Abstract
Background
Accelerated knee osteoarthritis (AKOA) is characterized by more pain, impaired physical function, and greater likelihood to receive a joint replacement compared to individuals who develop the typical gradual onset of disease. Prognostic tools are needed to determine which structural pathologies precede the development of AKOA compared to individuals without AKOA. Therefore, the purpose of this manuscript was to determine which pre-radiographic structural features precede the development of AKOA.
Methods
The sample comprised participants in the Osteoarthritis Initiative (OAI) who had at least one radiographically normal knee at baseline (Kellgren-Lawrence [KL] grade < 1). Participants were classified into 2 groups based on radiographic progression from baseline to 48 months: AKOA (KL grade change from < 1 to > 3) and No AKOA. The index visit was the study visit when participants met criteria for AKOA or a matched timepoint for those who did not develop AKOA. Magnetic resonance (MR) images were assessed for 12 structural features at the OAI baseline, and 1 and 2 years prior to the index visit. Separate logistic regression models (i.e. OAI baseline, 1 and 2 years prior) were used to determine which pre-radiographic structural features were more likely to antedate the development of AKOA compared to individuals not developing AKOA.
Results
At the OAI baseline visit, degenerative cruciate ligaments (Odds Ratio [OR] = 2.2, 95% Confidence Interval [CI] = 1.3,3.5), infrapatellar fat pad signal intensity alteration (OR = 2.0, 95%CI = 1.2,3.2), medial/lateral meniscal pathology (OR = 2.1/2.4, 95%CI = 1.3,3.4/1.5,3.8), and greater quantitative knee effusion-synovitis (OR = 2.2, 95%CI = 1.4,3.4) were more likely to antedate the development of AKOA when compared to those that did not develop AKOA. These results were similar at one and two years prior to disease onset. Additionally, medial meniscus extrusion at one year prior to disease onset (OR = 3.5, 95%CI = 2.1,6.0) increased the likelihood of developing AKOA.
Conclusions
Early ligamentous degeneration, effusion/synovitis, and meniscal pathology precede the onset of AKOA and may be prognostic biomarkers.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
While knee osteoarthritis (OA) is typically is a gradually progressive disorder, a subset of individuals develop an accelerated form of the disease that is defined by the rapid onset and progression of disease within 4 years, and oftentimes within 12 months [1,2,3]. Accelerated knee OA (AKOA) represents a greater personal burden compared to typical knee OA, because individuals with AKOA are more likely to report frequent knee pain and greater self-reported global impact of arthritis (i.e., 0–10 global rating scale), as well as present with decreased physical function performance (e.g. slower walking and chair-stand pace) [2]. Additionally, AKOA represents an increased economic burden, as individuals with AKOA are more likely to receive pharmacological/surgical treatments and knee replacements compared with individuals with typical knee OA [4]. Therefore, developing prognostic tools that can distinguish between people who will develop AKOA are needed to lessen the personal and economic burden of this disease.
There is preliminary evidence that alterations in the meniscus and subchondral bone may characterize the onset of AKOA [5]. However, since knee OA is a disease that affects all structures of the joint [6], a more in-depth investigation of alterations in pre-radiographic structural features is needed. Magnetic resonance (MR) imaging offers a comprehensive assessment that evaluates cartilage, subchondral bone, meniscus, ligaments, tendons, and synovium. The annual MR image assessments of the Osteoarthritis Initiative (OAI) in individuals with radiographically normal knees allows for the unique ability to monitor early pre-radiographic structural alterations prior to the rapid decline in joint health associated with AKOA.
The primary purpose of this analysis was to determine which pre-radiographic structural features at key OAI visits precede the radiographic development of AKOA. Due to the rapid radiographic decline in joint health in individuals with AKOA, we hypothesize that early degenerative changes in the cruciate ligaments, extensor mechanism, and proximal gastrocnemius tendons would be associated with the future onset of AKOA. Furthermore, we hypothesized the effusion/synovitis and the presence of meniscal pathology would be associated with the onset of AKOA. Additionally, we explored which combination of pre-radiographic structural features may best discriminate which individuals will develop AKOA. The results of these analyses will indicate which pre-radiographic structural features may be ideal prognostic imaging markers for future AKOA development. These imaging markers will be imperative for selecting individuals at risk for AKOA to assess and deploy prevention strategies for incident AKOA.
Methods
Study design and participant selection
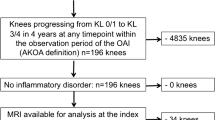
We identified individuals for this study using radiographic data from the OAI baseline and first 4 annual follow-up visits. The OAI is a multicenter (Memorial Hospital of Rhode Island, The Ohio State University, University of Maryland and Johns Hopkins University, and the University of Pittsburgh) cohort study that recruited 4796 adults with or at risk for symptomatic knee OA between February 2004 and May 2006 [7]. Institutional review boards at all OAI clinical sites and the OAI coordinating center (University of California, San Francisco) approved the OAI study. Participants provided informed consent prior to participation.
For this study, readers assessed 12 knee features on MR images at the OAI baseline visit, as well as at specific timepoints relative to the onset of disease (i.e. 2 and 1 years prior to onset of disease). Key features include semi-quantitative readings (i.e. collateral ligaments, cruciate ligaments, extensor mechanism, gastrocnemius tendons, infrapatellar fat pad signal intensity alteration, menisci) and quantitative measures (i.e. effusion-synovitis, bone marrow lesion [BML], and cartilage).
Participant selection
Participants in all groups were identified based on annual radiographs from the baseline to the 48-month OAI visit [3]. All groups had at least one knee with no radiographic knee OA at baseline (Kellgren-Lawrence [KL] < 1). Individuals who developed AKOA were defined as having one knee progress to advanced-stage knee OA (KL Grade = 0/1 to 3/4, definitive osteophyte and joint space narrowing) within 48 months (n = 125) [3]. Individuals with typical knee OA experienced a more gradual onset of OA and were defined as having one knee increase in KL grade within 48 months (i.e. KL = 0 to 1, 0 to 2, 1 to 2; n = 187). Individuals were defined as having no knee OA if both knees had no change in KL grade from baseline to the 48-month OAI visit (n = 1325). Individuals in the typical and no knee OA group were randomly matched to the AKOA group based on sex. Each group had 125 participants. For data analysis, we combined the typical knee OA and no knee OA groups into a single “no AKOA” group to allow for a comparison between individuals who would and would not develop AKOA [8].
Index knee
The index knee in individuals with AKOA or typical knee OA was defined as the first knee to meet the definition of AKOA or typical knee OA, respectively. The index knee in individuals with no knee OA was the same knee as that person’s matched member of the AKOA group.
Index visit
For individuals with AKOA or typical knee OA, the index visit was defined as the visit when a person first met the definition for AKOA or typical knee OA. For someone with no knee OA the index visit was the same visit as that person’s matched member of the incident AKOA group. The index visit could be at a 12-, 24-, 36-, or 48-month OAI visit.
Knee radiographs
To determine group assignment, we used readings of bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs obtained at baseline and each annual follow-up visits [3]. Central readers blinded to group assignment scored the KL Grade of each knee (KL = 0 to 4). The intrarater reliability agreement for the KL grades was good (weighted κ = 0.70 to 0.80) [9]. These data are publicly available (files: kXR_SQ_BU##_SAS [versions 0.6, 1.6, 3.5, 5.5, and 6.3]) [10].
MR imaging
MR acquisition
All semi-quantitative and quantitative analyses were conducted in index knees at the OAI baseline visit, as well as at 2 and 1 years prior to the index visit. MR images were acquired with one of four identical Siemens (Erlangen, Germany) Trio 3-Tesla MR systems at each clinical site using the OAI MR imaging protocol [10, 11]. The two musculoskeletal radiologists (RW, JM) performing semi-quantitative scoring were provided all the sequences acquired on each index knee at each visit (e.g., sagittal intermediate-weighted, turbo spin echo, fat-suppressed MR sequence; coronal intermediate-weighted, turbo spine echo, sequence without fat suppression, 3-dimensional dual-echo steady-state sequence). The quantitative measures of BML and effusion-synovitis were performed using a sagittal intermediate-weighted, turbo spin echo, fat-suppressed MR sequence: field of view = 160 mm, slice thickness = 3 mm, skip = 0 mm, flip angle = 180 degrees, echo time = 30 ms, recovery time = 3200 ms, 313 × 448 matrix, x resolution = 0.357 mm, y resolution = 0.511 mm, and total slice number = 37. Cartilage damage index was quantified using a 3-dimensional dual-echo steady-state sequence: field of view = 140 mm, slice thickness = 0.7 mm, skip = 0 mm, flip angle = 25 degrees, echo time = 4.7 ms, recovery time = 16.3 ms, 307 × 384 matrix, x resolution = 0.365 mm, y resolution = 0.456 mm, and total slice number = 160. These sequences have been described in detail elsewhere [10].
Semi-quantitative structural features
For all semi-quantitative and quantitative outcomes, the readers were blinded to group assignment and were unblinded to the order of time. Two musculoskeletal radiologists (RW:255 cases, JM:120 cases) performed the semi-quantitative MR readings. Readers had good agreement on the presence of each pathology among 25 cases: prevalence-adjusted and bias-adjusted kappa were 0.41 to 0.75 except for the posterior horn of the medial meniscus where the prevalence-adjusted and bias-adjusted kappa was fair at 0.25 (50% agreement).
The radiologists assessed the integrity of anterior/posterior cruciate ligaments, medial/lateral collateral ligaments, extensor mechanism, and gastrocnemius proximal tendons and noted if the structures appeared normal or degenerative. Degenerative tissue was defined as the presence of abnormal intrinsic high-signal intensity within the substance of the ligaments or tendon without discrete tear. Degenerative cruciate ligament pathology combined the presence of anterior or posterior cruciate ligament degenerative pathology. Degenerative collateral ligament pathology combined the presence of medial or lateral collateral ligament degenerative pathology.
The radiologists scored infrapatellar fat pad signal intensity alteration using the MR Imaging Osteoarthritis Knee Score grading system (i.e., normal, mild, moderate, and severe) [12]. Infrapatellar fat pad signal intensity was recoded as absence (i.e., normal) or presence (i.e., mild, moderate, and severe).
The radiologists scored medial and lateral meniscus extrusion using the MR Imaging Osteoarthritis Knee Score grading system (i.e., Grade 0: < 2 mm, Grade 1: 2 to 2.9 mm, Grade 2: 3 to 5 mm, and Grade 3: > 5 mm) [12]. Meniscal extrusion was recoded as absence (i.e., Grade 0) or presence (i.e., > Grade 1).
The radiologists used the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine meniscal tear classification, which was modified for MR imaging [13], to assess the body, posterior/anterior horn of each meniscus as: normal, degeneration, horizontal, flap horizontal, vertical longitudinal, radial, morphologic deformity, maceration, complex, or vertical flap tear. Meniscal pathology was recoded as absence (i.e., normal or degeneration without tear) and presence (i.e., horizontal, flap horizontal, vertical longitudinal, radial, morphologic deformity, maceration, complex, or vertical flap tear). For the medial/lateral menisci, pathology in the three regions meniscal tears of different morphologies were combined into the same variable. The medial/lateral menisci were considered pathologic if pathology was present in any of the three regions.
Quantitative structural features
Effusion-synovitis volume
We used a customized semi-automatic software to measure knee effusion-synovitis. Two readers (JBD and a visiting fellow) used the software to mark the first and last MR slice that included bone, the proximal border of the patella, and the apex of the fibular head on a central slice. The software then automatically segmented effusion-synovitis between these limits based on an existing threshold. The senior reader (JBD) then manually adjusted the threshold to change the effusion-synovitis boundaries and removed areas of high signal intensity that were not effusion-synovitis (e.g., subchondral cysts, blood vessels). The senior reader demonstrated excellent intra-reader reliability (ICC3,1 = 0.96). A total knee effusion-synovitis volume (in cm3) was used for data analysis.
Bone marrow lesion volume
One reader (ACS) measured tibiofemoral BML volume with a semi-automated segmentation method [14, 15]. The only manual step required the reader to identify crude boundaries of the tibia and femur in each slice of the MR images. The boundary furthest from the articular surfaces was marked just prior to the epiphyseal line or at the edge of the bone and soft tissue. The program then automatically identified the precise bone boundaries and performed a thresholding and curve evolution process twice to segment areas of high signal intensity, which may represent a BML. We eliminated false-positive regions by operationally defining a BML based on 2 criteria: 1) the distance between a BML to the articular surface should be < 10 mm; 2) a BML needed to span more than one MR image. The study principal investigator (JBD) reviewed all measurements with both timepoints on screen simultaneously. Our reader demonstrated excellent intra-reader reliability (ICC3,1 = 0.91). A total tibiofemoral BML volume (in cm3) was used for data analysis.
Cartilage damage index
The validated cartilage damage index (CDI) was used to quantify tibiofemoral cartilage size [16, 17]. One reader (JED) manually marked the bone-cartilage boundary on specific knee slices that are automatically selected based on the width of the knee. The reader then measured cartilage thickness at predefined informative locations, which the software automatically located. The software then computed the CDI for the medial femur, lateral femur, medial tibia, and lateral tibia by summing the products of cartilage thickness, cartilage length (anterior-posterior), and voxel size from 9 informative locations in each compartment. All measurements were reviewed by study principal investigator. Our reader demonstrated excellent intra-reader reliability (ICC3,1 = 0.86 to 0.99). The sum of all four tibiofemoral compartment CDI values was divided by the participant’s height to calculate a normalized total tibiofemoral CDI that was used for data analysis.
Clinical data
Demographic and other participant characteristics were acquired based on a standard protocol. We extracted age, body mass index, global impact rating, frequent knee pain and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain at the OAI baseline visit. The data are publicly available (Files: allclinical0#; version 0.2.2, 1.2.1, 3.2.1, 5.2.1, and 6.2.1) [11].
Data analysis
For the continuous quantitative outcomes, the variables in the entire cohort were separated into tertiles and converted to a dichotomous variable to compare the worst tertile (i.e. largest BML and effusion-synovitis, smallest CDI) to the combination of the other two tertiles to facilitate the interpretation of the odds ratios.
Statistical analysis
Primary analysis
Are Early Pre-Radiographic Structural Features Associated with the Onset of Accelerated Knee Osteoarthritis?
Separate logistic regression models were used to determine which pre-radiographic structural features at OAI baseline were more likely to antedate the development of AKOA compared to individuals not developing AKOA (i.e. referent group). Additionally, we conducted the same analyses for each structural outcome at 2 and 1 years prior to the index visit. The results are presented as odds ratios (ORs) and 95% confidence intervals (95% CIs). To control for multiple comparisons, we utilized a statistically significant p-value corrected for the number of structural features utilized in the primary OAI baseline analysis (p < 0.05/12 = 0.004).
Secondary analysis
Which Combination of Baseline Pre-Radiographic Structural Features Most Associate with the Onset of Accelerated Knee Osteoarthritis?
To explore which combination of pre-radiographic structural features characterize AKOA, we performed a backward stepwise logistic regression where the outcome was AKOA or no AKOA (referent group) at the OAI baseline. Separate models also were conducted for 2 and 1 years prior to the index visit. All 9 semi-quantitative and 3 quantitative pre-radiographic structural features were included in the analysis at each time point. The ability for the combination of pre-radiographic structural features to discriminate between AKOA status was quantified with the C statistic [18]. The discriminatory ability of a model based on the C statistic was classified as: very poor (C < 0.50), poor (0.50 < C < 0.70), good (0.70 < C < 0.80), and strong (0.80 < C < 1.00) [19].
All analyses were performed unadjusted, as the purpose of this investigation was to specifically determine the prognostic capability of baseline structural features at associating with future development of incident AKOA. Due to missing MR images at different OAI visits, there are different sample sizes depending on the analysis: OAI baseline (n = 354), 2 years prior to onset (n = 248), 1 years prior to onset (n = 354). There are uneven sample sizes at the different time points because some participants are unable to have a 2 year prior to onset visit (i.e., index visit at the 1-year OAI visit). We conducted a sensitivity analysis for the OAI baseline and 1 year prior to onset analyses limiting the sample to the 248 participants in the 2 years prior to onset analysis. All analyses were performed with SAS Enterprise 7.15 (Cary, NC, USA).
Results
Table 1 details the demographics for each group.
Primary analysis
Are Pre-Radiographic Structural Features Associated with the Onset of Accelerated Knee Osteoarthritis?
At the OAI baseline visit, degenerative cruciate ligaments (OR = 2.15; 95%CI = 1.34, 3.45; p = 0.002 Table 2), infrapatellar fat pad signal intensity alteration (OR = 1.98; 95%CI = 1.24, 3.15; p = 0.004), medial meniscal pathology (OR = 2.14; 95%CI = 1.33, 3.43; p = 0.002), lateral meniscal pathology (OR = 2.36; 95%CI = 1.47, 3.79; p = 0.0004), and large effusion-synovitis volume (effusion cutoff > 9.5cm3; OR = 2.15; 95%CI = 1.35, 3.43; p = 0.001) were more likely to antedate the development of AKOA when compared to those that did not develop AKOA.
At 2 years prior to disease onset, the same structural features from the OAI baseline analysis were more common in individuals prior to the development of AKOA when compared to those that did not develop AKOA (Table 3). The effusion cutoff at 2 years prior to disease onset was 9.8cm3.
At 1 year prior to disease onset, all significant features from the OAI baseline analysis were more common in individuals prior to the development of AKOA when compared to those that did not develop AKOA (Table 4). Additionally, we found that the presence of medial meniscus extrusion (OR = 3.52; 95%CI = 2.07, 6.00) increased the likelihood of developing AKOA when compared to individuals who did not develop AKOA. The effusion cutoff at 2 years prior to disease onset was 11.9cm3.
Secondary analysis
Which Combination of Baseline Pre-Radiographic Structural Features Most Associate with the Onset of Accelerated Knee Osteoarthritis?
At the OAI baseline, the combination of medial meniscal pathology, degenerative cruciate ligaments, greater effusion-synovitis volume, and lateral meniscal pathology provided good discrimination between individuals that would develop AKOA within the next four years and individuals that would not develop AKOA (C-statistic = 0.70).
At 2 years prior to disease onset, the pre-radiographic structural features included in the OAI baseline analysis plus degenerative collateral ligaments provided good discrimination between individuals that would develop AKOA within the next four years and individuals that would not develop AKOA (C-statistic = 0.76).
At 1 year prior to disease onset, the combination of medial meniscal pathology, degenerative cruciate ligaments, greater effusion-synovitis volume, infrapatellar fat pad signal intensity alteration, and medial meniscal extrusion provided good discrimination between individuals that would develop AKOA within the next four years and individuals that would not develop AKOA (C-statistic = 0.77).
The sensitivity analyses in the OAI baseline and the one year prior to disease cohorts that limited the sample size to the 248 individuals (i.e., participants included in the 2 years prior to disease cohort) did not alter the findings of any of the analyses.
Discussion
In this longitudinal study we found that several structural pathologies preceding the onset of radiographic knee OA increased the risk for the subsequent development of AKOA compared to individuals who did not develop AKOA. At the OAI baseline visit, the presence of degenerative ligaments, effusion-synovitis, and meniscal pathology were identified as pre-radiographic structural features that identified an increased risk of AKOA development over the next four years. Medial meniscal extrusion was additionally associated with AKOA at 1 year before the onset of disease. Thus, these pre-radiographic structural features, especially these more proximate findings, antedate the development of AKOA and may help identify individuals likely to develop AKOA in the near future.
These results were consistent, and not attenuated, even when mutually adjusted for in multivariate models, as we observed that the combination of medial meniscal pathology, degenerative cruciate ligaments, and the greatest tertile of quantitative knee effusion-synovitis volume (> 9.5cm3) at OAI baseline were associated with the future development of AKOA. Furthermore, the pre-radiographic structural features that associated with AKOA depended on the proximity of time between the image assessment and the onset of disease. Specifically, at 2 years prior to disease development, lateral meniscal pathology and degenerative collateral ligaments were also associated with AKOA and included in the multivariate model. At the year prior to disease development, infrapatellar fat pad signal intensity alteration and medial meniscal extrusion were associated with AKOA and included in the multivariate model. Therefore, depending on the time to disease onset, different combinations of pre-radiographic structural features may be most indicative of future AKOA development and may help us eventually determine their risk of AKOA over 1, 2 or 4 years.
We consistently observed that regardless of time, the presence of meniscal pathology was associated with future AKOA development. These findings complement previous research where we observed that incident AKOA was often characterized by medial meniscal tears with moderate-severe extrusion or changes in meniscal size [5], as well as other studies that have observed that meniscal pathology [20] and meniscal extrusion [21] were related to knee OA onset. Additionally, individuals with medial meniscal extrusion at the year prior to disease development are approximately 3.5 times more likely to develop AKOA. Meniscal extrusion was not associated with AKOA at any other timepoints. This indicates that medial meniscal extrusion may be a later finding that becomes relevant in the year prior to the onset of advanced-stage disease (KL = 3 or 4). While our analyses limit us from making causal inferences, previous biomechanical studies [22, 23] have observed that medial meniscal pathology results in increased tibiofemoral contact pressure and alterations in knee kinematics which may lead to overloading of the knee joint. Additionally, meniscal pathology and meniscal extrusion are key risk factors for fast cartilage loss [24,25,26]. Therefore, disruption of the medial meniscus may be related to the rapid decline in joint health which is why there is an association with future AKOA development.
Our results indicate that regardless of time, individuals with cruciate ligament degeneration are more than twice as likely to develop AKOA. Despite the apparent importance of cruciate ligament degeneration, the main semi-quantitative whole joint scoring systems only assess for acute tear and do not provide an indicator of cruciate ligament degeneration [27, 28], even though previous findings have observed an association between degenerative cruciate ligaments and symptomatic KOA [29]. The main function of the cruciate ligaments are to facilitate rotational and translational stability to the knee joint [30], and degenerative cruciate ligaments present with altered fiber arrangement and collagen composition [31, 32]. Additionally, individuals with cruciate ligament degeneration present with greater severity of cartilage damage, bone marrow lesions, subchondral cysts, and lateral meniscus pathology when compared to individuals with normal cruciate ligaments [33]. While we are unable to make definitive claims based on our results, cruciate ligament degeneration could be early evidence of a maladaptation to loading or due to aberrant joint loading created by impaired ligamentous function that increases knee instability or laxity [34]. Future studies are needed to confirm whether the presence of knee instability increases the risk for incident AKOA. If instability is present, then it may explain the increased meniscal damage and large effusion-synovitis commonly observed among knees that develop AKOA [5].
Knee effusion-synovitis volume may be the pre-radiographic structural feature most strongly associated with future AKOA development. At 2 years prior to disease development, individuals with effusion-synovitis greater than 11.9 cm3 are about 3 times more likely to develop AKOA, with this likelihood increasing to ~ 5.2 times in the year prior to the onset of advanced-stage disease. Effusion-synovitis has previously been observed to precede radiographic knee OA, and is theorized to be a consequence of early, underlying damage occurring within the knee [20, 35]. Therefore, all of the pathologic and degenerative tissues in the joint may contribute to greater knee effusion-synovitis. Furthermore, the effusion-synovitis may be a secondary sign of maladaptation to loading that is stressing other tissues in the joint. This study offers new knowledge indicating that individuals in the greatest tertile effusion-synovitis prior to OA onset are more likely to develop AKOA when compared to individuals with less effusion-synovitis. Individuals with AKOA have greater pain than those with typical knee OA [2] and this may be partially attributable to their large effusion-synovitis, which is associated with increased pain [36, 37]. Therefore, the association between AKOA and increased pain may be mediated by greater knee effusion-synovitis. However, future investigations are needed to determine the specific mechanisms leading to increased pain and effusion-synovitis in individuals with knee OA.
This study provides a critical initial step in determining which pre-radiographic structural features may serve as future prognostic imaging markers of AKOA; however, there are some limitations that must be discussed. Our analyses are not able to provide evidence of specific causal pathways between the identified pre-radiographic structural features and AKOA development, but only that the presence of these features precedes the eventual AKOA development. Future studies are needed to confirm that these structural features are prognostic and mechanistically involved in the development of AKOA. This study indicates which individual pre-radiographic structural features may increase the risk of AKOA development, however, we are unable to confirm how each pathologic finding is dependent on another. Therefore, future studies are needed to determine if each structural feature is a different pathway to the same outcome (i.e. AKOA), if the features are various manifestations due to the same underlying process, or if there are particular combinations of features leading to AKOA. We assessed knee effusion-synovitis using non-contrast enhanced MR images even though contrast-enhanced MR images are considered the gold standard. Due to the possible complications, increased price, lack of clinical feasibility in a pre-radiographic population the non-contrast MR was selected for the OAI protocol [10]. However, even when using the non-contrast MR imaging, we observed significant associations between knee effusion-synovitis and AKOA development. Meniscal tears of different morphologies were collapsed into the same variable of meniscal pathology due to small sample sizes of the individual types of tears. Since different meniscal tears have different biomechanical significance to the knee [38], future studies should attempt to individually determine the significance of specific meniscal tears. Two musculoskeletal radiologists performed the semi-quantitative structural readings with an uneven distribution of cases (RW = 225, JM = 120); however, our readers demonstrated good agreement that is similar to previous semi-quantitative readings [39].
Conclusions
In conclusion, this study indicates specific early structural features (e.g. degenerative ligaments, effusion/synovitis, and meniscal) that may be evidence of early maladaptation to loading that precedes the onset of AKOA. These findings should be considered as potential prognostic biomarkers that warrant further study.
Abbreviations
- AKOA:
-
Accelerated knee osteoarthritis
- BML:
-
Bone marrow lesions
- CDI:
-
Cartilage damage index
- CI:
-
Confidence intervals
- ICC:
-
Intraclass correlation coefficients
- KL:
-
Kellgren-Lawrence
- MR:
-
Magnetic resonance
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis Initiative
- OR:
-
Odds ratio
References
Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2014;66(11):1673–9.
Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL, McAlindon TE. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the osteoarthritis initiative. Clin Rheumatol. 2016;35(6):1565–71.
Driban JB, Stout AC, Lo GH, Eaton CB, Price LL, Lu B, Barbe MF, McAlindon TE. Best performing definition of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Ther Adv Musculoskelet Dis. 2016;8(5):165–71.
Davis JE, Liu SH, Lapane K, Harkey MS, Price LL, Lu B, Lo GH, Eaton CB, Barbe MF, McAlindon TE, et al. Adults with incident accelerated knee osteoarthritis are more likely to receive a knee replacement: data from the osteoarthritis initiative. Clin Rheumatol. 2018;37(4):1115–8.
Driban JB, Ward RJ, Eaton CB, Lo GH, Price LL, Lu B, McAlindon TE. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: data from the osteoarthritis initiative. Clin Anat. 2015;28(6):792–9.
Hunter DJ. Osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):801–14.
Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging--the osteoarthritis initiative. Nat Rev Rheumatol. 2012;8(10):622–30.
Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE, Lu B, Lo GH, Duryea J, Barbe MF. Risk factors can classify individuals who develop accelerated knee osteoarthritis: data from the osteoarthritis initiative. J Orthop Res. 2018;36(3):876–80.
Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–57.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil. 2008;16(12):1433–41.
The osteoarthritis initiative. https://oai.nih.gov.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthr Cartil. 19(8):990–1002.
Anderson AF, Irrgang JJ, Dunn W, Beaufils P, Cohen M, Cole BJ, Coolican M, Ferretti M, Glenn RE Jr, Johnson R, et al. Interobserver reliability of the International Society of Arthroscopy, knee surgery and Orthopaedic sports medicine (ISAKOS) classification of meniscal tears. Am J Sports Med. 2011;39(5):926–32.
Pang J, Driban JB, Destenaves G, Miller E, Lo GH, Ward RJ, Price LL, Lynch JA, Eaton CB, Eckstein F, et al. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the osteoarthritis initiative. BMC Musculoskelet Disord. 2013;14:3.
Driban JB, Price L, Lo GH, Pang J, Hunter DJ, Miller E, Ward RJ, Eaton CB, Lynch JA, McAlindon TE. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker--longitudinal relationships with pain and structural changes: data from the osteoarthritis initiative. Arthritis Res Ther. 2013;15(5):R112.
Zhang M, Driban JB, Price LL, Lo GH, Miller E, McAlindon TE. Development of a rapid cartilage damage quantification method for the lateral tibiofemoral compartment using magnetic resonance images: data from the osteoarthritis initiative. Biomed Res Int. 2015;2015:634275.
Zhang M, Driban JB, Price LL, Harper D, Lo GH, Miller E, Ward RJ, McAlindon TE. Development of a rapid knee cartilage damage quantification method using magnetic resonance images. BMC Musculoskelet Disord. 2014;15:264.
Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, Katz JN, Kent Kwoh C, Kraus VB, Hunter DJ. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health osteoarthritis biomarkers consortium. Arthritis Rheumatol. 2016;68(10):2422–31.
Hosmer DW, Lemeshow S. Applied logistic regression, 2nd Edition edn. New York: John Wiley & Sons; 2000.
Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, Boudreau RM, Guermazi A. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 2015;67(8):2085–96.
Emmanuel K, Quinn E, Niu J, Guermazi A, Roemer F, Wirth W, Eckstein F, Felson D. Quantitative measures of meniscus extrusion predict incident radiographic knee osteoarthritis--data from the osteoarthritis initiative. Osteoarthr Cartil. 2016;24(2):262–9.
Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90(9):1922–31.
Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124–9.
Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, Nevitt MC, Felson DT, Hughes LB, El-Khoury GY, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772–80.
Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, Guermazi A, Grigorian M, Gale D, Felson DT. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801.
Guermazi A, Eckstein F, Hayashi D, Roemer FW, Wirth W, Yang T, Niu J, Sharma L, Nevitt MC, Lewis CE, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the multicenter osteoarthritis study. Osteoarthr Cartil. 2015;23(12):2191–8.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthr Cartil. 2011;19(8):990–1002.
Roemer FW, Eckstein F, Guermazi A. Magnetic resonance imaging-based semiquantitative and quantitative assessment in osteoarthritis. Rheum Dis Clin N Am. 2009;35(3):521–55.
McIntyre J, Moelleken S, Tirman P. Mucoid degeneration of the anterior cruciate ligament mistaken for ligamentous tears. Skelet Radiol. 2001;30(6):312–5.
Zlotnicki JP, Naendrup JH, Ferrer GA, Debski RE. Basic biomechanic principles of knee instability. Curr Rev Musculoskelet Med. 2016;9(2):114–22.
Hasegawa A, Otsuki S, Pauli C, Miyaki S, Patil S, Steklov N, Kinoshita M, Koziol J, D'Lima DD, Lotz MK. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012;64(3):696–704.
Kumagai K, Sakai K, Kusayama Y, Akamatsu Y, Sakamaki K, Morita S, Sasaki T, Saito T, Sakai T. The extent of degeneration of cruciate ligament is associated with chondrogenic differentiation in patients with osteoarthritis of the knee. Osteoarthr Cartil. 2012;20(11):1258–67.
Hovis KK, Alizai H, Tham SC, Souza RB, Nevitt MC, McCulloch CE, Link TM. Non-traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the osteoarthritis initiative (OAI). Skelet Radiol. 2012;41(11):1435–43.
Amano K, Li Q, Ma CB. Functional knee assessment with advanced imaging. Curr Rev Musculoskelet Med. 2016;9(2):123–9.
Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, Hunter DJ. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390–5.
Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, Le Graverand MP, Hunter DJ, Group OAII. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil. 2009;17(12):1562–9.
Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, Felson DT. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7.
Jarraya M, Roemer FW, Englund M, Crema MD, Gale HI, Hayashi D, Katz JN, Guermazi A. Meniscus morphology: does tear type matter? A narrative review with focus on relevance for osteoarthritis research. Semin Arthritis Rheum. 2017;46:552–61.
Roemer FW, Eckstein F, Guermazi A. Magnetic resonance imaging-based Semiquantitative and quantitative assessment in osteoarthritis. Rheum Dis Clin N Am. 2009;35(3):521–55.
Acknowledgements
Not applicable.
Funding
These analyses were financially supported by a grant from the National Institutes of Health (grant no. 5 TL1 TR 1454–3) and a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01-AR065977. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. This work was also supported in part by the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90–020). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the OAI repository, https://oai.nih.gov
Author information
Authors and Affiliations
Contributions
MSH contributed to the analysis and interpretation of data and drafting/revisions of article. JED contributed to the analysis and interpretation of data and drafting/revising of article. BL contributed to the conception and design, analysis and interpretation of data, and revisions of article. LLP contributed to the analysis and interpretation of data, and revisions of article. RJW contributed to the conception and design, analysis and interpretation of data, and revisions of article. JWM contributed to the conception and design, analysis and interpretation of data, and revisions of article. CBE contributed to the conception and design, acquisition of data, analysis and interpretation of data, and revisions of article. GHL contributed to the analysis and interpretation of data and revisions of article. MFB contributed to the conception and design and revisions of article. MZ contributed to the analysis and revisions of article. JP contributed to the analysis and revisions of article. ACS contributed to the analysis and revisions of article. TEM contributed to the conception and design, acquisition of data, analysis and interpretation of data, and revisions of article. JBD contributed to the conception and design, analysis and interpretation of data, and drafting/revisions of article. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The OAI study was approved by institutional review boards at each OAI clinical site and the coordinating center: Memorial Hospital of Rhode Island Institutional Review Board, The Ohio State University’s Biomedical Sciences Institutional Review Board, University of Pittsburgh Institutional Review Board, University of Maryland Baltimore – Institutional Review Board, and Committee on Human Research at University of California, San Francisco. All participants provided written informed consent. The Tufts Medical Center Institutional Review Board deemed that the work done at our institution was not human research because we analyzed publicly available data.
Consent for publication
Not applicable.
Competing interests
JBD, MFB, and MSH are members of the Editorial Board of BMC Musculoskeletal Disorders. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Harkey, M.S., Davis, J.E., Lu, B. et al. Early pre-radiographic structural pathology precedes the onset of accelerated knee osteoarthritis. BMC Musculoskelet Disord 20, 241 (2019). https://doi.org/10.1186/s12891-019-2624-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-019-2624-y