Abstract
Background
High molecular weight (HMW) hyaluronic acid (HA) is a treatment option for knee osteoarthritis (OA). The efficacy of HMW-HA in knee OA is investigated extensively, but the effectiveness in patients in the working age is unknown. Nevertheless, the number knee OA patients in the working age is increasing. Surgical treatment options are less eligible in these patients and productivity losses are high. In this study the effectiveness of intra-articular HMW-HA added to regular non-surgical usual care in everyday clinical practice (UC) compared to UC over 52 weeks in symptomatic knee OA patients in the working age was investigated.
Methods
In this open labelled randomized controlled trial, subjects aged between 18 and 65 years with symptomatic knee OA (Kellgren and Lawrence I-III) were enrolled and randomized to UC + 3 weekly injections with HMW-HA (intervention) or UC only (control). The primary outcome was the between group difference in responders to therapy according to OMERACT-OARSI criteria after 52 weeks. These criteria include the domains pain, knee related function and patient’s global assessment (PGA). Function was evaluated with the KOOS questionnaire. Pain was assessed with the Numeric Rating Scale. Secondary outcome comprised the between group difference on the individual responder domains, as analysed with a random effects model. Odds Ratios (OR) were calculated by logistic regression analysis. Sensitivity analyses were performed.
Results
In total, 156 subjects were included (intervention group 77, control group 79). Subjects in the intervention group (HMW-HA + UC) were more often responder compared to the controls (UC). Depending on whether pain during rest or pain during activity was included in the responder domains, 57.1% versus 34.2% (p = 0.006) and 54.5% versus 34.2% (p = 0.015) was responder to therapy respectively. The results of the secondary outcome analyses show that scores on individual responder domains over all follow-up moments were statistically significant in favour of the intervention group in the domains pain during rest (δ 0.8, 95%CI 0.2; 1.4, p = 0.010), knee related function (δ − 6.8, 95%CI -11.9; − 1.7, p = 0.010) and PGA (δ − 0.7, 95%CI -0.9; − 0.4, p < 0.0001).
Conclusions
Intra-articular HMW-HA added to usual care is effective for knee OA in patients in the working age.
Trial registration
www.trialregister.nl, NTR1651, registered 2009-3-3.
Similar content being viewed by others
Background
Knee osteoarthritis (OA) is a chronic degenerative disease of the knee joint, causing pain, joint stiffness and functional impairment [1,2,3]. The lifetime risk on symptomatic knee OA is over 40% [4]. Next to health impairment and disability, knee OA is associated with substantial healthcare consumption and costs [1, 5, 6].
The initial pharmacological treatment for patients with symptomatic knee OA generally includes rapid-acting pain medication like acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs). NSAIDs have shown to be effective in pain reduction and functional improvement in the symptomatic treatment of knee OA [7,8,9].
Treatment with NSAIDs is related to an increased risk of serious gastrointestinal and cardiovascular side effects, indicating limited use of NSAIDs only [10, 11]. The safety profile of NSAIDs contradicts with the chronic character of knee OA in which prolonged symptomatic treatment is often required. Additionally, non-pharmacological interventions such as strength training, exercise and weight management are added to the treatment regime [12,13,14].
An alternative treatment for knee OA patients is intra-articular injection therapy with hyaluronic acid (HA) [15]. Intra-articular HA results in similar effects on pain reduction and improvement of function compared to NSAID use, without the aforementioned side effects [8, 16, 17]. The efficacy of intra-articular HA has been investigated extensively in randomized controlled trials (RCTs) and subsequently in various systematic reviews and meta-analyses [12, 15]. Peak effectiveness of a series of intra-articular HA is reached between 1 and 2 months and residual effects exist up to 6 months [15, 16, 18].
Limiting the results of meta-analyses to high quality trials only, the effect on pain is still clinically relevant in favour of intra-articular HA [14, 18]. There is increasing evidence that within the spectrum of available HA derivatives the efficacy of HA products with a high molecular weight (HMW) is superior to the efficacy of derivatives with a low molecular weight [19, 20].
The effectiveness of HMW-HA in knee OA patients in the working age has not been evaluated yet. Relevance lies in the fact that the number patients with knee OA in the working age is increasing and surgical treatment options like unicompartmental or total knee arthroplasty (TKA) are less eligible in these patients, especially when they are involved in a physically demanding occupation [21, 22]. The revision rate of knee arthroplasty in these patients is high and the life span of the prosthesis is limited [23]. Furthermore, the costs from loss of productivity at work due to symptomatic knee OA are high in patients in the working age [24]. In this population, the availability of an effective local therapy in everyday clinical care could thus offer important healthcare benefits next to possible economic benefits.
The aim of this study was to assess the effectiveness of intra-articular HMW-HA added to usual care (UC) compared to UC over a period of 52 weeks in symptomatic knee OA patients in the working age. We hypothesized that adding HMW-HA in patients with knee OA has a clinical relevant effect.
Alongside this effectiveness analysis, a parallel economic evaluation was performed which was published previously [25]. In this article we report that adding HMW-HA to the usual care results in an increase in quality of life. The increase is accompanied with an increase in costs. Ultimately this leads to a cost-effectiveness ratio of €9.100/ quality adjusted life years (QALY). Given the maximum willingness to pay for similar conditions to knee OA we conclude that intra-articular HMW-HA added to usual care for knee OA is probably cost-effective in the treatment of knee OA.
Methods
The current effectiveness evaluation and the previously published cost-effectiveness evaluation are both part of the VIScosupplementation for Knee osteoarthritis (VISK) study. The VISK study is registered at the Dutch trial register (www.trialregister.nl, NTR1651). The study protocol is available from the corresponding author on request.
The VISK study does not include a placebo group. In light of the evidence on the efficacy of HMW-HA in knee OA, we specifically sought to investigate the actual effectiveness of this intervention. Such a study design, in which the intervention is compared to what is considered regular care that is provided in an everyday clinical setting (without a placebo), is required to facilitate the parallel economic evaluation of the VISK study [26, 27].
Study sample
Inclusion of eligible subjects took place between May 2009 and May 2010 in 2 hospitals (1 academic, 1 non-academic) in The Netherlands. Consecutive knee OA patients at the outpatient orthopaedic department meeting the inclusion criteria were considered eligible. Patient’s age was set between 18 and 65 years, the latter being the pensionable age in The Netherlands at the inclusion period. Inclusion criteria were: pain > 3 months, mean pain severity ≥2 on the numeric rating scale (NRS), Kellgren & Lawrence (K&L) grade I to III in medial and/or lateral compartment.
Exclusion criteria were: intra-articular HA injections < 1 year, intra-articular steroid injection < 3 months, arthroscopy < 6 months, tibial osteotomy < 1 year, synovectomy, scheduled knee surgery < 1 year, varus/valgus deformity > 12 degrees, chondrocalcinosis, dermatologic knee disorders, allergy to HMW-HA components, (planned) pregnancy or lactation, inflammatory arthritis, severe hip OA, non-knee related regular analgesic use, daily oral steroid therapy, poor general health, conditions interfering with functional assessments, alcoholism, patients unable to attend follow-up and insufficient command of the Dutch language.
Sample size, randomization and masking
The sample size was calculated to detect a between group difference of 20% in the primary outcome parameter which was defined as response to therapy at 52 weeks according to OMERACT-OARSI criteria [28]. A power of 80% and an alpha of 0.05 resulted in a required sample size of 64 subjects per group (128 subjects in total). Anticipating a 20% dropout over 52 weeks, the final required sample size was set at 154 subjects.
Randomization took place after informed consent was signed. Concealed randomization was performed by computer generated lists with randomly assigned blocs of 2, 4, 6, 8 or 10 subjects. An independent employee not involved in any other part of the study performed the randomization. Stratification took place for radiologic degree of knee AO (K&L grade I/II versus grade III) and per orthopedic surgeons responsible for injections (2 per hospital, 4 in total).
The statistician and investigator responsible for assessment and analyses of the data were blinded for the treatment allocation. Due to the study design included subjects and orthopedic surgeons administering the study intervention could not be blinded.
Interventions
Subjects in the intervention group received 3 weekly intra-articular injections with Hylan G-F 20 (Sanofi S. A, Paris, France) added to usual care or usual care only. Hylan G-F 20 is the HMW-HA derivative with the highest molecular weight available for clinical use (6000 kDa. The injections were performed through the superolateral approach [29]. Usual care was defined accordingly to the guidelines on the treatment of knee OA of the Dutch Orthopedic Association. This guideline recommends several non-surgical treatment modalities including pain medication (eg acetaminophen or NSAIDs), physical therapy and lifestyle recommendations [12]. Treating physicians were encouraged to follow these guidelines, but no treatment restraints were imposed. Other treatments were allowed when deemed appropriate in order to maintain the pragmatic character of the trial.
Questionnaires
The follow-up was 52 weeks and data was collected through questionnaires by mail at baseline, 6, 13, 26, 39 and 52 weeks. Knee related function was assessed by the functioning in daily living scale of the Knee injury and Osteoarthritis Outcome Score (KOOS) [30, 31]. A normalized score from 0 (extreme symptoms) to 100 (no symptoms) was calculated for this subscale. Pain during rest and pain during activity was evaluated by the NRS, resulting in a score between 0 (no pain) and 10 (most severe pain) [32]. Patient’s global assessment (PGA) was assessed on a 5-point Likert scale on which subjects indicate the amount of improvement of their knee complaints compared to baseline (1. fair improvement, 2. moderate improvement, 3. no change, 4. moderate deterioration, 5. fair deterioration). Medication use and patient reported adverse events were monitored at all follow-up moments.
Outcomes
The primary outcome was defined as response to therapy at 52 weeks follow-up according to OMERACT-OARSI criteria. This variable presents the results of changes after treatment in three symptomatic domains (pain, function, and PGA) as a single variable [28]. Response to therapy according to the OMERACT-OARSI criteria is defined as ≥10% absolute improvement and ≥ 20% overall improvement at final follow-up in at least 2 of the 3 responder domains (pain, function and/or PGA); or ≥ 20% absolute improvement and ≥ 50% overall improvement in either the pain or function domain.
The secondary outcome comprised the between group difference over the whole follow-up period of the 3 individual primary outcome responder domains: pain, function, and PGA.
Statistical analyses
For the primary outcome, the difference in percentage of responders according to OMERACT-OARSI criteria between study groups after 52 weeks follow-up was calculated [28]. In the base case analyses two responder sets were investigated: 1. with pain during rest was included in the responder domains, next to function and PGA; and 2. with pain during activity included.
In order to minimize bias in favor of the intervention group, drop-outs and subjects lost to follow up were (regardless of their study results) considered non-responders in the intervention group, and (vice versa) responders in the control group in the final analyses.
Logistic regression analysis with responder as dependent variable and the intervention as independent variable were performed to calculate odds ratios (OR) including 95% confidence intervals (95%CI) after 52 weeks follow-up. The number needed to treat (NNT) to attain 1 responder was calculated (PASW statistics 17.0).
For the secondary outcome, scores on individual responder domains (pain during rest, pain during activity, knee related functioning in daily life, PGA) were analyzed over all follow-up moments by means of a random effects model with random intercept and slope. The baseline values of the variables and the treatment group were included in the model. In this way we obtained for each outcome an estimate for the between group difference in score on the relevant questionnaires (KOOS, NRS, Likert scale) over the whole follow-up period, including associated 95% CI (SAS 9.2, SAS Company).
Sixteen subjects divided over both study groups received knee related surgery during follow-up. This number was not foreseen and we therefore performed 2 additional sensitivity analyses to assess possible beneficial clinical effects on pain and function as a result of the surgery. These analyses were not specified in the VISK study protocol a priori. In these sensitivity analyses, subject receiving knee related were considered non-responder irrespective of their study results. [33] In the first additional analysis, subjects who received major knee related surgery (e.g. knee prosthesis implantation, high tibial osteotomy) during follow-up were considered non-responders. In the second additional analysis subjects receiving any knee surgery (major knee surgery plus minor knee surgery like arthroscopy or knee manipulation under general anesthesia) were considered non-responders. (PASW statistics 17.0) All analyses were performed according to the intention to treat principle. In order to generate unbiased estimates of the difference in effectiveness parameters across both treatment groups, we adjusted for baseline imbalances in and, if necessary, for unbalanced covariates.
Results
Study population
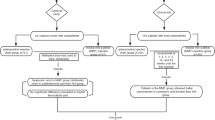
In total, 156 patients were included of which 77 subjects (mean age 53.6, standard deviation (SD) 8.6 range 20.9–64.6) in the intervention group and 79 subjects (mean age 54.8, SD 6.4, range 32.9–64.9) in the control group. The study flowchart is shown in Fig. 1. Additional characteristics of included subjects are shown in Table 1. One subject in the intervention group received only 1 out of 3 planned injections with HA due to a painful first injection and 1 subject refused the injections of HMW-HA after allocation to the intervention group. In the control group, 3 subjects were not motivated for further study participation after baseline measurements and randomization, and 1 subject was lost to follow-up. All subjects were retained in the analyses of their randomization groups. We adjusted for the baseline imbalances on pain and functioning in all analyses.
Primary outcome
In Table 2 the results on the primary outcome and the results of the sensitivity analyses are shown. Subjects in the intervention group were statistically significant more often responder to treatment arm they were randomized to compared to the control group. When pain during rest was included in the responder domains, 57.1% of the subjects in the intervention group were responder to therapy, against 34.2% in the control group (p = 0.006). With pain during activity included, 54.5% of the subjects was responders to therapy in the intervention group versus 34.2% of the controls (p = 0.015).
Secondary outcome
Over the whole follow-up period, we found statistically significant better scores in the intervention group in the domains pain during rest, knee related function, and PGA (Figs. 2, 3 and 4). These results where statistically significant for pain during rest (δ 0.8, 95%CI 0.2; 1.4, p = 0.010), knee related function (δ − 6.8, 95%CI -11.9; − 1.7, p = 0.010) and PGA (δ − 0.7, 95%CI -0.9; − 0.4, p < 0.0001). The intervention group also scored lower on the pain during activity score, but this difference was not statistically significant (δ 0.6, 95%CI 0; 1.2, p = 0.060).
Sensitivity analyses
Nine surgical procedures related to the study knee were performed in the intervention group during follow-up, versus 7 in the control group. Despite a slight decrease in the between group differences in responder percentages, the results of both additional analyses are still statistically significant in favour of the intervention group (Table 2).
Medication use
At baseline, more subjects used pain medication because of knee complaints in the intervention group with (53%) compared to the control group (42%). This difference decreased over time, resulting in similar usage of pain medication for both groups at final follow-up. The difference in pain medication users was not statistically significant on any of the time points during follow-up.
Adverse events
In the intervention group, more subjects reported any knee treatment related adverse events (AE) at 6 weeks (45% versus 18%. This difference was mainly due to flares or flare like symptoms of the study knee in this period (36% versus 10%), p > 0.001, number needed to harm (NNH) 4.0). The difference decreased at 13 weeks, and at 26 weeks the percentage of subjects reporting flares was similar in both groups. None of the between group differences on the following time points after 6 weeks were statistically significant. No septic arthritis of the study knee occurred in any of the subjects during follow-up.
The amount of non-treatment related AEs was similar in both groups during follow-up. An additional file shows the percentage of patients experiencing treatment and non-treatment related adverse events per study group per time point during study follow-up (see Additional file 1).
Discussion
This study is the first to investigate the effectiveness of HMW-HA added to usual care in subjects with clinical knee OA in the working age. We showed that adding intra-articular injections with a HMW-HA derivative to usual care treatment in an everyday clinical setting resulted in statistically significant more responders to therapy. It resulted in improvement of pain, function and PGA in these patients. The between group difference on 3 out of 4 of the individual responder domains (pain during rest, knee related function, PGA) was statistically significant and in favour of the intervention group. Subjects in the intervention group experienced more episodes of transient knee pain and/or swelling during the first 6 weeks.
In our study we specifically choose to include subjects in the working age (mean age 54) with a higher involvement in paid work (75%) [25]. By doing so we were able to investigate the effectiveness of HMW-HA in a population in which knee OA levels are rising and in which surgical treatments like arthroplasty are less eligible due to high revision rate and limited life span of the prosthesis [21,22,23]. We showed that in this population, intra-articular HMW-HA leads to clinically relevant improvement in pain, function and PGA. Since the costs from loss of productivity at work due to knee OA are high in patients in the working age, the treatment with HMW-HA could also result in certain economic benefits [24]. This was investigated in the parallel economic evaluation of the VISK study, in which we report that intra-articular HMW-HA in knee OA is probably cost-effective in this population [25].
To date, 2 other studies compared HMW-HA added to usual care to usual care only [34, 35]. The same HMW-HA derivative as in our study was investigated. Both studies imposed no limitation on maximum age at time of inclusion, which probably contributed to the relatively low proportion of subjects involved in a paid occupation (19 to 34%) [34, 35]. In the first study statistically significant more patients in the intervention group were responder to therapy at final follow-up of 9 months [35]. The percentage of responders was higher in both study groups compared to our study. Also no restriction on the radiologic degree of OA was imposed in this study and the minimal pain score at entry was higher (4 against 2) [35]. The inclusion of clinically more severe OA patients may have resulted in a larger percentage of responders in both groups since these patients are more likely to benefit from their treatment for knee OA. The second study reported statistically significant differences on pain, function and stiffness (WOMAC questionnaire), and on PGA in favour of the intervention group [34]. A decrease of 38% in the pain scale in the intervention group was reported, compared to a 13% decrease in the control group. K&L grade IV was excluded but multiple series of intra-articular injections with HMW-HA were allowed, in contrast to 1 series of HMW-HA in our study. The effectiveness results of our study are in line with the results of both aforementioned studies. Including our study, the results of the 3 studies showed that the primary effectiveness outcome parameters improve at least 20% when HMW-HA is added to the usual care treatment.
Intra-articular injections with HMW-HA are frequently accompanied by transient pain or swelling of the knee. The procedure itself also includes a risk of inducing septic arthritis [16, 17]. At 6 weeks, a statistically significant difference of subjects receiving HMW-HA in our study reported flares or flare-like symptoms of the study knee compared to the control group (35% vs 10%, p= > 0.001) in the control group. No septic arthritis occurred. These results on local adverse events (AE) are similar compared to other studies [34, 35]. In our opinion the reduction of knee pain and the improvement of function outweigh the increase of transient flare like symptoms.
The follow up of the VISK study was 52 weeks. Optimal pain decrease after administration of intra-articular HMW-HA is seen at about 3 months though [16, 18]. A shorter follow-up period, closer to the peak effectiveness, encloses the risk of underestimation of possible health effects. Effects on pain function and PGA can occur during a longer period than the peak effectiveness. A longer follow-up also allows for assessment of the course of these effects. To ensure that these matters were accounted for, the current follow-up period of 52 weeks was chosen.
This study has limitations that need to be addressed. The study design of the VISK study did not include a placebo group. Previous research showed that placebo effects in intra-articular HA studies are above average [36]. It is thus likely that part of the beneficial effect in the intervention group is explained by the placebo effect. There were 2 main reasons to opt for this specific study design without a placebo group. First, evidence from high quality studies in meta-analyses showed that HMW-HA is efficacious for knee OA [18,19,20]. The next logical step was to investigate the actual effectiveness of HMW-HA, thereby accepting the fact that part of the possible beneficial effects is probably explained by the placebo effect. Second, a study design in which the intervention (HMW-HA) is compared to the usual care treatment (and not to placebo) in an everyday clinical setting is required to be able to facilitate a parallel economic evaluation which was also part of the VISK study project [25,26,27].
The target population of our study can be described as secondary care patients with symptomatic and mild to moderate knee OA. We therefore included subjects with K&L grade I-III and a minimal VAS pain score of 2. Patients who were more likely to benefit from surgical therapy like TKA or osteotomy, or from rheumatologic treatment where excluded in this study (e.g. K&L grade IV, substantial varus/valgus deformation, inflammatory arthritis). We aimed to avoid measuring effects strongly related to other factors than the intervention itself (e.g. recent or planned knee surgery, daily steroid use) and to avoid possible harm due to the intervention (e.g. allergies, pregnancy). Applying these criteria may have consequences for the generalizability of the results. It is for example uncertain if the effectiveness results also extend to other patient groups who might benefit from HMW-HA treatment, like knee OA patients not fit for surgery who are in need of surgical therapy.
Conclusion
We conclude that intra-articular injections with HMW-HA added to usual care is effective in patients in the working age. It results in more responders to therapy and improvement in pain, function and PGA.
Abbreviations
- HA:
-
Hyaluronic acid
- HMW:
-
High molecular weight
- K&L:
-
Kellgren & Lawrence
- KOOS:
-
Knee injury and Osteoarthritis Outcome Score
- NRS:
-
Numeric rating scale
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PGA:
-
Patients global assessment
- RCT:
-
Randomized controlled trials
- TKA:
-
Total knee arthroplasty
- UC:
-
Usual care
- VISK study:
-
VIScosupplementation for Knee osteoarthritis study
References
Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S230–5.
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–96.
Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–13.
Dunlop DD, Manheim LM, Yelin EH, Song J, Chang RW. The costs of arthritis. Arthritis Rheum. 2003;49(1):101–13.
Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531–7.
Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54.
Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43(5):593–9.
Bruyere O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, Hochberg MC, Kanis JA, Kvien TK, Martel-Pelletier J, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of osteoporosis and osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44(3):253–63.
Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79.
Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3.
NOV (Dutch Orthopaedic Society): Richtlijn diagnostiek en behandeling van heup- en knie artrose (Guidelines in diagnostics and treatment of hip- and knee ostoarthritis). 2007.
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363–88.
Cooper C, Rannou F, Richette P, Bruyere O, Al-Daghri N, Altman RD, Brandi ML, Collaud Basset S, Herrero-Beaumont G, Migliore A, et al. Use of intraarticular hyaluronic acid in the Management of Knee Osteoarthritis in clinical practice. Arthritis Care Res (Hoboken). 2017;69(9):1287–96.
Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28–33.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
Ishijima M, Nakamura T, Shimizu K, Hayashi K, Kikuchi H, Soen S, Omori G, Yamashita T, Uchio Y, Chiba J, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16(1):R18.
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis--meta-analysis. Osteoarthr Cartil. 2011;19(6):611–9.
Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2015.
Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–91.
Ackerman IN, Kemp JL, Crossley KM, Culvenor AG, Hinman RS. Hip and knee osteoarthritis affects younger people, too. J Orthop Sports Phys Ther. 2017;47(2):67–79.
Sutton PM, Holloway ES. The young osteoarthritic knee: dilemmas in management. BMC Med. 2013;11:14.
Julin J, Jamsen E, Puolakka T, Konttinen YT, Moilanen T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis. A follow-up study of 32,019 total knee replacements in the Finnish arthroplasty register. Acta Orthop. 2010;81(4):413–9.
Hermans J, Koopmanschap MA, Bierma-Zeinstra SM, van Linge JH, Verhaar JA, Reijman M, Burdorf A. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res (Hoboken). 2012;64(6):853–61.
Hermans J, Reijman M, Goossens LM, Verburg H, Bierma-Zeinstra SM, Koopmanschap MA. A cost utility analysis of high molecular weight hyaluronic acid for knee osteoarthritis in everyday clinical care in patients in the working age. An economic evaluation of a randomized clinical trial. Arthritis Care Res (Hoboken). 2017.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care Programmes. 4th ed. Oxford: Oxford University Press; 2015.
Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, Briggs A, Sullivan SD. Cost-effectiveness analysis alongside clinical trials II-an ISPOR good research practices task force report. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18(2):161–72.
Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. OMERACT-OARSI initiative: osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12(5):389–99.
Hermans J, Bierma-Zeinstra SM, Bos PK, Verhaar JA, Reijman M. The most accurate approach for intra-articular needle placement in the knee joint: a systematic review. Semin Arthritis Rheum. 2011;41(2):106–15.
de Groot IB, Favejee MM, Reijman M, Verhaar JA, Terwee CB. The Dutch version of the knee injury and osteoarthritis outcome score: a validation study. Health Qual Life Outcomes. 2008;6:16.
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96.
Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022–4.
Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, Thabane M, Giangregorio L, Dennis B, Kosa D, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92.
Raynauld JP, Torrance GW, Band PA, Goldsmith CH, Tugwell P, Walker V, Schultz M, Bellamy N. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (part 1 of 2): clinical results. Osteoarthr Cartil. 2002;10(7):506–17.
Kahan A, Lleu PL, Salin L. Prospective randomized study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine. 2003;70(4):276–81.
Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–23.
Acknowledgements
We would like to thank Pim Luijsterburg for his help in designing the computer assisted randomization program. We furthermore would like to thank Roos Bernsen for her help with the statistical analyses.
Funding
The VISK study was supported by means of a grant of ZonMW [grant number 170992901], which is a non-commercial organisation for health research commissioned by the Dutch Ministry of Health, Welfare and Sport and the Netherlands Organisation for Scientific Research. ZonMW did not have any role in the study design, data collection, analyses, data interpretation or drafting of the manuscript The study intervention was purchased without any funding or discount from the manufacturer.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JH was responsible for all collection, analyses and interpretation of the data and drafted the manuscript. SBZ, JV and MR were responsible for the conception and design of the study and for interpretation of the data. They revised the manuscript critically for intellectual content. DN and PB were responsible for acquisition of the data, administering the study intervention at the outpatient clinic, and critically revised of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The VISK study was approved by the Medical Ethics Committee of the Erasmus University Medical Centre in Rotterdam (approval number NL24430.078.08). The study was performed in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from every subject included in the study.
Consent for publication
Not applicable.
Competing interests
Next to his employment as an orthopaedic surgeon at the Department of Orthopaedic Surgery of the Reinier de Graaf Hospital in Delft (The Netherlands), DN is a consultant orthopaedic surgeon for Zimmer Medical. This company was not involved in any part of the study. SBZ, PB, JV and MR are all employed by the Erasmus University Medical Centre Rotterdam and due to the nature of their work they are involved in several other study projects funded by research grants.
No financial support or other benefits from commercial sources or any other financial interests that any of the other authors may have, which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work, are reported for this article by any of the authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Adverse events per study group per time point. Displays the amount of subjects experiencing treatment related and non-treatment related adverse events and their nature. (DOCX 20 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hermans, J., Bierma-Zeinstra, S.M.A., Bos, P.K. et al. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: a randomised controlled trial. BMC Musculoskelet Disord 20, 196 (2019). https://doi.org/10.1186/s12891-019-2546-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-019-2546-8