;58(3):273–82.
Song K, Shi X, Wang H, et al. Can a nomogram help to predict the overall and Cancer-specific survival of patients with chondrosarcoma? [J]. Clin Orthop Relat Res. 2018;476(5):987–96.
Cipriano C, Griffin AM, Ferguson PC, et al. Developing an evidence-based Followup schedule for bone sarcomas based on local recurrence and metastatic progression [J]. Clin Orthop Relat Res. 2017;475(3):830–8.
Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data [J]. BMJ Open. 2012;2(5).
Van Maldegem AM, Gelderblom H, Palmerini E, et al. Outcome of advanced, unresectable conventional central chondrosarcoma [J]. Cancer. 2014;120(20):3159–64.
Nguyen MT, Jiang YQ, Li XL, et al. Risk Factors for Incidence and Prognosis in Chondrosarcoma Patients with Pulmonary Metastasis at Initial Diagnosis [J]. Med Sci Monit. 2019;25:10136–53.
Patel SR, Burgess MA, Papadopoulos NE, et al. Extraskeletal myxoid chondrosarcoma. Long-term experience with chemotherapy [J]. Am J Clin Oncol. 1995;18(2):161–3.
Leddy LR, Holmes RE. Chondrosarcoma of bone [J]. Cancer Treat Res. 2014;162:117–30.
Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database [J]. JAMA Surg. 2018;153(6):588–9.
Wang H, Chen X, Zhao J, et al. Predictive Nomogram for Midterm to Long-Term Prognosis in Patients with Papillary Renal Cell Carcinoma Based on Data from the Surveillance, Epidemiology, and End Results (SEER) Program [J]. Med Sci Monit. 2020;26:e921859.
Zhou G, Xiao K, Gong G, et al. A novel nomogram for predicting liver metastasis in patients with gastrointestinal stromal tumor: a SEER-based study [J]. BMC Surg. 2020;20(1):298.
Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 To 2003): an analysis of 2890 cases from the SEER database [J]. J Bone Joint Surg (Am Vol). 2009;91(5):1063–72.
Söderström M, Ekfors TO, Böhling TO, et al. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971-1990 [J]. Acta Orthop Scand. 2003;74(3):344–50.
Roos E, Van Coevorden F, Verhoef C, et al. Prognosis of primary and recurrent chondrosarcoma of the rib [J]. Ann Surg Oncol. 2016;23(3):811–7.
Li G, Tian ML, Bing YT, et al. Nomograms predict survival outcomes for distant metastatic pancreatic neuroendocrine tumor: A population based STROBE compliant study. Medicine. 2020;99(13):e19593.
Serenari M, Han KH, Ravaioli F, et al. A novel nomogram based on liver stiffness to predict the comprehensive complication index after liver resection in patients with hepatocellular carcinoma [J]. Dig Liver Dis. 2020;52(1):e17.
Zhang J, Pan Z, Zhao F, et al. Development and validation of a nomogram containing the prognostic determinants of chondrosarcoma based on the surveillance, epidemiology, and end results database [J]. Int J Clin Oncol. 2019;24(11):1459–67.
Song K, Song J, Shi X, et al. Development and Validation of Nomograms Predicting Overall and Cancer-Specific Survival of Spinal Chondrosarcoma Patients. Spine (Phila Pa 1976). 2018;43(21):E1281–e1289.
Gulia A, Puri A, Jain S, et al. Chondrosarcoma of the bone with nodal metastasis: the first case report with review of literature [J]. Indian J Med Sci. 2011;65(8):360–4.
Edwards JR, Williams K, Kindblom LG, et al. lymphatics and bone [J]. Hum Pathol. 2008;39(1):49–55.
Somers J, Faber LP. Chondroma and chondrosarcoma [J]. Semin Thorac Cardiovasc Surg. 1999;11(3):270–7.
Nota SP, Braun Y, Schwab JH, et al. The Identification of Prognostic Factors and Survival Statistics of Conventional Central Chondrosarcoma [J]. Sarcoma. 2015;2015:623746.
Wang Z, Chen G, Chen X, et al. Predictors of the survival of patients with chondrosarcoma of bone and metastatic disease at diagnosis [J]. J Cancer. 2019;10(11):2457–63.
Song K, Shi X, Liang X, et al. Risk factors for metastasis at presentation with conventional chondrosarcoma: a population-based study [J]. Int Orthop. 2018;42(12):2941–8.
Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: an assessment of outcome [J]. J Bone Joint Surg Am. 1999;81(3):326–38.
Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up [J]. Acta Orthop. 2011;82(6):749–55.
Van Praag Veroniek VM, Rueten-Budde AJ, Ho V, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas [J]. Surg Oncol. 2018;27(3):402–8.
Song K, Song J, Chen F, et al. Does resection of the primary tumor improve survival in patients with metastatic chondrosarcoma? [J]. Clin Orthop Relat Res. 2019;477(3):573–83.
Zhang SL, Wang WR, Liu ZJ, et al. Marital status and survival in patients with soft tissue sarcoma: A population-based, propensity-matched study [J]. Cancer Med. 2019;8(2):465–79.
Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the surveillance, epidemiology, and end results (SEER) database [J]. Clin Res Hepatol Gastroenterol. 2017;41(4):476–86.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (81260274) and the Science and Technology Research and Development Program of Liuzhou City (2014 J030405).
Author information
Authors and Affiliations
Contributions
CLY and ZHH designed the study. WLL performed the study and analyzed the data. WLL and CLY wrote the manuscript. WLL provided the expert consultations and clinical suggestions. STD, HSW, HTW, RLGW, ZRT and JYZ conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study is based on the SEER database and does not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Dong, S., Wang, H. et al. Risk analysis of pulmonary metastasis of chondrosarcoma by establishing and validating a new clinical prediction model: a clinical study based on SEER database. BMC Musculoskelet Disord 22, 529 (2021). https://doi.org/10.1186/s12891-021-04414-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04414-2