Abstract
Background
Obesity and musculoskeletal pain are strongly related, but there is emerging evidence that body fat, not body weight, may be a better indicator of risk. There is, therefore, a need to determine if body fat is associated with musculoskeletal pain as it may improve management strategies. The aim of this systematic review was to investigate the association between body fat and musculoskeletal pain.
Methods
Seven electronic databases were searched from inception to 8th January 2018. Cross-sectional and longitudinal studies investigating the association between measures of body fat and musculoskeletal pain were included. All included articles were assessed for methodological rigour using the Epidemiology Appraisal Instrument. Standardised mean differences (SMDs) and effect estimates were pooled for meta-analysis.
Results
A total of 10,221 citations were identified through the database searching, which after abstract and full-text review, yielded 28 unique articles. Fourteen studies were included in the meta-analyses, which found significant cross-sectional associations between total body fat mass and widespread pain (SMD 0.49, 95% CI 0.37–0.61, p < 0.001). Individuals with low-back pain and knee pain had a higher body fat percentage than asymptomatic controls (SMD 0.34, 95% CI 0.17–0.52, p < 0.001 and SMD 0.18, 95% CI 0.05–0.32, p = 0.009, respectively). Fat mass index was significantly, albeit weakly, associated with foot pain (SMD 0.05, 95% CI 0.03–0.06, p < 0.001). Longitudinal studies (n = 8) were unsuitable for meta-analysis, but were largely indicative of elevated body fat increasing the risk of incident and worsening joint pain. There was conflicting evidence for an association between body fat percentage and incident low-back pain (3 studies, follow-up 4–20 years). Increasing knee pain (1 study) and incident foot pain (2 studies) were positively associated with body fat percentage and fat mass index. The percentage of items in the EAI graded as ‘yes’ for each study ranged from 23 to 85%, indicating variable methodological quality of the included studies.
Conclusions
This systematic review and meta-analysis identified positive cross-sectional associations between increased body fat and widespread and single-site joint pain in the low-back, knee and foot. Longitudinal studies suggest elevated body fat may infer increased risk of incident and worsening joint pain, although further high-quality studies are required.
Similar content being viewed by others
Background
Musculoskeletal conditions, manifesting as pain in soft tissues and joints, are a leading cause of disability [1]. Worldwide, they are second only to mental and behavioral problems in contributing to the total years lived with disability [2]. Musculoskeletal pain can lead to an avoidance of physical activity [3] and weight gain [4]. Excessive weight gain may result in the development of obesity and there is a strong bidirectional relationship between obesity and musculoskeletal pain [5], but understanding how excessive body weight and pain are related is important as it guides therapy.
The implication that excessive loading of joints is directly related to pain likely oversimplifies the complex relationship between obesity and pain. This is demonstrated by an abundance of studies with often conflicting findings regarding the nature of the relationship between mechanical loading and pain [6,7,8,9,10]. Moreover, whilst ground impact forces are positively related to obesity, lean mass (i.e. muscle) is negatively associated with impact force and may be protective, suggesting that body tissues should not all be considered homogeneous [11].
Obesity is commonly defined as ≥30 kg/m2 on the body mass index (BMI) scale, which is calculated by dividing body weight (kg) by body height (m) squared. This scale, however, treats all body tissue as homogeneous and it does not account for either the type or the distribution of body weight [12]. The BMI is not a good measure of adiposity (body fatness) as it does not account for age or gender differences [13]. Furthermore, given the association between BMI-defined obesity and musculoskeletal pain extends to both weight-bearing [14] and non-weight-bearing joints [15], it follows that the mechanism underpinning this relationship may extend beyond excessive mechanical loading alone, which is implied with the BMI. Fat mass index (FMI) is a more relevant measure in having or predicting pain [16], suggesting the type of tissue is important. It is also now well-recognised that adipose tissue is an active endocrine organ that secretes many active cytokines and hormones [17], some of which may be related to the development of musculoskeletal pain.
Recent cross-sectional and longitudinal studies are beginning to highlight the important role of body composition in the development and worsening of joint pain [18,19,20]. Body composition can be analysed using a number of techniques including dual energy x-ray absorptiometry, bioelectrical impedance analysis and skin-fold thickness, although this method has challenges with increasing levels of obesity [21]. Whilst much attention is directed toward the strong association between BMI-defined obesity and musculoskeletal pain, there are metabolic [22, 23], structural [24] and psychological mechanisms [25] that may link adiposity and pain. There is, therefore, a need to determine whether body fat is associated with musculoskeletal pain as this understanding may improve management strategies. The aim of this systematic review was therefore to investigate the association between body fat and musculoskeletal pain.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [26]. This systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) on 12th August 2017 (http://www.crd.york.ac.uk/PROSPERO/), registration number: CRD42017074289.
Search strategy for identification of studies
The following databases were searched on 9th August 2017: Medline (Ovid); PubMed (non-Medline content only); Embase (OVID); Scopus; CINAHL (EBSCOhost); Cochrane Central Register of Controlled Trials; and Web of Science. All databases were searched from inception to current date. Reference lists from suitable papers were also investigated and included prior to applying exclusion and inclusion criteria. Broad MeSH terms and keywords were used, combining musculoskeletal pain and body composition. The search terms were broad to ensure capture of all relevant studies. Additional file 1 illustrates the full search strategy used for this systematic review, and minor modifications to search terms were required depending on the database searched. Database searching and registration for automatic e-alerts were also continued until the review was finalised (8th January 2018).
Following removal of duplicates, two reviewers (TPW and JBA) applied the predetermined selection criteria to all articles by reading the title and abstract alone. Where discrepancies between article selections existed, the reviewers discussed these discrepancies to form a consensus, a third reviewer was not required to arbitrate a consensus for this review. Articles were then assessed for eligibility by full-text review.
Eligibility criteria
Articles from English language, peer-reviewed, scientific journals were eligible for inclusion in this review if they reported studies that examined the association between body composition and musculoskeletal pain. Studies were included if all participants were aged at least 18 years, had musculoskeletal pain recorded via self-report or questionnaire (or were controls) and had an assessment of body fat. Studies specifically investigating participants with inflammatory conditions or autoimmune diseases were excluded. Further exclusion criteria were; unclear assessment of musculoskeletal pain or body composition, letters to the editor and editorials, opinion pieces and non-English language publications.
Assessment of methodological quality
All included articles were assessed for methodological rigour using the Epidemiology Appraisal Instrument (EAI) [27]. This tool has been shown to demonstrate good reliability and content validity [28]. A number of items from the EAI were omitted as they were not applicable to non-interventional studies (Questions 10,12,20,22-24,35,37,40) as per previous reviews of observational studies investigating musculoskeletal disorders [29, 30]. The covariates considered important for questions 11 and 36 were age, gender and a measure of psychological health. As it is not known if each question of the EAI is equally weighted, rather than providing a quality assessment score for each study, a summary score for each question is reported. A summary (%) of the number of questions a study scored ‘yes’ on is also reported.
Data extraction and analysis
To reduce the risk of bias, author and publication details were removed prior to data extraction. Where available the relevant data (means, medians, standard deviations (SDs), odds ratios (ORs), relative risks (RRs), confidence intervals (CIs) and p values) were recorded for each study. Where available, multivariable OR (95% CI) were extracted in preference to unadjusted OR (95% CI). For studies reporting means and standard deviations, effect sizes (Cohen’s d) and CIs were calculated. According to Cohen [31], effect sizes were interpreted as 0.2, small; 0.5, medium; and 0.8, large. Widespread pain was defined as ≥5 painful joints, which is modeled on the criteria of the American College of Rheumatology [32]. Multi-site pain was defined as > 1 but < 5 painful joints. For those studies investigating multi-site or widespread pain, the differences were calculated between the no pain group and the multi-site / widespread pain group. Meta-analysis was performed where more than one study reported on the same parameter, grouped by widespread or single-site pain location. Only the gender-specific sample size was used when entering gender-stratified data into the meta-analysis.
The OR and CIs, and SMD (Cohen’s d) were pooled for meta-analysis by the standard approach, weighted by the inverse variance method. Odds ratios and CIs were converted to SMDs for meta-analysis [33]. Statistical heterogeneity was assessed for each site using the I2 statistic. Potential publication bias was assessed graphically using a funnel plot [34] and Egger’s regression intercept for low-back pain, knee pain and foot pain. Both heterogeneity and publication bias were considered, accepting the fact that the power was low because of the small number of studies for each site. Sensitivity analysis was performed via the one-study removed test (removal of individual studies out of the model in turn), which gauges each study’s impact on the overall pooled effect size. A p-value less than 0.05 (two-tailed) was considered statistically significant. All analyses were conducted using Comprehensive Meta Analysis v3.0 (Biostat, NJ, USA).
Results
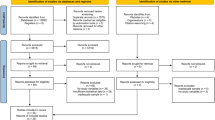
The initial literature search yielded a total of 10,221 citations, which was reduced to 5026 following the removal of duplicates. These 5026 articles were screened based on their title and abstract, where a further 4945 articles were excluded, leaving 81 articles that underwent full-text review. After 53 articles were excluded, 28 unique articles were included in this review [16, 18,19,20, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Twenty-two articles reported cross-sectional data [16, 18, 20, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] and eight articles [16, 19, 35, 54,55,56,57,58] provided longitudinal data (two articles reported both cross-sectional and longitudinal data [16, 35]). Four articles used participants from the same study [18,19,20, 40], and there were three other instances of articles using data, reporting different outcomes, from the same study [47, 58, 35, 39, 55] and [38, 42], leaving 21 unique studies. The regions with multiple studies using the same parameter were widespread, low-back, knee and foot and thus these were included in the quantitative analysis (n = 14) [16, 35,36,37, 39,40,41,42,43, 47,48,49, 51, 52]. All of the studies included in the meta-analysis were cross-sectional. There were fewer longitudinal studies, with most studies only investigating one site (other than low-back) with variable follow up time, therefore these data did not undergo meta-analysis. Details of study selection have been recorded (Fig. 1) following the guidelines set by PRISMA.
Study characteristics
A variety of sites for musculoskeletal pain were investigated, including the neck, low-back, knee and foot. The low-back was the most common region investigated, with 15 articles including this site in their analysis [18, 20, 35,36,37,38, 43, 46,47,48, 51, 52, 56,57,58]. Three articles investigated the association between multi-site / widespread pain and body composition [18, 35, 36], while another investigated multiple regions, but stratified the analysis by these regions [37]. One study [54] used body composition as a predictor for any injury and thus a specific region was not investigated. Body composition was analysed with dual energy x-ray absorptiometry in 13 articles [16, 18,19,20, 35, 36, 38,39,40,41,42,43, 55], bioelectrical impedance analysis in 10 articles [37, 44,45,46,47,48,49,50, 56, 57], and skin fold thickness in 5 articles [51,52,53,54, 58]. Body composition was generally reported as a percentage of body fat (17/28 articles) [37, 39, 43,44,45,46,47,48,49,50,51,52, 54,55,56,57,58]. The cross-sectional articles consisted of; population-based (n = 7), clinic-based (n = 7), musculoskeletal pain (n = 5), occupational-based (n = 2) and unknown (n = 1). The longitudinal articles were largely population-based (n = 6) along with occupational-based (n = 1) and military-based (n = 1). The longitudinal articles varied in follow-up from 3 months [54] to > 20 years [58], but most were between 3 and 5 years.
Participant characteristics
The studies included in this systematic review reported on 12,942 participants, with studies from Asia, Europe, South America and Australia. Both men and women were represented in most studies, although gender-specific studies accounted for > 35% of the total [38, 41, 42, 46, 47, 49, 52, 54, 57, 58]. Mean age in the cross-sectional studies ranged from 20.7 years [50] to 74.4 years [41], while the longitudinal studies ranged from 19.0 years [54] to 64.6 years [16]. Most cross-sectional studies included participants with mean BMIs of < 30 kg/m2, however four included participants with a mean BMI of > 30 kg/m2 [18, 20, 40, 53]. The mean BMI of the participants from the longitudinal studies ranged from 20.8 kg/m2 [54] to 29.6 kg/m2 [19].
Methodological quality assessment
The results of the methodological quality assessment are provided in Additional file 2. The summary scores for each question ranged from 4 to 96%, with 14/34 questions scoring above 50%. The percentage of items in the EAI graded as ‘yes’ for each study ranged from 23 to 85%, indicating variable methodological quality of the included studies. There were common, strong themes among the studies with the clear descriptions of the aims, study design and results reported in most studies (> 85%). There were, however, a number of consistent methodological limitations; the reliability and validity of the instruments used was often under-reported, a sample size calculation was mostly not reported (96%) and the generalisability of the findings was questionable in over 80% of the included studies. Whilst there was adjustment for a number of other variables e.g. smoking, physical activity, self-reported arthritis, adjustment for all of the important confounding variables was reported in less than 30% of the articles [16, 18, 19, 35, 38, 40, 42, 56]. One article considered psychological health alone [44], one article considered age alone [58], six articles considered both age and gender [20, 36, 37, 41, 55, 57]. Only three articles [16, 40, 42] provided data that were adjusted for the important confounding variables (age, gender, psychological health) that were also used in the meta-analyses.
Meta-analysis
Meta-analysis of cross-sectional single-site and widespread pain studies found significant associations between body fat and pain (Figs. 2, 3, 4 and 5), summarised in Table 1. There was a positive medium effect size between total body fat mass and widespread pain (SMD 0.49, 95% CI, 0.37–0.61, p < 0.001 and I2 < 0.001, p = 0.366). Single-site musculoskeletal pain also had positive associations with body fat. Low-back pain and body fat percentage had a combined small-medium effect size (SMD 0.34, 95% CI 0.17–0.52, p < 0.001), but there was a significant level of heterogeneity (I2 = 91.21, p < 0.001). Body fat percentage and knee pain had a small effect (SMD 0.18 95% CI, 0.05–0.32, p = 0.009 and I2 < 0.001, p = 0.941), while the pooled FMI and foot pain had a small effect (SMD 0.05, 95% CI, 0.03–0.06, p < 0.001 and I2 < 0.001, p = 0.564).
Sensitivity analysis
The association between knee pain and body fat percentage was not significant when the data pertaining to women from Scott et al. [39] was removed from the meta-analysis, (SMD 0.16, 95% CI -0.02-0.34, p = 0.075), suggesting that the relationship may be mediated by gender. All other sites remained significant when one study was removed from the respective model.
Publication bias
No significant publication bias was detected for studies reporting foot pain or knee pain, with Egger’s regression intercept (95% CI) of 0.75 (− 2.38–3.87), p = 0.412 and − 0.61 (− 10.87–9.66), p = 0.589, respectively. There was however a potential for publication bias detected for studies reporting low-back pain with Egger’s regression intercept (95% CI) of 3.44 (1.57–5.33), p = 0.004. Widespread pain was reported in only two studies and was therefore not amenable for the funnel plot test or Egger’s regression intercept.
Cross-sectional studies not included in the meta-analysis
The cross-sectional studies not included in the meta-analysis (due to the type of data or the parameter used) were generally concordant with the overall findings (Table 1), with the reasons for exclusion in Additional file 3. Multi-site pain (3 sites) was associated with FMI in the study by Brady et al. [18]. Neck pain was associated with body fat percentage in one study [45], while temporomandibular pain was not [44]. The large study by Chou et al. [38] that investigated low-back pain used the same sample as reported by Butterworth et al. [42] who investigated foot pain, with both finding FMI, but not FFMI, to be significantly associated with pain. Celan et al. [46] studied the relationship between body fat percentage and low-back pain, but the only data provided were mean body fat percentage, without confidence intervals or standard deviations and therefore these data were not amenable for the meta-analysis. Iizuka et al. [37] investigated multiple regions separately (neck / shoulder, back and low-back) and their associations between body fat percentage. Whilst we felt it appropriate to include the low-back region in the meta-analysis, we did not include the neck / shoulder and the back region with the other studies given the difficulty with delineating these regions, particularly the low-back region from the back region, but we did include the neck / shoulder region in Table 1. Other studies investigating low-back pain generally found increased fat mass was associated with pain. The smaller studies that investigated both knee and shin pain found non-significant associations between pain and body fat mass.
Longitudinal studies
Findings from the longitudinal studies (Table 2) were consistent with the overall theme identified in the cross-sectional studies, finding increased levels of body fat predicted future musculoskeletal pain. Higher baseline FMI was predictive of foot pain in the short term (less than 3 years) [16, 19] in data from both a community cohort (OR 1.06, 95% CI 1.02–1.11) and a musculoskeletal study (OR 1.28, 95% CI 1.04–1.57). In the knee, Jin et al. [55] found an association between increased fat mass and an increased relative risk (RR) of pain in either lying in bed, (RR 1.47, 95% CI 1.12–1.93) or sitting (RR 1.46, 95% CI 1.10–1.95), although knee pain when weight-bearing was not associated with fat mass. More frequent knee pain at 5.1 years follow-up was positively associated with higher total fat mass, and there was an increased risk (95% CI) of consistent (RR 1.89, 95% CI 1.43–2.51) and fluctuating knee pain (RR 1.78, 95% CI 1.41–2.25). A five-year longitudinal study by Pan et al. [35] found a significant trend across three time-points for fat mass and multisite pain, with the number of painful sites significantly associated with total body fat mass over 5 years. There was, however, some discordance between the relationship of body composition and low-back pain, but the larger studies found fat mass to be a predictor of increased pain and disability following multiple adjustments [56,57,58]. A twin study by Dario et al. [57] did not find a significant relationship between body fat and the risk of chronic low-back pain in women (n = 314), however a larger study (n = 4986) by Hussain et al. [56] found higher body fat at baseline to be predictive of both high pain intensity and high disability in women and men at 5 years follow-up. Hashimoto et al. [58] also found that men in the fourth quartile of body fat percentage had a significant risk of chronic back pain at > 20 years follow up when adjusting for age, smoking, alcohol consumption and maximal oxygen uptake (OR 2.12, 95% CI 1.13–3.98). One study found that the risk of developing injury during a three-month training program increased in women with an increased body fat percentage (OR 1.16, 95% CI 1.00–1.34) [54].
Discussion
This is the first review to systematically appraise and synthesise studies examining the relationship between body fat and musculoskeletal pain. This review included single- and multi-site joint pain and the meta-analyses demonstrated significant associations between increased fat mass and widespread pain, low-back pain, knee pain and foot pain. There was also emerging evidence from longitudinal studies that elevated body fat may infer an increased risk of incident or worsening joint pain. Thus, musculoskeletal pain may be a manifestation of excessive fat mass, which exists beyond excessive mechanical loading.
The association between fat mass and widespread pain is perhaps the most important finding of this review. Single-site pain may be confounded by local biomechanical factors or trauma, whereas widespread pain may be due to the pervasive nature of excessive adipose tissue on pain, extending beyond local tissue disease to include how pain may be perceived centrally [59]. The study by Pan et al. [35] found both cross-sectional and longitudinal associations with widespread pain and they adjusted for psychological health in the longitudinal analysis, which is particularly important given the bidirectional relationship between depression and pain [25]. Whilst depressive symptoms are undoubtedly more common in those with excessive adiposity, there were independent associations between body fat and pain, particularly in the foot [16, 40, 42]. The foot is the first site in the body to modulate ground reaction forces, where the bones and soft tissues are subjected to bending and torsional loads [60]. The weak pooled estimate for the association between foot pain and body fat may be attributed to the fact that three of the four articles included in the meta-analysis adjusted for age, gender and depression and normalised fat mass for height, while the other article also matched on age, gender and BMI. This therefore suggests that unless FMI is associated with specific changes to foot mechanics, which seems unlikely, that the association of foot pain with obesity may be metabolically mediated. It is important to note that the magnitude of the effects were small to medium in size, suggesting a relatively modest potential contribution of fat mass to musculoskeletal pain amongst other known physiological and psychological factors.
A number of proposed pathways can explain the association between body fat and musculoskeletal pain, including the up-regulation of cytokines secreted by adipose tissue, referred to as adipokines. Leptin, a pro-inflammatory adipokine predominately expressed by subcutaneous adipose tissue [61] is associated with bodily pain in women [62] and leptin levels in both serum [63] and synovial fluid [64] are associated with osteoarthritis, particularly in women. Leptin has functional receptors on articular chondrocytes, and may be involved with cartilage generation [65]. Leptin signaling, however, may be blunted with adiposity, through a regulative negative feedback loop [66]. Interestingly, excessive adiposity may increase leptin secretion, which in turn may compromise its ability to repair joint cartilage by a down-regulation in receptor expression [67]. This theory is supported by an observational study investigating knee joint changes using magnetic resonance imaging, where reduced cartilage volume, a hallmark of osteoarthritis, is associated with increased leptin [68]. Thus, leptin may be associated with structural joint changes that, at the very least may predispose the joint to further cartilage failure and pain.
Other suggested mechanisms linking adipose tissue with pain, including subclinical inflammation [69, 70]. Tumour necrosis factor-alpha (TNF-α) is a cytokine involved in the inflammatory cascade. It is a therapeutic target for the management of inflammatory arthropathies, and is primarily produced by activated macrophages, but it is also secreted by adipose tissue [17]. Systemic inflammation is up-regulated with obesity with the acute inflammatory phase marker, C-reactive protein (CRP), higher in obese people [71]. The increase in inflammation may be in response to over-nutrition initiating an immune response [72], particularly linked to the consumption of dietary fats [73]. Moreover, elevated TNF-α, along with other inflammatory mediators and markers are associated with chronic pain [74]. Elevated synovial TNF-α levels are also predictive of pain severity and a poor outcome following temporomandibular joint surgery [75]. Furthermore, elevated serum levels of TNF-α and interleukin-6 (IL-6) are associated with less improvement to treatment in those with chronic pain [76] and TNF-α may moderate the relationship between chronic back pain and depressive symptoms [77].
Systemic inflammation related to adiposity has been linked to other structural joint changes and this may be one phenotype that contributes to osteoarthritis [78]. In the knee, both TNF-α and IL-6 have been associated with knee cartilage loss [24] and elevated IL-6 is a predictor of radiographic osteoarthritis [79], suggesting a link between low-level inflammation and osteoarthritis pathogenesis. Tendinopathy has also been linked with dietary fats, adiposity and inflammation [80, 81], highlighting that obesity may not necessarily be only related to excessive load. Clearly elevated body fat is linked with structural changes and pain in multiple regions and may explain the known link between elevated BMI and osteoarthritis in non-weight-bearing joints such as the hands [15]. Future work to investigate if there is a true discordance between fat mass and fat-free mass may help strengthen the notion that body composition is more meaningful measure of risk for musculoskeletal pain.
This review should be considered in light of certain limitations. Firstly, given the lack of homogeneity in follow-up time, we were unable to undertake a meta-analysis on longitudinal associations between musculoskeletal pain and body fat. Secondly, despite the considerable variability in the quality of the articles included in this study, a number of items assessed with the EAI would have scored higher had they been explicitly reported, such as the reliability and validity of the tools used to assess pain and body composition. A number of the tools are known to be both reliable and valid, but unfortunately this was not reported by the authors. Thirdly, the case-definition for pain did vary between studies and thus while we did perform a meta-analysis by region those with stricter criteria may under-report the prevalence, incidence or progression of pain. Fourthly, the pooled estimates of the meta-analyses are small to medium in size, suggesting a weak to moderate effect which should be taken into consideration. Finally, this review focused on the association between body fat and pain, but it did not investigate whether lean mass was inversely related to pain. However, this is the first review to systematically appraise and synthesise studies examining the relationship between body fat and musculoskeletal pain.
Conclusion
This systematic review has demonstrated that increased body fat is positively associated with widespread pain, low-back pain, knee pain and foot pain. Meta-analysis found positive cross-sectional associations between increased body fat and widespread and single-site joint pain in the low-back, knee and foot. Evidence from longitudinal studies suggests elevated body fat may infer increased risk of incident and worsening joint pain, although further high-quality studies are required.
Abbreviations
- BIA:
-
Bioelectrical impedance analysis
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- cm:
-
Centimetre
- CRP:
-
C-reactive protein
- DXA:
-
Dual-energy X-ray absorptiometry
- EAI:
-
Epidemiology Appraisal Instrument
- FFMI:
-
Fat-free mass index
- FMI:
-
Fat mass index
- IL-6:
-
Interleukin-6
- NJ:
-
New Jersey
- OR:
-
Odds ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR:
-
Relative risk
- SMD:
-
Standardised mean differences
- TNF-α:
-
Tumour necrosis factor-alpha
- USA:
-
United States of America
References
Storheim K, Zwart JA. Musculoskeletal disorders and the global burden of disease study. Ann Rheum Dis. 2014;73:949–50.
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–223.
Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32.
Ferguson S, Al-Rehany L, Tang C, Gougeon L, Warwick K, Madill J. Self-reported causes of weight gain: among prebariatric surgery patients. Can J Diet Pract Res. 2013;74:189–92.
Cameron AJ, Magliano DJ, Dunstan DW, Zimmet PZ, Hesketh K, Peeters A, et al. A bi-directional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes. 2012;36:295–303.
Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57:1254–60.
Hurwitz DE, Ryals AR, Block JA, Sharma L, Schnitzer TJ, Andriacchi TP. Knee pain and joint loading in subjects with osteoarthritis of the knee. J Orthop Res. 2000;18:572–9.
Sullivan J, Burns J, Adams R, Pappas E, Crosbie J. Plantar heel pain and foot loading during normal walking. Gait Posture. 2015;41:688–93.
Coenen P, Kingma I, Boot CRL, Bongers PM, van Dieën JH. Cumulative mechanical low-back load at work is a determinant of low-back pain. Occup Environ Med. 2014;71:332–7.
Bakker EW, Verhagen AP, Lucas C, Koning HJ, Koes BW. Spinal mechanical load: a predictor of persistent low back pain? A prospective cohort study. Eur Spine J. 2007;16:933–41.
Villarrasa-Sapiña I, Serra-Añó P, Pardo-Ibáñez A, Gonzalez LM, García-Massó X. Relationship between body composition and vertical ground reaction forces in obese children when walking. Clin Biomech (Bristol, Avon). 2017;41:77–81.
Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–66.
Pasco JA, Nicholson GC, Brennan SL, Kotowicz MA. Prevalence of obesity and the relationship between the body mass index and body fat: cross-sectional, population-based data. PLoS One. 2012;7:e29580.
Zhou ZY, Liu YK, Chen HL, Liu F. Body mass index and knee osteoarthritis risk: a dose-response meta-analysis. Obesity (Silver Spring). 2014;22:2180–5.
Jiang L, Xie X, Wang Y, Wang Y, Wang Y, Lu Y, et al. Body mass index and hand osteoarthritis susceptibility: an updated meta-analysis. Int J Rheum Dis. 2016;19:1244–54.
Walsh TP, Gill TK, Evans AM, Yaxley A, Shanahan EM, Hill CL. Association of fat Mass and Adipokines with Foot Pain in a community cohort. Arthritis Care Res (Hoboken). 2016;68:526–33.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Brady SR, Mamuaya BB, Cicuttini F, Wluka AE, Wang Y, Hussain SM, et al. Body composition is associated with multisite lower body musculoskeletal pain in a community-based study. J Pain. 2015;16:700–6.
Butterworth PA, Urquhart DM, Cicuttini FM, Menz HB, Strauss BJ, Proietto J, et al. Fat mass is a predictor of incident foot pain. Obesity (Silver Spring). 2013;21:E495–9.
Urquhart DM, Berry P, Wluka AE, Strauss BJ, Wang Y, Proietto J, et al. Young investigator award winner: increased fat mass is associated with high levels of low back pain intensity and disability. Spine (Phila Pa 1976). 2011;36:1320–5.
Gray DS, Bray GA, Bauer M, Kaplan K, Gemayel N, Wood R, et al. Skinfold thickness measurements in obese subjects. Am J Clin Nutr. 1990;51:571–7.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S.
Gold MS, Flake NM. Inflammation-mediated hyperexcitability of sensory neurons. Neurosignals. 2005;14:147–57.
Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil. 2010;18:1441–7.
Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM. Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–73.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Genaidy AM, Lemasters GK, Lockey J, Succop P, Deddens J, Sobeih T, et al. An epidemiological appraisal instrument – a tool for evaluation of epidemiological studies. Ergonomics. 2007;50:920–60.
Crowe M, Sheppard L. A review of critical appraisal tools show they lack rigor: alternative tool structure is proposed. J Clin Epidemiol. 2011;64:79–89.
Nix S, Smith M, Vicenzino B. Prevalence of hallux valgus in the general population: a systematic review and meta-analysis. J Foot Ankle Res. 2010;3:21.
Uden H, Scharfbillig R, Causby R. The typically developing paediatric foot: how flat should it be? A systematic review. J Foot Ankle Res. 2017;10:37.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9.
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–72.
Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychol Bull. 1995;117:167–78.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Pan F, Laslett L, Blizzard L, Cicuttini F, Winzenberg T, Ding C, et al. Associations between fat mass and multisite pain: a five-year longitudinal study. Arthritis Care Res (Hoboken). 2017;69:509–16.
Yoo JJ, Cho NH, Lim SH, Kim HA. Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents. Arthritis Rheumatol. 2014;66:3511–20.
Iizuka Y, Iizuka H, Mieda T, Tajika T, Yamamoto A, Ohsawa T, et al. Association between neck and shoulder pain, back pain, low back pain and body composition parameters among the Japanese general population. BMC Musculoskelet Disord. 2015;16:333.
Chou L, Brady SR, Urquhart DM, Teichtahl AJ, Cicuttini FM, Pasco JA, et al. The association between obesity and low back pain and disability is affected by mood disorders: a population-based, cross-sectional study of men. Medicine (Baltimore). 2016;95:e3367.
Scott D, Blizzard L, Fell J, Jones G. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res (Hoboken). 2012;64:30–7.
Tanamas SK, Wluka AE, Berry P, Menz HB, Strauss BJ, Davies-Tuck M, et al. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res (Hoboken). 2012;64:262–8.
Walsh TP, Arnold JB, Gill TK, Evans AM, Yaxley A, Hill CL, et al. Foot pain severity is associated with the ratio of visceral to subcutaneous fat mass, fat-mass index and depression in women. Rheumatol Int. 2017;37:1175–82.
Butterworth PA, Menz HB, Urquhart DM, Cicuttini FM, Landorf KB, Pasco JA, et al. Fat mass is associated with foot pain in men: the Geelong osteoporosis study. J Rheumatol. 2016;43:138–43.
Sakai Y, Matsui H, Ito S, Hida T, Ito K, Koshimizu H, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia. 2017;3:195–200.
Jordani PC, Campi LB, Circeli GZ, Visscher CM, Bigal ME, Gonçalves DA. Obesity as a risk factor for temporomandibular disorders. J Oral Rehabil. 2017;44:1–8.
Yalcinkaya H, Ucok K, Ulasli AM, Coban NF, Aydin S, Kaya I, et al. Do male and female patients with chronic neck pain really have different health-related physical fitness, depression, anxiety and quality of life parameters? Int J Rheum Dis. 2017;20:1079–87.
Celan D, Turk Z. The impact of anthropometric parameters on the incidence of low back pain. Coll Antropol. 2005;29:101–5.
Dario AB, Ferreira ML, Refshauge K, Sánchez-Romera JF, Luque-Suarez A, Hopper JL, et al. Are obesity and body fat distribution associated with low back pain in women? A population-based study of 1128 Spanish twins. Eur Spine J. 2016;25:1188–95.
Toda Y, Segal N, Toda T, Morimoto T, Ogawa R. Lean body mass and body fat distribution in participants with chronic low back pain. Arch Intern Med. 2000;160:3265–9.
Ozer Kaya D, Düzgün I, Baltaci G. Differences in body fat mass, muscular endurance, coordination and proprioception in woman with and without knee pain: a cross-sectional study. Acta Orthop Traumatol Turc. 2014;48:43–9.
Sabeti V, Khoshraftar Yazdi N, Bizheh N. The relationship between shin splints with anthropometric characteristics and some indicators of body composition. J Sports Med Phys Fitness. 2014; [Epub ahead of print]
Hodselmans AP, Dijkstra PU, Geertzen JHB, van der Schans CP. Nonspecific chronic low back pain patients are deconditioned and have an increased body fat percentage. Int J Rehabil Res. 2010;33:268–70.
Spyropoulos P, Chronopoulos E, Papathanasiou G, Georgoudis G, Koutis H, Kompoti A. Chronic low back pain and function of Greek office workers. J Back Musculoskelet Rehabil. 2008;21:129–35.
Sutbeyaz ST, Sezer N, Koseoglu BF, Ibrahimoglu F, Tekin D. Influence of knee osteoarthritis on exercise capacity and quality of life in obese adults. Obesity (Silver Spring). 2007;15:2071–6.
Kodesh E, Shargal E, Kislev-Cohen R, Funk S, Dorfman L, Samuelly G, et al. Examination of the effectiveness of predictors for musculoskeletal injuries in female soldiers. J Sports Sci Med. 2015;14:515–21.
Jin X, Ding C, Wang X, Antony B, Laslett LL, Blizzard L, et al. Longitudinal associations between adiposity and change in knee pain: Tasmanian older adult cohort study. Semin Arthritis Rheum. 2016;45:564–9.
Hussain SM, Urquhart DM, Wang Y, Shaw JE, Magliano DJ, Wluka AE, et al. Fat mass and fat distribution are associated with low back pain intensity and disability: results from a cohort study. Arthritis Res Ther. 2017;19:26.
Dario AB, Loureiro Ferreira M, Refshauge K, Luque-Suarez A, Ordoñana JR, Ferreira PH. Obesity does not increase the risk of chronic low back pain when genetics are considered. A prospective study of Spanish adult twins. Spine J. 2017;17:282–90.
Hashimoto Y, Matsudaira K, Sawada SS, Gando Y, Kawakami R, Kinugawa C, et al. Obesity and low back pain: a retrospective cohort study of Japanese males. J Phys Ther Sci. 2017;29:978–83.
Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, Afari N. Chronic pain, overweight, and obesity: findings from a community-based twin registry. J Pain. 2010;11:628–35.
Kirby KA. Longitudinal arch load-sharing system of the foot. Rev Esp Podol. 2017;28:e18–26.
Shimizu H, Shimomura Y, Hayashi R, Ohtani K, Sato N, Futawatari T, et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int J Obes Relat Metab Disord. 1997;21:536–41.
Younger J, Kapphahn K, Brennan K, Sullivan SD, Stefanick ML. Association of Leptin with body pain in women. J Women's Health (Larchmt). 2016;25:752–60.
Zhang P, Zhong ZH, Yu HT, Liu B. Significance of increased leptin expression in osteoarthritis patients. PLoS One. 2015;10:e0123224.
Lübbeke A, Finckh A, Puskas GJ, Suva D, Lädermann A, Bas S, et al. Do synovial leptin levels correlate with pain in end stage arthritis? Int Orthop. 2013;37:2071–9.
Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjörnsson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190–7.
Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–64.
Liu ZJ, Bian J, Liu J, Endoh A. Obesity reduced the gene expressions of leptin receptors in hypothalamus and liver. Horm Metab Res. 2007;39:489–94.
Ding C, Parameswaran V, Cicuttini F, Burgess J, Zhai G, Quinn S, et al. Association between leptin, body composition, sex and knee cartilage morphology in older adults: the Tasmanian older adult cohort (TASOAC) study. Ann Rheum Dis. 2008;67:1256–61.
Festa A, D'Agostino R, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407–15.
Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7.
Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, et al. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:674–9.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43.
Teng KT, Chang CY, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J. 2014;13:12.
Parkitny L, McAuley JH, Di Pietro F, Stanton TR, O'Connell NE, Marinus J, et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology. 2013;80:106–17.
Shafer DM, Assael L, White LB, Rossomando EF. Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J Oral Maxillofac Surg. 1994;52:786–91. discussion 791–2.
Lasselin J, Kemani MK, Kanstrup M, Olsson GL, Axelsson J, Andreasson A, et al. Low-grade inflammation may moderate the effect of behavioral treatment for chronic pain in adults. J Behav Med. 2016;39:916–24.
Wang H, Ahrens C, Rief W, Gantz S, Schiltenwolf M, Richter W. Influence of depression symptoms on serum tumor necrosis factor-α of patients with chronic low back pain. Arthritis Res Ther. 2010;12:R186.
Appleton CT, Hawker GA, Hill CL, Pope JE. Editorial: “weighing in” on the Framingham osteoarthritis study: measuring biomechanical and metabolic contributions to osteoarthritis. Arthritis Rheumatol. 2017;69:1127–30.
Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum. 2009;60:2037–45.
Scott A, Zwerver J, Grewal N, de Sa A, Alktebi T, Granville DJ, et al. Lipids, adiposity and tendinopathy: is there a mechanistic link? Critical review. Br J Sports Med. 2015;50:984–8.
Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum. 2009;61:840–9.
Acknowledgements
TPW is funded by a Nursing and Allied Health Scholarship and Support Scheme funded by the Commonwealth Department of Health and administered by Services for Australian Rural and Remote Allied Health. JBA is currently supported by a National Health and Medical Research Council Early Career Research Fellowship (ID: 1120560).
We would like to thank Mr. Pawel Skuza, consultant statistician, for his advice on the data analysis.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
TPW, JBA, AME, AY and EMS conceived the study and its design. TPW, JBA and RAD undertook the database searching and initial analysis. TPW drafted the initial manuscript, JBA and AY provided critical appraisal of the first draft and all authors provided input into the review draft and agreed on the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Ovid Medline search strategy. Example of database search strategy. (DOCX 73 kb)
Additional file 2:
Quality assessment. Quality assessment of the articles included in the systematic review. (DOCX 140 kb)
Additional file 3:
Reasons for exclusion from meta-analysis. Reasons for exclusion of cross-sectional studies from the meta-analyses. (DOCX 135 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Walsh, T.P., Arnold, J.B., Evans, A.M. et al. The association between body fat and musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet Disord 19, 233 (2018). https://doi.org/10.1186/s12891-018-2137-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-018-2137-0