Abstract
Background
There is no consensus concerning the optimal casting technique for displaced distal radius fractures (DRFs) following closed reduction. This study evaluates whether a splint or a circumferential cast is most optimal to prevent fracture redisplacement in adult patients with a reduced DRF. Additionally, the cost-effectiveness of both cast types will be calculated.
Methods/design
This multicenter cluster randomized controlled trial will compare initial immobilization with a circumferential below-elbow cast versus a below-elbow plaster splint in reduced DRFs. Randomization will take place on hospital-level (cluster, n = 10) with a cross-over point halfway the inclusion of the needed number of patients per hospital. Inclusion criteria comprise adult patients (≥ 18 years) with a primary displaced DRF which is treated conservatively after closed reduction. Multiple trauma patients (Injury Severity Score ≥ 16), concomitant ulnar fractures (except styloid process fractures) and patients with concomitant injury on the ipsilateral arm or inability to complete study forms will be excluded. Primary study outcome is fracture redisplacement of the initial reduced DRF. Secondary outcomes are patient-reported outcomes assessed with the Disability Arm Shoulder Hand score (DASH) and Patient-Rated Wrist Evaluation score (PRWE), comfort of the cast, quality of life assessed with the EQ-5D-5L questionnaire, analgesics use, cost-effectiveness and (serious) adverse events occurence. In total, 560 patients will be included and followed for 1 year. The estimated time required for inclusion will be 18 months.
Discussion
The CAST study will provide evidence whether the type of cast immobilization is of influence on fracture redisplacement in distal radius fractures. Extensive follow-up during one year concerning radiographic, functional and patient reported outcomes will give a broad view on DRF recovery.
Trial registration
Registered in the Dutch Trial Registry on January 14th 2020. Registration number: NL8311.
Similar content being viewed by others
Background
Displaced distal radius fractures (DRF’s) are very common in the adult population and their incidence is still increasing because of the ageing population [1, 2]. In the Netherlands, the incidence of DRFs is estimated at 20 per 10.000 persons per year [3]. Two-thirds of DRFs in adults are displaced and require closed reduction [4]. After successful reduction, DRFs are generally immobilized using a non-circular splint or a circumferential cast. Unfortunately, a large number of reduced DRF’s (32–64%) redisplace during cast immobilization in the first treatment weeks [5,6,7]. Whereas redisplacement of DRFs was previously accepted or reduced in a second attempt, nowadays these redisplaced fractures are generally treated surgically [8, 9].
Although surgical reduction and fixation generally results in a satisfying outcome, preventing fracture redisplacement and thereby preventing surgery would be the preferred scenario. Therefore, it is important to discover factors that could predict or prevent fracture redisplacement. Studies focusing on this topic are mostly assessing non-modifiable factors predicting fracture redisplacement [5,6,7, 10]. Known factors enhancing the risk on fracture displacement are female gender, age > 60 years and fractures with dorsal comminution [10]. However, it is unknown if type of cast immobilization is of influence.
Existing literature concerning the role of cast immobilization on fracture re-displacement is scarce and inconclusive [11]. Recently, Caruso et al. (2019) performed a randomized controlled trial comparing the effect of above-elbow casting and below-elbow casting on maintaining reduction and found similar results in both groups [12]. Patient-reported outcome after 1-year follow-up did not differ amongst both treatment groups. A randomized controlled trial by Wik et al. (2009) evaluated fracture alignment during 5 weeks follow-up in 72 patients with displaced DRF’s treated with dorsal splinting versus circumferential casting [13]. The circumferential casting group showed a significantly better result for radial length at 5 weeks but no difference concerning dorsal angulation. Recently, our research group conducted a retrospective study in 500 patients with reduced DRFs and found significantly less fracture redisplacement in fractures treated with circumferential casting compared with splinting, namely 17% versus 29% [14].
The choice for a splint or circumferential cast is often based on the treating physician’s preference [11]. A splint could be considered easy and quick to apply which is favorable at busy emergency departments, but it can loosen easily. One argument for not applying a circumferential cast initially after reduction is the risk of pain and comprised circulation due to swelling. Several studies however found none or mild differences in pain complaints comparing circumferential casting with splinting [13, 15, 16].
This study aims to clarify if type of cast immobilization influences maintaining fracture alignment in reduced adult distal radius fractures. A cluster randomized controlled trial is designed to compare the treatment of reduced DRFs with a splint or a circumferential cast. Radiological, functional and patient-reported outcomes are studied during a one-year study period.
Methods/design
This manuscript is written according to the Consolidated Standards for Reporting Trials (CONSORT statement) and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT guidelines) [17, 18].
Objectives
The primary objective is to assess which type of cast, a splint versus a circumferential cast, is most optimal to prevent fracture redisplacement in adult patients with a reduced DRF.
As secondary objectives, we assess which type of casting results in fewer surgical interventions and complications. Thereby we will assess cost-effectiveness, the comfort of the cast, pain scores, functional outcome, patient-reported outcome and quality of life.
Design, participants, interventions and outcomes
Study design and randomization
For this study, a multicenter cluster randomized design is used. All participating hospitals are located in the Netherlands and include the following 10 centres; Alrijne hospital (Leiderdorp), Elisabeth-Tweesteden hospital (Tilburg), Erasmus MC University Medical Center (Rotterdam), Franciscus hospital, location Gasthuis and Vlietland (Rotterdam and Schiedam), Haga Teaching hospital (Den Haag), Haaglanden Medical Center (Den Haag), IJsselland hospital (Capelle aan den IJssel), Maasstad hospital (Rotterdam), Reinier de Graaf Gasthuis (Delft) and St. Antonius Hospital (Utrecht and Nieuwegein).
Two types of cast immobilization will be compared in this trial. Randomization at patient level will be challenging because of the 24/7 availability of the Emergency Department (ED), leading to a high number of treating physicians. To overcome the potential of many protocol violations, we chose to randomise on hospital-level with a cross-over point halfway the needed inclusions per hospital (i.e. after 31 inclusions). This means all patients in one hospital will receive the same intervention, which will change after half of the number of patients are included. This cross-over design is used to overcome potential non-eligibility of both groups because patient populations can differ amongst hospitals. Secondly, possible bias due to existing experience with one of the techniques in each hospital will be diminished. Before the start of the study, an independent researcher not involved in the study, randomly allocated the starting treatments amongst the participating hospitals.
Study population
Participants and recruitment
The study population will consist of adult patients who visit the ED of participating hospitals with a distal radius fracture needing reduction. Fractures who should be reduced conform to the Dutch guideline meet one or more of the following criteria: > 15° of dorsal angulation, > 20° of volar angulation, < 15° of radial inclination, > 3 mm of radial shortening and > 2 mm intra-articular step-off or gap.
Patients that match the inclusion criteria are informed about the study by the treating physician before closed reduction. All patients receive written information, namely the patient information folder (PIF) and a short information folder containing the study aim, contact information of the local hospital and general information about cast immobilization. Because of the acute status of a sustained fracture, patients will be asked for participation in the study directly after diagnosis of a displaced DRF. Written informed consent is obtained before the study procedure starts.
Inclusion criteria
-
Age ≥ 18 years
-
Distal radius fracture requiring closed reduction
Exclusion criteria
-
Concomitant ulnar fracture (styloid process fracture not encountered)
-
Multiple trauma patients (Injury Severity Score (ISS) ≥ 16)
-
Concomitant injuries to the ipsilateral extremity, interfering with the treatment of the DRF
-
Inability to complete study forms due to any mental status or insufficient understanding of the Dutch language
Study procedures and timeline
Measurements will take place at 7 time points as shown in Table 1. These time points are: baseline (T0), 1 week (T1), 2 weeks (T2), 5 weeks (T3), 3 months (T4), 6 months (T5) and 1 year (T6) after inclusion in the study. At T0, baseline characteristics will be gathered. An inclusion form will be filled in by the treating physician providing fracture and treatment-specific information. Second, patients receive a questionnaire, providing predominantly patient and injury-specific information. The list of baseline characteristics is shown in Table 2. Patients receive questionnaires at T1 to T6. These questionnaires are carried out by email with the use of data capture system GemsTracker [19]. GemsTracker (GEneric Medical Survey Tracker) is a secure web-based application for distribution of questionnaires and forms during clinical research and quality registrations. Receiving questionnaires on paper is optional, as well as telephone interviews. Reminders will be automatically sent. When the patient does not respond to emails, we will contact the patient by telephone. Posterior-anterior (PA) and lateral radiographs of the wrist will be taken at T0 (before and after reduction) and during follow-up at T1 –T3. Physical examination of the wrist will take place at T4 and will be performed by the researcher. If patients are not able to visit the hospital, a home visit will be offered to improve the follow-up.
Interventions
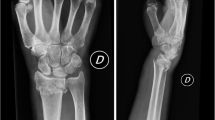
We will compare two casting options that are applied directly after reduction: a plaster of Paris envelope splint (further called splint) versus a below-elbow forearm cast (further called circumferential cast). Both interventions are shown in Figs. 1 and 2. The splint or circumferential cast will be applied by an ED nurse, a cast technician, the physician or a physician assistant. Both interventions will be implemented by education and training. Education will be available at the ED’s at all times by means of an instruction video and an instruction poster. The need for training of both casting techniques is evaluated before the start of the study and differs amongst participating hospitals. We chose hospital individualized training instead of a general training program because participating hospitals differ in: the currently used treatment, the experience of ED nurses with both casting techniques and the availability of cast technicians at the ED. In case extra training is needed, training is organized by local cast technicians, in accordance with the research group.
Outcome
Primary outcome
The primary outcome of this study is the occurrence of fracture re-displacement of the initial reduced DRF assessed on PA and lateral radiographs at 1, 2 and 5 weeks after reduction. Displacement of the radius is defined by the Dutch guideline: > 15° of dorsal angulation, > 20° of volar angulation, < 15° of radial inclination, > 3 mm of radial shortening and > 2 mm intra-articular step-off or gap. Measurements will be digitally carried out in the Picture Archiving and Communication System (PACS) on standard PA and lateral radiographs of the wrist. PA and lateral radiographs will be taken conform standardised procedures. An Intraclass Correlation Coefficient (ICC) will be calculated to assess inter- and intra-observer variability in radiograph measurements. For this, 50 radiographs will be measured twice by BB and NS.
Secondary outcomes
An overview of measurements is shown in Table 1.
-
Comfort of the cast assessed using a self-developed questionnaire at T1 and T2.
-
Severity of pain evaluated with the Numeric Rating Scale (NRS) at T1 to T6.
-
Patient-reported recovery of function assessed using the Quick Disabilities of the Arm, Shoulder and Hand questionnaire (Q-DASH) and the Patients-Rated Wrist Hand Evaluation questionnaire (PRWHE). Both questionnaires are validated for assessing functional outcome in patients with a DRF [20,21,22]. Questionnaires will be carried out at T3 to T6.
-
Quality of life will be assessed using the 5-level EuroQol (EQ-5D-5L) [23] scoring questionnaire at T1 (pre- and postfracture state) and T3 to T6.
-
Cost-effectiveness will be measured using the Medical Consumption Questionnaire (iMCQ) and the Production Consumption Questionnaire (iPCQ) [24]. Questionnaires will be sent at T3 to T6.
-
Recovery of function will be evaluated by physical examination at T4.
-
Range of motion (ROM) of the wrist will be measured with a goniometer. ROM concerns dorsal flexion, volar flexion, radial deviation, ulnar deviation, pronation and supination.
-
Grip strength will be measured with a Jamar hydraulic hand dynamometer. Patients get three attempts on both sides. The maximum score for each side is used in the analyses.
-
Specific testing of the wrist and hand will be performed namely:
-
distal radioulnar joint (DRUJ) stability tested with the DRUJ ballottement test [25]
-
opposition of the thumb using the Kapandji score [26]
-
finger stiffness will be measured with finger-to-palm distance, tested from the tip of the finger to the distal palmar crease when the fingers are in maximal active flexion [27]
-
ROM and grip strength on the injured side will be compared with the uninjured side.
-
-
Number of conversions to surgical treatment and other (serious) adverse events will be monitored and are listed at 1.9.
Modified follow-up by partial exclusion
Patients are recruited before fracture reduction because immobilization takes place immediately after reduction. A part of included patients will receive surgical fixation in the first treatment week, due to unsuccessful fracture reduction. Consequently, it is unavoidable to include patients who will eventually be unsuitable to answer the primary research question. Patients receiving surgical fixation before the first follow-up appointment (T1) will not be encountered in the needed patient numbers. Recovery of these patients will be evaluated by questionnaires only (Fig. 3).
CAST study follow-up flowchart. *Type of treatment depends on randomization status of the hospital. μ Unacceptable alignment conform the Dutch guideline: > 15° of dorsal angulation, > 20° of volar angulation, < 15° of radial inclination, > 3 mm of radial shortening and > 2 mm intra-articular step-off or gap. ∆ Cast immobilization, at least until the first control radiographs are taken. £ All measurements encountered in Table 1. ¥ All questionnaires encountered in Table 1
(serious) adverse events reporting
All adverse events reported by the patient or observed by the treating physician or researcher will be recorded. Serious adverse events (SAE) will be reported through the web portal ToetsingOnline of the Central Committee on Research Involving Human Research (Dutch CCMO) to the Medical Research Ethics Committee of Erasmus Medical Center in Rotterdam, which approved the protocol. SAE reporting will take place within 7 days after the sponsor has first knowledge of a serious adverse event resulting in death or is life-threatening. All other SAE will be reported within 15 days.
Adverse events are defined as:
-
Cast or splint related problems
-
Fracture redisplacement treated with surgical reduction and fixation
-
Complex regional pain syndrome (CRPS) defined conform the Budapest criteria
-
Disabling fracture mal-union or non-union
Study related serious adverse events are defined as:
-
Compartment syndrome
Sample size
The sample size calculation is based on the results of our recent retrospective study [14]. We hypothesize that fracture redisplacement occurs in 10% of circumferential casted patients versus 20% in splinted patients. A total of 500 patients is needed to detect superiority of a circumferential cast. The sample size calculation is based on a mixed-effects logistic regression, to account for clustering using a random intercept for the hospitals. The intra-class correlation coefficient between the different hospitals for the proportion of fracture redisplacement is assumed to be 0.06, which is generally reported in the literature for hospital processes. From the expected proportions of redisplacement in the two groups, we calculated the hospital-specific log odds of secondary displacement in the circular cast group, equal to − 2.275 and log odds ratio of redisplacement between the two groups equal to 0.84. This calculation is based on the formula that links the cluster-specific coefficients in the mixed-effects logistic regression with the population coefficients averaged over the hospitals. With a power of 93%, 50 patients per hospital need to be included, resulting in a total number needed of 500 (10 participating hospitals). Additionally, we also calculated the needed number of patients using a two-sample test for proportions. Using the same assumptions, namely difference between groups, a significance level of 0.05 and a power of 90%. This resulted in 490 needed patients in total. Accounting for a 10% loss to follow-up, a total of 560 (280 patients per group) are required.
To improve awareness of the study in all participating centers and thereby advert for reaching targeted sample size, a local investigator is appointed in each hospital. This local investigator is a physician who supervises the study. This person is easily accessible and will promote the study on a regular base. We send a newsletter every 2 months to inform about the study progression.
Data analysis
General descriptive statistics will be performed on baseline patient and fracture characteristics. Patients will be analyzed according to the intention-to-treat principle.
Primary outcome
The primary outcome, fracture re-displacement, will be analyzed using a mixed-effects logistic regression. To account for clustering, a random intercept for the hospitals will be used. Fixed effects will be the covariates we adjust for as reported in the literature, namely age, presence of osteoporosis, and fracture characteristics. If new prognostic factors will be identified and reported in the literature, these factors will also be added as covariates.
Secondary outcomes
For secondary outcomes, trends between baseline and follow-up time points (T0-T6) will be assessed using linear mixed models for repeated measures. This accounts for comfort of the cast, recovery of function and grip strength, pain severity (NRS), Q-DASH scores, PRWHE and EQ-5D-5L scores. The number of conversions to surgical fixation and complications will be determined using Fisher Exact or Chi-square test, depending on the magnitude of results.
Cost-effectiveness analysis
Both cost-effectiveness (CEA) and cost-utility (CUA) analysis will be performed from a societal perspective. For the calculation of medical costs, we will use charges as published in Dutch guidelines as a proxy of real costs. The unit per price of the cast and splint application in patients with DRF will be calculated with the micro-costing method. Intramural costs (i.a. additional diagnostics, number of hospital visits, in case of hospital admission the length of stay etc.) are collected from the electronic health record. Productivity costs will be registered in detail by the iPCQ. The iMCQ and the iPCQ are validated by the Institute of Medical Technology Assessment (Erasmus University, Rotterdam, The Netherlands).
The difference in costs and effects of a circumferential cast instead of a splint will be calculated as incremental cost-effectiveness ratio (ICER). The primary effect outcome measures will be the number of re-displacements for the CEA and quality-adjusted life years (QALY) for the CUA. QALYs will be measured, based on the Dutch tariff for the EQ-5D-5L.
The sensitivity analysis will assess the robustness of the results to changes in costs and effect parameters. Bootstrapping with 5000 replications will be used to estimate 95% confidence intervals around cost differences and the uncertainty surrounding the ICERs. This will be graphically presented on cost-effectiveness planes and acceptability curves using the net benefit framework [28, 29]. For the time horizon of 1 year, discounting is not necessary.
Data management
All data are handled confidentially and anonymized in compliance with the Dutch Personal Data Protection Act. Personal data of participants will be changed by a study number. This number is used for all study documentation, study reports and publications. The key of this study number will be handled by an independent researcher. During the study period, all data will be collected and managed using GemsTracker electronic data capture tools hosted at Erasmus Medical Center [19]. Paper case report forms are entered in GemsTracker by the researcher and the original paper case forms will be filed in the investigator site file at the recruiting hospital. All data is stored for 15 years.
Data monitoring
Since the study is labelled as low risk, a data safety monitoring board is not required. However, the study will be monitored at least once a year by an independent monitoring board. A written report will be available from all monitors.
The investigator will submit a progress report to the accredited Medical Research Ethics Committee of Erasmus Medical Center in Rotterdam throughout the clinical trial annually. This will consist of the date of inclusion of the first subject, numbers of subjects included and numbers of subjects that have completed the trial, SAEs, other problems and amendments.
Dissemination
We plan to present the study results at (inter) national conferences and submit the manuscript to general peer-review journals. We aim to implement the study results in the Dutch guideline for DRFs.
Discussion
This study is an open-label trial. Allocated treatments are visually different for the treating physician and the patient. Randomization status will therefore not be blinded.
Heterogeneity of the study population concerning fracture characteristics and age could be pointed out as a limitation. However, this pragmatic study tries to represent the actual patient population.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DRF:
-
Distal radius fracture
- ED:
-
Emergency Department
- PIF:
-
Patient Information Folder
- ISS:
-
Injury Severity Score
- PA:
-
Posterior-anterior
- PACS:
-
Picture Archiving and Communication System
- ICC:
-
Intraclass Correlation Coefficient
- NRS:
-
Numeric Rating Scale
- Q-DASH:
-
Quick Disabilities of the Arm, Shoulder and Hand
- PRWHE:
-
Patients-Rated Wrist Hand Evaluation
- EQ-5D-5L:
-
5-level EuroQol
- iMCQ:
-
Medical Consumption Questionnaire
- iPCQ:
-
Production Consumption Questionnaire
- ROM:
-
Range of motion
- DRUJ:
-
Distal radioulnar joint
- SAE:
-
Serious adverse event
- CCMO:
-
Central Committee on Research Involving Human Research
- CRPS:
-
Complex regional pain syndrome
- CEA:
-
Cost-effectiveness analysis
CUA
Cost-utility analysis
- ICER:
-
Incremental cost-effectiveness ratio
- QALY:
-
Quality-adjusted life year
References
Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–7. https://doi.org/10.1016/j.injury.2006.04.130.
MacIntyre NJ, Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther. 2016;29(2):136–45. https://doi.org/10.1016/j.jht.2016.03.003.
Bentohami A, Bosma J, Akkersdijk GJ, van Dijkman B, Goslings JC, Schep NW. Incidence and characteristics of distal radial fractures in an urban population in the Netherlands. Eur J Trauma Emerg Surg. 2014;40(3):357–61.
Brogren E, Petranek M, Atroshi I. Incidence and characteristics of distal radius fractures in a southern Swedish region. BMC Musculoskelet Disord. 2007;8(1):48. https://doi.org/10.1186/1471-2474-8-48.
Mackenney PJ, McQueen MM, Elton R. Prediction of instability in distal radial fractures. J Bone Joint Surg Am. 2006;88(9):1944–51. https://doi.org/10.2106/JBJS.D.02520.
Jung HW, Hong H, Jung HJ, Kim JS, Park HY, Bae KH, et al. Redisplacement of distal radius fracture after initial closed reduction: analysis of prognostic factors. Clin Orthop Surg. 2015;7(3):377–82. https://doi.org/10.4055/cios.2015.7.3.377.
Makhni EC, Ewald TJ, Kelly S, Day CS. Effect of patient age on the radiographic outcomes of distal radius fractures subject to nonoperative treatment. J Hand Surg Am. 2008;33(8):1301–8. https://doi.org/10.1016/j.jhsa.2008.04.031.
van der Linden W, Ericson R. Colles’ fracture. How should its displacement be measured and how should it be immobilized? J Bone Joint Surg Am. 1981;63(8):1285–8. https://doi.org/10.2106/00004623-198163080-00009.
Jo YH, Lee BG, Kim JH, Lee CH, Kim SJ, Choi WS, et al. National Surgical Trends for distal radius fractures in Korea. J Korean Med Sci. 2017;32(7):1181–6. https://doi.org/10.3346/jkms.2017.32.7.1181.
Walenkamp MM, Aydin S, Mulders MA, Goslings JC, Schep NW. Predictors of unstable distal radius fractures: a systematic review and meta-analysis. J Hand Surg Eur Vol. 2016;41(5):501–15. https://doi.org/10.1177/1753193415604795.
Handoll HH, Madhok R. Conservative interventions for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2002;2:CD000314.
Caruso G, Tonon F, Gildone A, Andreotti M, Altavilla R, Valentini A, et al. Below-elbow or above-elbow cast for conservative treatment of extra-articular distal radius fractures with dorsal displacement: a prospective randomized trial. J Orthop Surg Res. 2019;14(1):477. https://doi.org/10.1186/s13018-019-1530-1.
Wik TS, Aurstad AT, Finsen V. Colles' fracture: dorsal splint or complete cast during the first 10 days? Injury. 2009;40(4):400–4. https://doi.org/10.1016/j.injury.2008.08.044.
Berger AC, Barvelink B, Reijman M, Gosens T, Kraan GA, De Vries MR, et al. Does circumferential casting prevent fracture redisplacement in reduced distal radius fractures? A retrospective multicenter study. (unpublished work). 2020.
O'Connor D, Mullett H, Doyle M, Mofidi A, Kutty S, O'Sullivan M. Minimally displaced Colles' fractures: a prospective randomized trial of treatment with a wrist splint or a plaster cast. J Hand Surg Br. 2003;28(1):50–3. https://doi.org/10.1054/JHSB.2002.0864.
Helweg J, Boesen J, Hellberg S, Thyregod HC. Colles' fracture. Therapeutic results and use of resources. A comparative study between 2 departments in the same county. Ugeskr Laeger. 1991;153(8):576–8.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c332. https://doi.org/10.1136/bmj.c332.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586. https://doi.org/10.1136/bmj.e7586.
GemsTracker, copyright©. latest release 2019. Available from: https://gemstracker.org/. Accessed 1 Feb 2020.
Tsang P, Walton D, Grewal R, MacDermid J. Validation of the QuickDASH and DASH in patients with distal radius fractures through agreement analysis. Arch Phys Med Rehabil. 2017;98(6):1217–22 e1. https://doi.org/10.1016/j.apmr.2016.11.023.
Changulani M, Okonkwo U, Keswani T, Kalairajah Y. Outcome evaluation measures for wrist and hand: which one to choose? Int Orthop. 2008;32(1):1–6. https://doi.org/10.1007/s00264-007-0368-z.
Kleinlugtenbelt YV, Krol RG, Bhandari M, Goslings JC, Poolman RW, Scholtes VAB. Are the patient-rated wrist evaluation (PRWE) and the disabilities of the arm, shoulder and hand (DASH) questionnaire used in distal radial fractures truly valid and reliable? Bone Joint Res. 2018;7(1):36–45. https://doi.org/10.1302/2046-3758.71.BJR-2017-0081.R1.
Hung MC, Lu WS, Chen SS, Hou WH, Hsieh CL, Wang JD. Validation of the EQ-5D in patients with traumatic limb injury. J Occup Rehabil. 2015;25(2):387–93. https://doi.org/10.1007/s10926-014-9547-0.
Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van RL. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18(6):753–8. https://doi.org/10.1016/j.jval.2015.05.009.
Moriya T, Aoki M, Iba K, Ozasa Y, Wada T, Yamashita T. Effect of triangular ligament tears on distal radioulnar joint instability and evaluation of three clinical tests: a biomechanical study. J Hand Surg Eur Vol. 2009;34(2):219–23. https://doi.org/10.1177/1753193408098482.
Kapandji A. Clinical test of apposition and counter-apposition of the thumb. Ann Chir Main. 1986;5(1):67–73. https://doi.org/10.1016/S0753-9053(86)80053-9.
Torok KS, Baker NA, Lucas M, Domsic RT, Boudreau R, Medsger TA Jr. Reliability and validity of the delta finger-to-palm (FTP), a new measure of finger range of motion in systemic sclerosis. Clin Exp Rheumatol. 2010;28(2 Suppl 58):S28–36.
Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves--facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–15. https://doi.org/10.1002/hec.903.
Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Mak. 1998;18(2 Suppl):S68–80. https://doi.org/10.1177/0272989X98018002S09.
Acknowledgements
The CAST study group consists of the following persons:
Ninka Slebioda 1 , Mathieu M E Wijffels 2 , Marna G Bouwhuis 3 , Flip van Beek 4 , Daphne A van Rijssel 5 , Mark R de Vries 6 , Michiel Leijnen 7 , Peer van der Zwaal 8 , Merel van Loon 9 , Alexander P A Greeven 10 , Lenneke Scholtens 11 , Ruud LM Deijkers 12 , Marike C Kokke 13 , Milko M M Bruijninckx 14
-
1.
Department of Orthopedic Surgery, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
-
2.
Trauma Research Unit, Department of Surgery, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
-
3.
Department of Emergency Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands
-
4.
Department of Trauma Surgery, Franciscus Hospital, Rotterdam, The Netherlands
-
5.
Department of Emergency Medicine, Reinier de Graaf Gasthuis, Delft, The Netherlands
-
6.
Department of Trauma Surgery, Reinier de Graaf Gasthuis, Delft, The Netherlands
-
7.
Department of Trauma Surgery, Alrijne Hospital, Leiderdorp, The Netherlands
-
8.
Department of Orthopedic Surgery, Haaglanden Medical Center, The Hague, The Netherlands
-
9.
Department of Emergency Medicine, Haaglanden Medical Center, The Hague, The Netherlands
-
10.
Department of Trauma Surgery, Haga Hospital, The Hague, The Netherlands
-
11.
Department of Emergency Medicine, Haga Hospital, The Hague, The Netherlands
-
12.
Department of Orthopedic Surgery, Haga Hospital, The Hague, The Netherlands
-
13.
Department of Trauma Surgery, St. Antonius Hospital, Nieuwegein, The Netherlands
-
14.
Department of Trauma Surgery, IJsselland Hospital, Capelle aan den IJssel, The Netherlands
Trial status
Study inclusion is ongoing and has started in May 2020.
Funding
A grant is received from ZonMw (project number: 852002021), a Dutch organization for health research and care innovation. ZonMw was not involved in the study design, data collection, analysis and interpretation of data, nor in writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
MR and JC drafted the study idea and secured funding. MR, JC, TG, VB, GK, NS, WI, SP and BB were involved in the study design. JC, VB, GK, NS, BB and the CAST study group are involved with the implementation of the protocol and data collection. BB is conducting the study. SP will perform the cost-effectiveness analyses. WI will aid with the study implementation. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study will be conducted according to the principles of the Declaration of Helsinki (64th version, October 2013) and was approved by the Medical Research Ethics Committee of Erasmus Medical Center in Rotterdam (reference number: project 2019_0528). Written informed consent is obtained from all participants before the study procedure starts. The trial is registered in the Dutch Trial Registry (registration number NL8311). In all participating hospitals, the local responsible authorities approved the conduct of the study. In case of important protocol modifications (e.g. new or cancelling of a recruiting hospital, change in outcomes) this will be communicated towards the Medical Research Ethics Committee of Erasmus Medical Center in Rotterdam.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barvelink, B., Reijman, M., Schep, N.W.L. et al. The CAST study protocol: a cluster randomized trial assessing the effect of circumferential casting versus plaster splinting on fracture redisplacement in reduced distal radius fractures in adults. BMC Musculoskelet Disord 22, 370 (2021). https://doi.org/10.1186/s12891-021-04238-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04238-0