Abstract
Background
3D printing technology in hospitals facilitates production models such as point-of-care manufacturing. Orthopedic Surgery and Traumatology is the specialty that can most benefit from the advantages of these tools. The purpose of this study is to present the results of the integration of 3D printing technology in a Department of Orthopedic Surgery and Traumatology and to identify the productive model of the point-of-care manufacturing as a paradigm of personalized medicine.
Methods
Observational, descriptive, retrospective and monocentric study of a total of 623 additive manufacturing processes carried out in a Department of Orthopedic Surgery and Traumatology from November 2015 to March 2020. Variables such as product type, utility, time or materials for manufacture were analyzed.
Results
The areas of expertise that have performed more processes are Traumatology, Reconstructive and Orthopedic Oncology. Pre-operative planning is their primary use. Working and 3D printing hours, as well as the amount of 3D printing material used, vary according to the type of product or material delivered to perform the process. The most commonly used 3D printing material for manufacturing is polylactic acid, although biocompatible resin has been used to produce surgical guides. In addition, the hospital has worked on the co-design of customized implants with manufacturing companies.
Conclusions
The integration of 3D printing in a Department of Orthopedic Surgery and Traumatology allows identifying the conceptual evolution from “Do-It-Yourself” to “POC manufacturing”.
Similar content being viewed by others
Background
3D printing has emerged as a disruptive technology in Orthopedic Surgery and Traumatology [1, 2]. In this area, it has been used to create customized biomodels (replicas of patient anatomy), devices, instruments and implants, to plan and simulate complex surgical procedures, or as a teaching or communication tool [3]. The increase in accessibility of this technology has moved hospitals towards the implementation of their own in-house 3D printing programs, intending to create knowledge internally and to reduce both delivery times and costs of the 3D printed models [4]. In this scenario, a manufacturing university hospital may act as a hub that may combine in-house 3D printing with distributed production. This idea arises not to compete against the traditional medical industry, but to generate value in personalized medicine. There are several references to this approach, where professional teams with the necessary resources have developed knowledge based on their individual experience, allowing a qualitative leap in patient-centered medicine [5,6,7].
The integration of this technology within the hospitals started with a “Do it yourself” approach, where realistic reproductions were 3D printed by innovative individuals at a minimum cost. These initial steps have now led to new production models in which the manufacturing hospitals are identified as network hubs, reconciling in-house manufacturing and outsourcing. This solution strengthens collaborative work, ensures that hospital manufacturing becomes the standard of care, and efficiently adjust available resources to the existing limitations [5, 8]. This change of paradigm frees hospitals from the restrictions imposed by the commercial catalog, allowing them to propose, manufacture, evaluate and participate in emerging lines such as custom-made implants manufacturing [9,10,11,12] or tissue bio-printing [13,14,15].
New production models such as “point-of-care (POC) manufacturing” provide for this, making it possible to respond to space needs or expensive installations, to bring together the technical competence profile in industrial aspects, or to keep its portfolio of services up to date without depending on the obsolescence of the machines and manufacturing materials, which is very rapid for this type of technology [5].
The purpose of this study is to present the results of the integration of 3D printing technology in a Department of Orthopedic Surgery and Traumatology and to identify the POC productive model as a paradigm of personalized medicine, allowing to conciliate indication and surgical planning with the design and manufacture of specific patient solutions.
Methods
In order to define the possibilities of integrating 3D printing technology in a Department of Orthopedic Surgery and Traumatology, we present an observational, descriptive, retrospective, and monocentric study which includes the additive manufacturing processes carried out in the Department at Hospital General Universitario Gregorio Marañón (Madrid. Spain). The time interval starts from the creation of the Advanced Planning and 3D Manufacturing Unit (UPAM3D) in November 2015 to March 2020, date on which the UPAM3D projects were temporarily interrupted as a result of the COVID-19 pandemic.
An additive manufacturing process allows transforming a digital model into a real and tangible three-dimensional product. 3D digital models can be obtained from different sources: digital radiological studies, three-dimensional scanning, computational design (CAD) or reverse engineering. When the digital model is ready, the products are built layer by layer, using different technologies and materials depending on the final application [16, 17]. Figure 1 summarizes the 3DP workflow in a patient with a sacral tumor, enabling the fabrication of the biomodel and surgical guides for tumor resection.
In this study, we have defined the following activity variables to analyze the additive manufacturing process:
-
Area of expertise: it identifies the specific area in the department that originated the activit. Possible values are Traumatology, Upper Limb, Spine, Foot and Ankle, Pediatric orthopedic, Reconstructive, and Oncology, Basic research, and some specific research lines (3DP research, University collaborative projects).
-
Required product: The products can be 3D printed biomodel, surgical guide/instrument for an interventional procedure, or an instrument for surgical navigation.
-
Product utility: This variable takes into account the primary utility of the product. Although these products have great value as a communication tool in most cases, it has been described as such only when communication was the essential utility of the product. Other utilities have been preoperative planning, intraoperative utility, instrumental or research.
-
Material delivered to perform the process: this identifies the original information provided by the requesting user. It may be a medical record number or radiological images in DICOM files [MRN/DICOM], a 3D digital model design [3D model], or other materials such as a drawing or a tool with design improvement suggestions, which need to be reverse-engineered to obtain a 3D Model.
-
Work time: Time allocated by the facultative team and technical team to develop the project, which includes obtaining and designing the 3D model, preparing it for the printing process, and post-processing the product after manufacturing before delivery.
-
3D Printing time: Time required by the 3D printer to manufacture the product.
-
Quantity of 3D Printing material: Quantity of material (in grams) used by the printer for each of the products obtained.
-
Type of 3D printing material: Type of material used to manufacture the product, that depends on the 3D printing technology used. Two types of technology were used in this study: FDM, where the printer deposits fused material (different plastics) layer by layer, and stereolithography (SLA), in which a laser photo-polymerizes a resin converting it into solid material.
Quantitative variables have been described with centralization measures (mean and median); qualitative variables as numbers and percentages.
Results
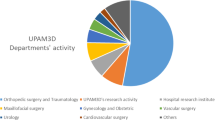
A total of 623 additive manufacturing processes have been carried out in the Orthopedic Surgery and Traumatology Department during the studied time. Research lines (Basic research, 3DP research, University collaborative projects) show the most significant activity (38.84%). Other areas of expertise that carried out a substantial number of processes were Orthopedics Oncology (21.86%) and Traumatology (20.06%). Although the number of annual processes has been similar, a different evolution by area of expertise is observed, with a larger number of processes identified in specific research lines (3DP research, University collaborative projects) in the first two years. The detailed data is shown in Table 1.
The products requested were 3D printed biomodels in 87.32% of cases and surgical positioning guides in 10.75%. The remaining cases were patient-specific instruments for surgical interventions, in which 3D printing hybridization with surgical navigation or augmented reality provided added value [18, 19] (Fig. 2). The utility has been different depending on the type of product manufactured. 3D printed biomodels have been used in communication, planning, or research. On the other side, the usefulness of positioning guides or instruments has mainly been intraoperative (Table 2).
A specific analysis of areas of expertise has shown that the types of products required in each area are different (Table 3). In areas such as Traumatology, Upper Limb or Pediatric Orthopedic, 3D Printed Biomodel has been the requested product in all cases. However, Foot and Ankle, Orthopedic Oncology or Reconstructive Surgery, requested other products such as guides or patient-specific instruments in 75, 33.01, and 16.24% of cases, respectively.
Material delivered to perform the process was MRN / DICOM (45.59%) or 3D Model (45.26%). In the remaining cases (9.15%), other materials were delivered (Fig. 3). A different percentage distribution is evidenced in delivery material according to areas of expertise and types of products required. The detailed data is shown in Table 3.
Total working hours were 7410, with an average of 11.89 h (median 3 h) per process. The operating time of the 3D printers was 6277 h, with an average of 10.08 h (median 5 h) per process. However, the working time and 3D printing time have been different according to the type of product required and the delivery material (Table 4). The annual distribution of working and printing hours shows two lines that cross during the study period, due to a greater number of working hours in the first years with increased printing times in recent years (Fig. 4). This evolution has been mainly due to the increasing complexity of the products that are printed, combined with the decreasing times in the segmentation and image processing necessary to obtain virtual 3D models.
3D printing material consumed was 59,555 g, with an average of 95.59 g (median of 50 g) per process, with 96.5% of the material used on FDM 3D printers. 3D printing material most widely used was PLA (84%). This is a rigid material that replicates bone structures with great realism. But many others have been used, either flexible such as Filaflex (thermoplastic elastomer based on polyurethane and certain additives) which allows replica of vascular structures, solid organs or muscular-tendon structures, or support materials such as PVA (polyvinyl alcohol) to optimize the post-processing and improve the quality of the printed object. The demand and production capacity of positioning guides or patient-specific instruments has led to the development of biocompatible materials, basically resins, certified for medical use that allow manufacturing these products with SLA technology. During this period, 2.29% of the products have been printed with SLA, representing 3.5% of the total material (Fig. 5).
The experience and accreditation as a manufacturing university hospital have made it possible to work with different companies in the sector, participating in the co-design of personalized implants [20] (Fig. 6), and collaborating with different research groups in bone and cartilage tissue bio-impression lines [21].
Discussion
3D technology is helping to address some of the growing complexities in healthcare, while enabling a more sustainable future as a scalable and cost-effective technology. Understood as a patient-specific process, it allows for greater efficiency throughout the entire value chain to improve results for the patient, and doing it right the first time (GIRFT methodology) through a higher level of customization and predictability [4, 22,23,24].
Hospital General Universitario Gregorio Marañón is a pioneer in the transversal implementation of hospital 3D printing, incorporating an “in-house” medical 3D printing laboratory integrated into the clinical workflow of more than 20 medical-surgical specialties. As a university manufacturing hospital, it is licensed to manufacture medical devices in compliance with the international standard ISO 13485 for quality management systems for medical products. This has allowed the Orthopaedic Surgery and Traumatology Department to network and coordinate production with traditional orthopedic implant manufacturers and research centers [25,26,27,28,29,30].
A university hospital includes students or doctors in training, not replacing universities but complementing training, enriching the academic environment. In the same way, a manufacturing university hospital does not replace factories. In a manufacturing university hospital, 3D printing goes hand-in-hand with translational research and teaching, acting as an accelerator for clinical innovation. 3D technology is a great tool for teaching and medical simulation, which is also carried out efficiently and in a personalized way, since training models can be manufactured that reproduce specific pathologies of real medical cases.
The integration of 3D printing into the clinical workflow has allowed complete control and monitoring of the process, from the indication to the manufacture of a customized medical-surgical solution. This adds significant value in the manufacture of guides and instruments or even customized implants, integrating 3D design as part of the therapeutic planning process, and 3D printing as part of the surgical approach [31,32,33].
The indicators described in this study allow evaluation and proposal of specific corrective actions according to the results obtained. Annual production by areas of expertise has changed during the study period. Identification and optimization of specific software and hardware, materials or manufacturing parameters have been research objectives, which has required not only implementation of a high number of processes in first two years, but also an increase in working time of technical and medical staff assigned to the achievement of these processes.
It is important to identify the areas of expertise that have the greatest potential for the integration of 3D printing technology. The Radiological Society of North America 3D printing group (3D Special Interest Group RSNA) has reviewed and classified the clinical cases in which it is more efficient to use 3D printed biomodels, and has concluded that in simple fractures the role of 3D printing is not as useful as in complex fractures, hip dysplasia or bone tumours with joint involvement [34]. In our study, Reconstructive Surgery, which included deformities, degenerative joint pathology, infections or arthroplasties, Traumatology, which managed fractures of any anatomical location or age of presentation, and Orthopedic Oncology, represented 53.77% of the global activity. If we take into account that 38.84% was research activity, the remaining areas of expertise accounted for 7.39% (46 cases) of the total activity. With these findings, it is important to highlight the role of the manufacturing university hospital allowing the adaptation and optimization of response times, of great relevance in areas such as Traumatology or Orthopedic Oncology, where traditional manufacturing presents restrictions such as process outsourcing or associated costs.
The utility of 3D printed biomodels for preoperative planning has been of great interest in recent years [35]. In our study, 87.32% of the required products were 3D printed biomodels used not only for surgical planning but also for communication or research.
The availability of machines for “in-house” manufacturing by means of FDM or SLA technologies has allowed the production of 3D Printed Biomodels, Surgical Guides and Patient-specific Instruments, and the collaborative work with manufacturing companies has facilitated the co-design and production of patient-specific implants. In this way, complete traceability can be maintained over each stage of creation without interrupting the workflow. It allows attending in times of very tight therapeutic windows and with the solvency of a multidisciplinary team that accumulates valuable experience and knowledge of the patient that would otherwise remain fragmented. This is enabled by the point-of-care manufacturing model. It is also in line with the regulatory framework of this technology applied to personalized medicine, which identifies the prescribing physician as the final responsable for the process, including the design of the custom-made product [36,37,38,39].
Conclusions
This study identifies the possibilities of integrating 3D printing technology in a Department of Orthopedic Surgery and Traumatology. This experience allowed us to identify the conceptual evolution of the hospital workflow in additive manufacturing processes from “Do it yourself” to “POCM”. A descriptive monocentric study makes it difficult to extrapolate the results, so it is essential to propose specific multicenter studies and consensus documents.
Availability of data and materials
The authors declare that they have followed their centre’s protocols on the publication of patient data. All data analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D:
-
Three dimensional
- 3DP:
-
Three dimensional printing
- COVID-19:
-
Coronavirus disease 2019
- DICOM:
-
digital imaging and communication on medicine
- FDM:
-
Fused deposition modeling
- GIRFT:
-
Getting It Right First Time
- ISO:
-
International Organization for Standardization
- MRN:
-
medical record number
- PLA:
-
polylactic acid
- POC:
-
point of care
- POCM:
-
point of care manufacturing
- PVA:
-
polyvinyl alcohol
- SLA:
-
Stereolithography
- UPAM3D:
-
Advanced Planning and 3D Manufacturing Unit
References
Vaishya R, Patralekh MK, Vaish A, Agarwal AK, Vijay V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J Clin Orthopaed Trauma. 2018;9(3):194–201. https://doi.org/10.1016/j.jcot.2018.07.006.
Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: a technological marvel. J Clin Orthopaed Trauma. 2018;9(3):260–8. https://doi.org/10.1016/j.jcot.2018.07.022.
Auricchio F, Marconi S. 3D printing: clinical applications in orthopaedics and traumatology. EFORT Open Rev. 2016;1(5):121–7. https://doi.org/10.1302/2058-5241.1.000012.
Ballard DH, Mills P, Duszak R Jr, Weisman JA, Rybicki FJ, Woodard PK. Medical 3D printing cost-Savings in Orthopedic and Maxillofacial Surgery: cost analysis of operating room time saved with 3D printed anatomic models and surgical guides. Acad Radiol. 2019;27(8):1103–13. https://doi.org/10.1016/j.acra.2019.08.011.
SME Annual Report 2018. Medical Additive Manufacturing 3D Printing. In: https://www.sme.org/globalassets/sme.org/media/white-papers-and-reports/2018-sme-medical-am3dp-annual-report.pdf.
Mayo Clinic. Anatomical 3D Printing Lab. In: https://www.mayoclinic.org/es-es/departments-centers/anatomic-modeling-laboratories/overview/ovc-20473121.
L’Institut d’Investigació i Innovació Parc Taulí. 3D Lab. In: http://www.tauli.cat/es/institut/plataformes-i-serveis/laboratori-3d/.
Hospital for Special Surgery 2019. HSS 3D Printing Lab for Complex, Personalized Ortho Implants. In: https://news.hss.edu/hss-opening-3d-printing-lab-for-complex-personalized-ortho-implants/.
Liu W, Shao Z, Rai S, Hu B, Wu Q, Hu H, et al. Three-dimensional-printed intercalary prosthesis for the reconstruction of large bone defect after joint-preserving tumor resection. J Surg Oncol. 2020;121(3):570–7. https://doi.org/10.1002/jso.25826.
Angelini A, Trovarelli G, Berizzi A, Pala E, Breda A, Ruggieri P. Three-dimension-printed custom-made prosthetic reconstructions: from revision surgery to oncologic reconstructions. Int Orthop. 2018;43(1):123–32. https://doi.org/10.1007/s00264-018-4232-0.
Fang C, Cai H, Kuong E, Chui E, Siu YC, Ji T, et al. Surgical applications of three-dimensional printing in the pelvis and acetabulum: from models and tools to implants. Unfallchirurg. 2019;122(4):278–85. https://doi.org/10.1007/s00113-019-0626-8.
Angelini A, Kotrych D, Trovarelli G, Szafrański A, Bohatyrewicz A, Ruggieri P. Analysis of principles inspiring design of three-dimensional-printed custom-made prostheses in two referral centres. Int Orthop. 2020;44(5):829–37. https://doi.org/10.1007/s00264-020-04523-y.
Dhawan A, Kennedy PM, Rizk EB, Ozbolat IT. Three-dimensional bioprinting for bone and cartilage restoration in Orthopaedic surgery. J Am Acad Orthop Surg. 2019;27(5):e215–26. https://doi.org/10.5435/jaaos-d-17-00632.
Midha S, Dalela M, Sybil D, Patra P, Mohanty S. Advances in three-dimensional bioprinting of bone: Progress and challenges. J Tissue Eng Regen Med. 2019. https://doi.org/10.1002/term.2847.
Shahabipour F, Ashammakhi N, Oskuee RK, Bonakdar S, Hoffman T, Shokrgozar MA, et al. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res. 2020;216:57–76. https://doi.org/10.1016/j.trsl.2019.08.010.
Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3D printing in medicine. 3D printing in. Medicine. 2017;3(1):1. https://doi.org/10.1186/s41205-016-0009-5.
Green N, Glatt V, Tetsworth K, Wilson LJ, Grant CA. A practical guide to image processing in the creation of 3D models for orthopedics. Tech Orthop. 2016;31(3):153–63. https://doi.org/10.1097/bto.0000000000000181.
Pascau J, Moreta-Martinez R, Garcia-Mato D, Garcia-Sevilla M, Perez-Mananes R, Calvo-Haro J. Augmented reality in computer assisted interventions based on patient-specific 3D printed reference. Healthcare Technol Letters. 2018;5(5):162–6. https://doi.org/10.1049/htl.2018.5072.
Moreta-Martinez R, García-Mato D, García-Sevilla M, Pérez-Mañanes R, Calvo-Haro JA, Pascau J. Combining Augmented Reality and 3D Printing to Display Patient Models on a Smartphone. J Vis Exp. 2020;155:e60618. https://doi.org/10.3791/60618.
Instituto tecnológico de Canarias. Ingeniería biomédica. Hitos. Primeros implantes óseos a medida en España. 2013. In: https://www.itccanarias.org/web/es/areas/ingenieria-biomedica.
Ahlfeld T, Cubo-Mateo N, Cometta S, Guduric V, Vater C, Bernhardt A, et al. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. CS Appl Mater Interfaces. 2020;11:12557–72 https://doi.org/10.1021/acsami.0c00710.
Wu C, Deng J, Li T, Tan L, Yuan D. Percutaneous pedicle screw placement aided by a new drill guide template combined with fluoroscopy: an accuracy study. Orthop Surg. 2020;12(2):471–9. https://doi.org/10.1111/os.12642.
Gregory TM, Alkhaili J, Silvera J, Vitis B, Chaves C, Gregory J. 3D printing technology for the classification of complex distal humerus fractures. Ann Joint. 2018;3:96. https://doi.org/10.21037/aoj.2018.10.05.
Yang L, Shang X-W, Fan J-N, He Z-X, Wang J-J, Liu M, et al. Application of 3D printing in the surgical planning of Trimalleolar fracture and doctor-patient communication. Biomed Res Int. 2016:1–5. https://doi.org/10.1155/2016/2482086.
Pérez-Mañanes R, Calvo-Haro J, Arnal-Burró J, Chana-Rodríguez F, Sanz-Ruiz P, Vaquero-Martín J. Nuestra experiencia con impresión 3D doméstica en Cirugía Ortopédica y Traumatología. Hazlo tú mismo. Rev Latinoam Cirugía Ortop. 2016;1(2):47–53. https://doi.org/10.1016/j.rslaot.2016.06.004.
Pérez-Mañanes R, Arnal J, et al. 3D surgical printing cutting guides for open-wedge high Tibial osteotomy: DIY. J Knee Surg. 2016;29(08):690–5. https://doi.org/10.1055/s-0036-1572412https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0036-1572412.
Arnal J, Pérez-Mañanes R, et al. Three dimensional-printed patient-specific cutting guides for femoral variation osteotomy: do it yourself. Knee. 2017 Dec;24(6):1359–68. https://doi.org/10.1016/j.knee.2017.04.016https://www.ncbi.nlm.nih.gov/pubmed/28978460.
Chana F, Pérez-Mañanes R, et al. 3D surgical printing and pre contoured plates for acetabular fractures. Injury. 2016. pii: S0020–S1383(16): 30427–2. doi: https://doi.org/10.1016/j.injury.2016.08.027https://www.ncbi.nlm.nih.gov/pubmed/27599393.
García-Vázquez V, Pérez-Mañanes R, Calvo JA, García-Mato D, Cuervo-Dehesa M, Desco M, et al. Desktop 3D printing in medicine to improve surgical navigation in acral tumors. Int J CARS. 2016;11(Suppl 1):S262–3.
García-Vázquez V, Rodríguez-Lozano G, Pérez-Mañanes R, Calvo JA, Moreta-Martínez R, Asencio JM, et al. Surgical navigation and 3D printing in hemipelvic osteotomy. Int J CARS. 2017;12(Suppl 1):S106–7.
Chepelev L, Wake N, Ryan J, Althobaity W, Gupta A, et al. Radiological Society of North America (RSNA) 3D printing special interest group (SIG): guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D printing in. Medicine. 2018;4(1):11. https://doi.org/10.1186/s41205-018-0030-y.
Woo S-H, Sung M-J, Park K-S, Yoon T-R. Three-dimensional-printing Technology in hip and Pelvic Surgery: current landscape. Hip Pelvis. 2020;32(1):1–10. https://doi.org/10.5371/hp.2020.32.1.1.
Xia R, Zhai Z, Chang Y, Li H. Clinical applications of 3-dimensional printing Technology in hip Joint. Orthop Surg. 2019;11(4):533–44. https://doi.org/10.1111/os.12468.
Henckel J, Holme TJ, Radford W, Skinner JA, Hart AJ. 3D-printed patient-specific guides for hip Arthroplasty. J Am Acad Orthop Surg. 2018;26(16):e342–8. https://doi.org/10.5435/jaaos-d-16-00719.
Morgan C, Khatri C, Hanna SA, Ashrafian H, Sarraf KM. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: a systematic review and meta-analysis. World J Orthop. 2019;11(1):57–67. https://doi.org/10.5312/wjo.v11.i1.57.
Hurst EJ. 3D printing in healthcare: emerging applications. J Hosp Librariansh. 2016;16(3):255–67. https://doi.org/10.1080/15323269.2016.1188042.
Otero JJ, Vijverman A, Mommaerts MY. Use of fused deposit modeling for additive manufacturing in hospital facilities: European certification directives. J Cranio-Maxillofac Surg. 2017;45(9):1542–6. https://doi.org/10.1016/j.jcms.2017.06.018.
Morrison RJ, Kashlan KN, Flanangan CL, Wright JK, Green GE, Hollister SJ, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci. 2015;8(5):594–600. https://doi.org/10.1111/cts.12315.
European Parliament, Council of the European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. In: https://www.emergogroup.com/sites/default/files/europe-medical-devices-regulation.pdf.
Acknowledgements
We would like to thank the Orthopaedic Surgery and Traumatology department, workers from the Advanced Planning and 3D Manufacturing Unit and all the members of the Hospital 3d Printing Commission.
Authors’s contributions
JACH and RPM conceived the idea of the study; JACH, JP, LMS, PSR,CSP JVM and RPM contributed to the study design; LMS, PSR and CSP performed the statistical analysis; JACH, JP, JVM and RPM took part in the interpretation of the results; and JACH and RPM critically revised manuscript drafts. All authors read and approved the final version of the manuscript.
Disclosures
The authors declare that the work is original and is not being evaluated by any other scientific journal.
Funding
Analysis and interpretation of the data supported by Project PI18/01625 (Ministerio de Ciencia, Innovación y Universidades, Instituto de Salud Carlos III) and European Regional Development Fund (“Una manera de hacer Europa”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors state that no experiments on humans or animals have been conducted for this research. The authors declare that patient data do not appear in this article and that they are in possession of the patients’ informed consent for participation in the study and publication of the results.
Consent for publication
The authors declare they are in possession of the patients’ informed consent for participation in the study and publication of the results.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Calvo-Haro, J.A., Pascau, J., Mediavilla-Santos, L. et al. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: from “do it yourself” to “point of care manufacturing”. BMC Musculoskelet Disord 22, 360 (2021). https://doi.org/10.1186/s12891-021-04224-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04224-6