Abstract
Background
The number of hip fractures and resulting post-surgical outcome are a major public health concern and the incidence is expected to increase significantly. The acute recovery phase after hip fracture surgery in elder patients is often complicated by severe pain, high morphine consumption, perioperative blood loss with subsequent transfusion and delirium. Postoperative continuous-flow cryocompression therapy is suggested to minimize these complications and to attenuate the inflammatory reaction that the traumatic fracture and subsequent surgical trauma encompass. Based on a pilot study in patients undergoing total hip arthroplasty for osteoarthritis, it is anticipated that patients treated with continuous-flow cryocompression therapy will have less pain, less morphine consumption and lower decrease of postoperative hemoglobin levels. These factors are associated with a shorter hospital stay and better long-term (functional) outcome.
Methods/design
One hundred and sixty patients with an intra or extracapsular hip fracture scheduled for internal fixation (intramedullary hip nail, dynamic hip screw or cannulated screws) or prosthesis surgery (total hip or hemiarthroplasty) will be included in this prospective, open-label, parallel, multicenter, randomized controlled, clinical superiority trial. Patients will be allocated to two treatment arms: group ‘A’ will be treated with continuous-flow cryocompression therapy and compared to group ‘B’ that will receive standard care. Routine use of drains and/or compressive bandages is allowed in both groups. The primary objective of this study is to compare acute pain the first 72 h postoperative, measured with numeric rating scale for pain. Secondary objectives are: (non-) morphine analgesic use; adjusted postoperative hemoglobin level; transfusion incidence; incidence, duration and severity of delirium and use of psychotropic medication; length of stay; location and duration of rehabilitation; functional outcome; short-term patient-reported health outcome; general and cryotherapy related complications and feasibility.
Discussion
This is the first randomized controlled trial that will assess the analgesic efficiacy of continuous-flow cryocompression therapy in the acute recovery phase after hip fracture surgery.
Trial registration
www.trialregister.nl, NTR4152 (23rd of August 2013)
Similar content being viewed by others
Background
Hip fractures are one of the most important causes of long-term disability and a significant public health issue [1]. Incidence varies on a global scale [2, 3] but incidence is estimated to vary between 414 and 957 per 100,000 inhabitants in the USA [4]. Due to aging of the population in general a significant increase in hip fracture numbers is expected [5].
Hip fractures are a serious condition associated with high morbidity, one-year mortality rates up to 29 % [2, 4], severe pain [6, 7] and significant decline in functional status [8–11]. Less than 50 % of (semi-) independent living elders return to their pre-fracture habitat [11, 12]. Among the most serious of complications is the onset of delirium, which has a particular high incidence of 45 % in hip fracture populations [13–15]. Possibly because this condition is known to be extremely painful [15–18] and patients with pain are nine times more likely to develop a delirium [18–20] and furthermore take longer to ambulate and consequently, have longer length of stay [9].
Upon admission 40 % of hip fracture patients is anemic [21] and observed intraoperative blood loss is systematically underestimated; up to six-fold in excess in extracapsular fractures [22]. The continued postoperative decline in hemoglobin levels results from, among others, surgical site bleeding [22]. This yields a specifically high transfusion rate in this frail elderly patient category where use anticoagulants or thrombocyte aggregation inhibitors is relatively prevalent [23]. Besides (postoperative) surgical site bleeding, the fracture trauma and subsequent surgical stabilization procedure induce a inflammatory response causing leakage of plasma proteins and migration of inflammatory cells that lead to local peripheral vasodilatation and increased capillary permeability, ultimately causing edema [24]. Edema gradually develops during the first week post surgery, is more pronounced in intertrochanteric fractures and correlates well with reduced knee extension strength and functional performance [25, 26]. In an attempt to reduce edema and postoperative hemorrhaging compressive wound dressings are applied [27–29]. Dynamic pneumatic compressive devices not only reduce blood loss, edema and offer a better (hemo) dynamic profile in the deep venous and lymph system, it also exerts analgesic effects and reduces the inflammatory response when combined with a cryotherapy adjunct [30–35].
In a pilot study on 30 patients in a Dutch teaching hospital an apparatus with continuous-flow cryotherapy combined with intermittent compression was used in the postoperative setting of hip arthroplasty for end-stage osteoarthritis [36]. A trend towards lower visual analogue scale (VAS) pain scores and less morphine use was observed, and patients receiving continuous-flow cryocompression (CFC) therapy had statistical significant less decline in postoperative hemoglobin levels. In two randomized controlled trials evaluating continuous-flow cryotherapy (without a compression adjunct) in 45 total hip [37] and 208 total hip and knee arthroplasty [38] patients, lower pain scores were observed and less morphine was used. Furthermore length of stay was 1.4 days shorter when continuous-flow cryotherapy was applied after hip arthroplasty in 74 patients [39].
Currently all published trials studying CFC therapy focus on semi-elective procedures such as anterior cruciate ligament reconstruction and total knee arthroplasties [40]. To our knowledge, no randomized controlled trial exists evaluating the efficacy of CFC therapy in the acute postoperative recovery phase of hip fracture patients [40]. As hip fracture patients have duplicate trauma, severe pain, fracture site bleeding with related inflammation and associated soft tissue damage they are expected to benefit most from CFC therapy.
Aim
The aim of the current study is to evaluate the efficacy of CFC therapy on pain in the first 72 postoperative hours of hip fracture patients. The secondary aim is to evaluate the effects on (non-) morphine analgesic use; postoperative hemoglobin level; transfusion incidence; delirium incidence and severity; use of psychotropic medication; length of stay; short-term location and duration of rehabilitation; functional outcome; short-term patient-reported health outcome; general and cryotherapy related complications. Furthermore the feasibility of a cryocompression device on orthopedic/surgery wards is assessed.
We hypothesized that: 1) CFC therapy will lower perceived pain levels and morphine consumption; and 2) will reduce postoperative blood loss and transfusion incidence; and 3) reduced pain by CFC therapy will lead to lower delirium incidence and 4) enhance functional recovery, leading to shorter length of stay in postoperative hip facture patients.
Methods
Study design
This study is designed as a prospective, open-label, parallel, multicenter, 1:1 randomized controlled, clinical superiority trial in accordance with CONSORT and SPIRIT guidelines [41, 42]. Eight orthopedic, surgery and/or geriatric departments of three middle sized teaching hospitals and one academic hospital in the Netherlands will participate: The Spaarne Gasthuis, Hoofddorp and Haarlem; Noordwest Ziekenhuisgroep, Alkmaar; Amstelland Hospital, Amstelveen; VU University Medical Center, Amsterdam.
Randomization
A balanced 1:1 block (size six) randomization stratified by center will be performed directly after surgery, using Research Manager (RM; version 4.5.0.1), a web-based computer program (Fig. 1). A research nurse who is not involved in the study generates the allocation sequence with RM. Intramedullary hip nail (IMHN), dynamic hip screw (DHS) and cannulated screws are grouped for block randomization, as are total hip arthroplasty (THA) and hemiarthroplasty (HA). The intervention group ‘A’ will receive CFC therapy postoperative; the control group ‘B’ will receive standard care. Due to the nature of the study no blinding can exist as patients, family and physicians notice the use and settings of CFC therapy. Physicians randomize participants with a digital logon to the web-based system or the physician can contact the coordinating investigator and he will randomize the included participant (see Table 1 for criteria).The Informant Questionnaire for the Cognitive Decline in the Elderly (IQ-CODE) questionnaire is administered when the clinician has doubts about the cognitive state that may compromise CFC therapy sessions or study measurements (Table 1). Ideally a MMSE would be used for this purpose, however due frequently perceived pain at the accident and emergency A&E department and consequently administered narcotic analgesics, the hetero anamnestic IQ-CODE is used instead. The coordinating investigator contacts the participating hospitals on a daily basis by telephone or physically to ensure adequate and appropriate enrollment of participants and completeness of data. A list of excluded patients will be drafted.
GRAPES-trial flowchart. * Patient enrollment is allowed up to 6 h postoperative. IMHN: intramedullary hip nail; DHS: dynamic hip screw; screws: cannulated screws; THA: total hip arthroplasty; HA: hemiarthroplasty; SF-12: Short Form-12; EQ-5D-3L: EuroQol; TUGT: Timed Up and Go Test; DEMMI: De Morton Mobility Index; SPPB: Short Physical Performance battery
Standard care
On arrival at the accident and emergency (A&E) department patients receive acetaminophen intravenously, diclofenac (if not contraindicated) and morphine subcutaneously or fentanyl intravenously until numerical rating scale (NRS)-pain scores have dropped below 4. Upon admission the local protocol is started (Table 2). At all centers the 'as needed' medication is administered when the NRS-pain score is 4 or higher and no excessive (opioid-induced) sedation is present. Patients who are deemed able are given intravenous patient controlled analgesia (PCA) pumps with morphine (Table 2). In patients older than 70 years the bolus setting is reduced by 50 %. Preferably patients are operated on using spinal anesthesia. In order to put the patient in an upright position for spinal anesthesia administration femoral blocking with short-acting analgesics can be used. All centers use bupivacaine 0.5 % between 2.0 and 3.0 ml administered at the lumbar level. Additional (long acting) analgesics administered during surgery are noted. The participating centers adhere to the Dutch national guidelines for surgical technique for the various fracture types [43]. Postoperatively, on the day of surgery or the first postoperative day physical therapy is commenced once or twice daily. Physical therapy sessions are usually 30 min long. In the period in which the focus of this study lies physical therapy focuses on strengthening of quadriceps and gluteal musculature, walking and making transfers.
Study apparatus and treatment schedule
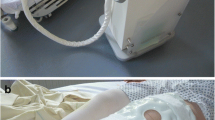
Continuous-flow cryocompression therapy is applied by using the ‘Game Ready system’ (GRS; CoolSystems Alameda, California). Through an anatomically designed hip/groin wrap covering most of the thigh and pelvis up to the iliac crest, the GRS simultaneously delivers both adjustable continuous-flow cryotherapy and intermittent compression through a portable control unit filled with ice and water. The machine has four pressure settings: no pressure, low pressure (5–15 mm Hg), medium pressure (5–50 mm Hg) and high pressure (5–75 mm Hg). Temperature can be adjusted between 4 and 13 °C and is indicated by one, two or three snowflakes. The lowest temperature is started and maintained throughout the study if feasible. Pressure is started at ‘low’ and is increased one step per 4 treatments (Table 3). Depending on the end of surgery patients will be categorized in three treatment schedules with respective start and end-times (Table 3). If patients are uncomfortable the appropriate adjunct will be adjusted stepwise until a comfortable setting is reached, deviations are noted. Adjustments are recorded and comfortable settings are maintained according to the discomfort flowchart (Fig. 2). Patients will be treated between 10 and 12 times during the first 72 postoperative hours, each cycle lasting 30 min. Preferably, treatment cycles and control measurements during the first 72 postoperative hours are performed at fixed moments: 8:00 h, 12:00 h, 16:30 h, and 21:30 h. The GRS-wrap is only in place when CFC therapy is administered and applied/removed by the nurse.
Co-interventions
In case of discomfort the appropriate adjunct is adjusted accordingly (Fig. 2) and supplemental analgesics are given as needed (Table 2). In both the intervention group receiving CFC therapy and in the control group no restrictions are made towards regularly used static compressive bandages and/or wound drains (with or without autologous re-infusion). Geriatricians are consulted in a standard fashion in patients aged 70 years and over.
Admission
Patient demographic data are noted on admission as well as American Society of Anesthesiologists (ASA) class, current NRS-pain, delirium observation screening (DOS)-score, activities of daily living (ADL)-Katz score and the New Mobility Score (NMS; Table 4) [44–46]. Time of injury and arrival at the A&E department is noted. Patient delay intervals longer than 6 h are actively explored and reasons for delay are clarified e.g. if a patient was prone and unable to call emergency services.
A standard preoperative venous blood sample is taken in which hemoglobin, hematocrit and mean corpuscular volumes values are measured (Table 4). Upon admission the local hospital analgesic and antithrombotic protocol is started (Table 2). A geriatrician is consulted preoperatively in patients who are older than 70 years and will focus on: polypharmacy, risk reduction of falls, prevention and/or treatment of delirium, optimization of nutritional status. The ADL-Katz score and delirium risk assessment score (DRAS) [47] are completed upon admission; furthermore precipitating factors for delirium (bladder infection and/or retention, fixation, sleep deprivation, electrolyte abnormalities including renal function impairment) are noted throughout the hospital stay. After the operation the surgeon notes the intraoperative blood loss, the type of implant, if applicable intraoperative complications, randomizes the patient and determines the patient’s treatment schedule.
Cryocompression therapy and clinical measurements
Intervention patients start with CFC therapy 6 h postoperative, until 72 h postoperatively. A trained ward nurse will conduct the pain assessments when the patient is in bed or stationary in a chair for at least five minutes. Directly before and directly after CFC therapy vital signs including NRS-pain are measured by the ward nurse, when the patient is prone in bed for at least five minutes before commencing and five minutes after cessation of CFC therapy. In control patients only NRS-pain is measured at the same time as intervention patients would normally be assessed. All pain measurements are continued throughout the first postoperative 72 h for control and intervention patients alike (Table 4). Three times daily DOS-scores [48] will be obtained; in case of a DOS-score higher or equal to three, geriatricians diagnose and subsequently monitor delirium severity with the delirium rating scale revised 1998 (DRS-R-98) [49] on a daily basis or until the score drops below 12.25 out of 39 (Table 4). Time and amount of administered (psychotropic) medication will be documented. The nurse and ward doctor inspect the wound and dressing in accordance with routine care. Blood samples are taken at postoperative day one and three, and post-transfusion if applicable (Table 4). The timed up and go test (TUGT) is performed before discharge (Table 4). The TUGT will be administered in all patients who are able. If no weight bearing is allowed or patients are physically unable, than the test will be postponed to the outpatient visit. The mini mental state examination (MMSE) is administered for data stratification purposes [50–52], if patients are delirious during admission the MMSE is postponed to the outpatient visit.
In order to document experiences with CFC therapy all treatment patients will fill in a questionnaire at discharge, which is specifically drafted for this study (Table 5). During the study a booklet will be made available into which staff can write down prevalent occurring technical GRS-related problems.
Outpatient visit
At the single outpatient study visit between six to eight weeks, the following parameters are assessed; current NRS-pain; analgesic use; mobility status by the TUGT, de Morton Mobility Index (DEMMI) and the Short Physical Performance Battery (SPPB); current living situation (rehabilitation or at home); wound status and complications (thrombosis, consolidation, infection, readmission). Furthermore the self-assessment health questionnaires EuroQol(EQ)-5D-3 L and Short Form (SF)-12 are completed. If not administered during admission the MMSE is completed (Table 4).
Data collection and handling
Upon inclusion patient characteristics are entered in RM and postoperative randomization takes place. A unique patient number is generated by RM, which will be used for further data collection on preformatted case report forms (CRF’s). Only the coordinating investigator has access to the key-file that can identify patients from RM-generated patient numbers, it will be stored at the coordinating center throughout and after completion of the trial and will not be shared. The Spaarne Gasthuis is the unrestricted owner of the final dataset; no contractual agreements exist in regard to publication policies. No interim analysis will be performed.
Data management team
The coordinating center comprises of: the principal investigator, the coordinating investigator, a research nurse, a research assistant, a clinical epidemiologist and statistician. The research assistant enters data in RM. The research nurse can be contacted via telephone to solve any (acute) problems that might occur and enters data in RM. The epidemiologist and statistician assist with data analyses and drafting of the manuscript. During the enrollment phase of the trial the coordinating investigator meets bimonthly with the individual principal investigators of the respective centers to assess the current inclusion rate, data quality, protocol adherence and prevalent occurring problems. During enrollment two meetings are planned to collectively assess problems and deliberate on how to instigate solutions to these problems. All participating personnel are updated on a monthly basis via a newsletter in which current inclusion rates are presented, and to point out points of attention and solutions to frequently observed problems. During physical visits CRF’s are collected, assessed for completeness and missing data is retrieved from patient medical records where possible; secondly the appropriateness of administered treatments and (functional) tests is assessed.
Data monitoring committee
Two independent data monitors are instigated and will monitor the trial at two of the five sites where highest inclusion rates are expected. Results of the monitor will be discussed in a committee independently of the investigators and the investigators will be informed both orally and in writing. Since the overall risk of participation in the trial is considered low by the REC/IRB no external safety overseers are required by national guidelines. If selected, external auditing is possible.
Outcome parameters
NRS-pain (primary)
The verbally administered 11-point (range 0–10) NRS-pain is a widely accepted assessment tool and has been validated in many different conditions, among is acute pain in the emergency department [53–55]. Reliability and validity has been thoroughly studied in (cognitively impaired) elderly and has shown to maintain acceptable clinometric characteristics [56–58]. NRS-pain scores are compared within the intervention group i.e. pre and post-treatment and compared between the intervention and control groups. Cumulated pain scores will be calculated for both groups by adding the (post-treatment) pain scores of the first 72 postoperative hours.
Analgesic use
All postoperative oral and parenteral administered narcotic analgesics will be converted and added up using accepted algorithms [59, 60] and compared after the last treatment day, at discharge and at the outpatient clinic. Total milligrams of acetaminophen and non-steroidal anti-inflammatory drugs (NSAID’s) are reported in the same fashion. At the outpatient visit patients will be asked about their current pain medication use.
Postoperative blood loss and transfusion incidence
The hemoglobin values from the obtained blood samples at postoperative day one and three will separately be detracted from the preoperative concentration and postoperative day three will be detracted from postoperative day one. An adjustment will be made for the intraoperative blood loss. Indications for transfusion are managed according to the Dutch national blood transfusion guideline [61]. Normovolemic ASA-one patients older than 60 years who lose blood at a single locus will be transfused at 5 mmol/l (8 g/dl). Patients unable to elevate cardiac output for hemodilution are transfused at 6 mmol/l (9.6 g/dl). Incidence of erythrocyte transfusion is noted and compared between groups.
Postoperative delirium
The DRS-R-98 is considered to be a valid and reliable instrument for delirium diagnosis and documenting delirium severity [49, 62]. It takes a trained nurse or physician five to ten minutes to assess the patient and comprise the DRS-R-98 score, and is ideal for longitudinal delirium follow-up. The sensitivity and specificity of the scale are reported to be 92 % and 86 % respectively. DRS-R-98 scores are compared within and between groups, and newly started psychotropic medication is compared.
Length of stay
Admission time is measured and expressed in two ways: from arrival time at the A&E department until discharge and from end of surgery to until discharge. The discharge criteria in all participating centers are: NRS-pain score below 5 without need for parenteral analgesics and an Elderly Mobility Scale (EMS) score 14 out of 20 or higher [63, 64]. If this some items were deemed unobtainable EMS scores are completed until transfer to a nursing home is arranged. If family members are able to foresee in certain home care aspects the patient can be discharged to his/her home. The fulfillment of this care by family is noted and corrected for afterwards.
Functional outcome
Timed Up and Go Test
The TUGT is a functional mobility test that measures the time it takes a patient to get up out of a chair, walk 3 m and return in a sitting position. The test is easy to use and has sufficient clinometric characteristics [65], can predict short-term risk of new falls [66] and functional outcome over time [67]. Detailed instructions are provided to the patient: rise from the chair (knees at 90° flexion) when you hear “go”, walk at safe speed to the mark on the floor and back, the time stops when you hit the chair with your buttocks. The physical therapist will demonstrate how to perform the TUGT once. The average of three tests will be the final TUGT outcome and no trial run is performed. Postoperative mobilization policy is noted and patients are stratified in three groups: no weight bearing, partial weight bearing or full weight bearing. Preferably no walking aid will be used during the test but if needed, the patient can use any walking aid available, the type of aid used will be recorded. The updated NMS is administered at inclusion for data stratification purposes [44, 45, 68]. NMS will be classified as low when NMS is 2-6 and scored high when NMS is 7–9 [46].
De Morton Mobility Index
The DEMMI is a measure of mobility that has been validated in hip fracture patients [69-71]. The DEMMI is administered by physician or physical therapist observation of the test subject’ physical performance, measured in 15 hierarchical domains (three bed, three chair, four static balance, two walking and three dynamic balance items), each measured on a two (able/unable) or three (able/partial/unable) point scale. It takes a trained person ten minutes to administer the test in elderly patients. The raw score is converted to an interval score.
Short Physical Performance Battery
The SPPB is composed of three tasks: a hierarchical balance task, a short walk at normal speed, and five repetitive chair stands. Low scores in the SPPB have predictive value for a wide range of health outcomes: mobility loss, disability, hospitalization, length of hospital stay, nursing home admission, and death [72-75]. Normative values of SPPB have been published for representative populations by five-year age groups and sex [73]. The strong and consistent association with health status measures demonstrated the validity of the SPPB [76].
Patient reported outcome measures
EQ-5D-3L
The EuroQol/EQ-5D-3L is a validated, generalized and standardized instrument comprising a VAS measuring self-rated health and a health status instrument, consisting of a three-level response (no problems, some problems and extreme problems) for five domains related to daily activities; (i) mobility, (ii) self- care, (iii) usual activities, (iv) pain and discomfort and (v) anxiety and depression. Responses to the health status classification system are converted into an overall score using a published utility algorithm for the Dutch population [77]. A respondent’s EQ-VAS gives self-rated health on a scale where the endpoints are labeled ‘best imaginable health state’ (100) and ‘worst imaginable health state’ (0).
Short Form-12
The SF-12 is the abbreviated version of the original SF-36 and scores quality of life in two domains, physical and mental health. It has shown good correlation with the SF-36 and proved valid in many conditions, amongst is orthopedic surgery [78].
Complications
The surgeon monitors all complications that may occur throughout admission, in general or cryotherapy-related. After discharge the coordinating investigator verifies if all adverse events are registered and recorded on the appropriate CRF. In case of serious adverse events relating to the treatment patients will no longer receive treatment but will remain in the study for follow-up, if feasible. Severe leakage i.e. multiple bandage swaps daily is noted and registered as an adverse event. Furthermore an intragroup analysis of vital signs is made to determine possible central effects of the administered therapy. Finally, at the outpatient visit patients are assessed for adverse events that may have occurred during rehabilitation. Additional to the patient questionnaire (Table 5), intervention patients are asked about their experiences with CFC therapy. An additional insurance is taken out to financially compensate participants if applicable. Adverse events are tabulated and reported in the final manuscript.
Feasibility
At the end of the study nursing staff will complete questionnaires specifically drafted for this study (Table 6) and the booklet of prevalent technical problems will be evaluated. A close out visit will be planned in which recommendations for further improvement of the machine will be discussed. Treatment failure i.e. discontinuation of treatment is reported and provided with reasons e.g. discomfort.
Statistics
Analysis of outcome parameters
All statistical analyses will be computed using the SPSS statistical package (IMB SPSS, Inc., Release 20.0.0.0, 64-bit edition). Statistical analysis will be performed according to the intention-to-treat principle. Baseline characteristics will be described in accordance with CONSORT guidelines [41] using means and standard deviation in case of normal distribution, and medians and interquartile ranges otherwise. Continuous variables will be checked for normality, visually and by using the Shapiro Wilk test. Our primary analysis focuses on the differences in NRS-pain scores between the study groups 24 h postoperative and will be analyzed by use of Student’s T or Mann–Whitney U tests in case of skewed distribution. To assess treatment effect over time, a mixed model analysis for repeated measures will be performed. This model allows missing data and adjustment for serious confounders and interactions. In case of skewed distributions and outliers non-parametric variants will be used (e.g. Mann Whitney U or Friedman tests). Secondary continuous outcome measures (EQ-5D-3L, SF-12, DRS-R-98 and hemoglobin) will be compared by use of Student’s t or Mann Whitney U tests. Ordinal variables will be analyzed using the Mann–Whitney test. Chi-squared tests will be performed in case of categorical variables. In case of important confounding, analysis will be adjusted to correct for these factors (by use of multiple regression analysis, linear or logistic). A p-value of < 0.05 will be considered statistically significant.
Sample size calculation
Sample size calculation is based on the primary outcome measure, NRS-pain at 24 h postoperatively. Subsequently, a mean NRS-pain score for postoperative day one was drawn from the Spaarne Gasthuis electronic patient dossier digital databanks and a standard deviation of 2.2 was calculated for pooled internal fixation and (hemi) arthroplasty for cervical and peritrochanteric fractures. The minimal clinical relevant difference for NRS-pain is 1.3 out of 10 [53]. The alpha was set at 5 % with a power of 90 % due to anticipated heterogeneity in regard to operating techniques, general care protocols but predominantly in regard to pain protocols between the participating centers (Table 2). An expected NRS failure rate of 10 % is anticipated together with expected missing data a drop out of 22.3 % is computed. The total number of included patients will be 160.
Dissemination policy
The trial outcome will be published as an international peer-reviewed article. Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the patient or may affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures, or significant administrative aspects will require a formal amendment to the protocol. Such amendment will be agreed upon by the REC/IRB and will be reported to www.trialregister.nl and to this journal.
Discussion
This multicenter study will evaluate the efficacy of intermittent application of continuous-flow cryocompression therapy administered in the first 72 postoperative hours after hip fracture surgery. Due to the multicenter design of the study the results can easily be translated to the general care of hip fracture patients. Conversely, some inequality in local hospital pain protocols is present but conversion through accepted algorithms [59, 60] make comparison feasible. Spinal anesthetized patients could have advantages over the general anesthesia group in the acute hours post-surgery in regard to pain scores and analgesic use since the effect of the latter ends immediately after surgery in contrast to the former that takes a few hours to wear off completely.
The varying types of fractures have different characteristics and demand a different surgical approach. Peritrochanteric fractures quite often have more extensive bony trauma and less soft-tissue trauma, and after surgical stabilization with a DHS or IMHN fracture micro-motion leads to prolonged and greater dynamic pain [6]. Conversely, in (hemi) arthroplasty the fracture-site is removed completely at the expense of increased (surgical-induced) soft-tissue trauma. Hence the efficacy of CFC therapy is likely to vary in patients with varying extent of soft-tissue trauma. Therefore stratification is implemented according to type of surgery that divides patients with greater surgical trauma ((hemi) arthroplasty) and lesser surgical trauma (cannulated screws, DHS, IMHN).
By nature, delirium is known to fluctuate over the day and a single DRS-R-98 measurement might not reach sufficient sensitivity for diagnosis. However with longitudinal follow-up of three times daily DOS-scores this problem is overcome for the most part. Delirium severity is assessed with more information gathered from family and personnel, over the past 24 h thereby making assessment adequate.
Currently three of the four participating hospitals have a geriatric trauma unit, in one of the hospitals with a geriatric trauma unit the care of the surgical geriatric patient is transmitted to the geriatrician in full except for wound assessments and related care. At this ward all patients receive prophylactic haloperidol 1 mg daily, and more if needed. At the hospital without a geriatric trauma unit a geriatrician is not readily available and unable to attend the clinic on a daily basis. Confounding of secondary outcomes as delirium, psychotropic medication and functional outcome can therefore not be ruled out.
When compared to the preoperative values, hemoglobin levels in collected blood samples at postoperative day one are usually much lower than one would expect from the observed blood loss during surgery. This gap called ‘the hidden blood loss’ is thought to arise from, among others gastro-intestinal bleeding [22, 79]. The amount of (hidden) blood loss is more severe when oral anticoagulants or thrombocyte aggregation inhibitors compromise a patient’s hemostasis and this loss may continue throughout the early postoperative period. Hemoglobin measured at postoperative day one is most likely, as opposed to the preoperative values, significantly diluted, thereby underestimating the actual hemoglobin concentration. Combined with the possibility of hidden blood loss throughout the hospital stay, care has to be taken when comparing the value of postoperative day one to day three.
The functional outcome assessment of patients who are not allowed to bear weight at the time of discharge is postponed to the outpatient clinic. At this visit they are allowed to bear weight for the first time in weeks. Testing directly after weight bearing for the first time can introduce confounding.
Finally, care has to be taken to interpret the allocation to rehabilitation facilities. For instance patients who have sufficient family members who can help out at home are more likely to be discharged home with or without homecare rather than be assigned to a rehabilitation clinic or nursing home with rehabilitation facilities.
Conclusion
The present study will provide evidence for the efficacy of continuous-flow cryocompression therapy applied after hip fracture surgery. Due to the duplicate trauma this condition encompasses these patients, who are generally aged 70 years and over, are expected to benefit most. Furthermore treatment feasibility is assessed and consequently, recommendations are made about which settings are best to employ.
Ethics and consent to participate
Permission has been obtained from the 'Medisch Ethische Toetsingscommissie Noord-Holland' regional medical ethics committee (NL45657.094.14) and the trial is registered at www.trialregister.nl on the 23rd of August 2013 with trial number: “NTR4152”. Patients with an intra or extracapsular hip fracture who meet the inclusion criteria and without apparent exclusion criteria (Table 1) are informed about the study both orally and in writing by the assessing physician e.g. the local ward doctor, staff member or consulting geriatricians. Depending on operating room capacity and patient characteristics patients will either first be transferred to the ward or will go directly to the operating room. Patients can be included pre and postoperatively within 6 h post surgery, before the first treatment starts 6 h postoperative (Fig. 1). Maximum time for consideration to participate in the study is set at 12 h. Patients approving to participate need to sign the informed consent form after which baseline characteristics are noted.
Consent to publish
Not applicable.
Availability of data and materials
Not applicable as this is a protocol of a study and no data are reported.
Abbreviations
- A&E:
-
accident and emergency
- ASA:
-
American Society of Anesthesiologists
- CFC:
-
continuous-flow cryocompression
- CRF:
-
case report form
- DEMMI:
-
de morton mobility index
- DHS:
-
dynamic hip screw
- DOS:
-
delirium observation screening
- DRAS:
-
delirium risk assessment score
- DRS-R-98:
-
delirium rating scale revised 1998
- EMS:
-
elderly mobility scale
- EQ-5D:
-
EuroQol-5D
- GRS:
-
game ready system
- HA:
-
hemiarthroplasty
- IQ-CODE:
-
informant questionnaire for the cognitive decline in the elderly
- IRB:
-
institutional review board
- MMSE:
-
mini mental state examination
- NMS:
-
new mobility score
- NRS:
-
numeric rating scale
- NSAID’s:
-
non-steroidal anti-inflammatory drugs
- PCA:
-
patient controlled analgesia
- PROM:
-
patient reported outcome measure
- REC:
-
Regional ethics committee
- RM:
-
research manager
- SF-12:
-
short form-12
- SF-36:
-
short form-36
- SPPB:
-
short physical performance battery
- THA:
-
total hip arthroplasty
- TKA:
-
total knee arthroplasty
- TUGT:
-
timed up and go test
- VAS:
-
visual analogue scale
References
Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33.
Haleem S, Lutchman L, Mayahi R, Grice J, Parker M. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39:1157–63.
Dhanwal DK, Dennison EM, Harvey NC, Cooper C. Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop. 2011;45:15–22.
Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–9.
Rosengren B, Karlsson M. The annual number of hip fractures in Sweden will double from year 2002 to 2050: projections based on local and nationwide data. Acta Orthop. 2014;85:234–7.
Foss NB, Kristensen MT, Palm H, Kehlet H. Postoperative pain after hip fracture is procedure specific. Br J Anaesth. 2009;102:111–6.
Pasero C, McCaffery M. Orthopaedic postoperative pain management. J Perianesth Nurs. 2007;22:160–74.
Magaziner J, Hawkes W, Hebel JR, Zimmerman SI, Fox KM, Dolan M, Felsenthal G, Kenzora J. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55:M498–507.
Morrison RS, Magaziner J, McLaughlin MA, Orosz G, Silberzweig SB, Koval KJ, Siu AL. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303–11.
Feldt KS, Oh HL. Pain and hip fracture outcomes for older adults. Orthop Nurs. 2000;19:35–44.
Vochteloo AJH, Moerman S, Tuinebreijer WE, Maier AB, De Vries MR, Bloem RM, Nelissen RGHH, Pilot P. More than half of hip fracture patients do not regain mobility in the first postoperative year. Geriatr Gerontol Int. 2013;13:334–41.
Ariza-Vega P, Jiménez-Moleón JJ, Kristensen MT. Change of residence and functional status within three months and one year following hip fracture surgery. Disabil Rehabil. 2014;36:685–90.
Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267–73.
Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Forstl H. Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–55.
Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808–16.
Pitimana-Aree S, Visalyaputra S, Komoltri C, Muangman S, Tiviraj S, Puangchan S, Immark P. An economic evaluation of bupivacaine plus fentanyl versus ropivacaine alone for patient-controlled epidural analgesia after total-knee replacement procedure: a double-blinded randomized study. Reg Anesth Pain Med. 2005;30:446–51.
Zaslansky R, Eisenberg E, Peskin B, Sprecher E, Reis DN, Zinman C, Brill S. Early administration of oral morphine to orthopedic patients after surgery. J Opioid Manag. 2006;2:88–92.
Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5.
Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9:19–27.
Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81.
Halm EA, Wang JJ, Boockvar K, Penrod J, Silberzweig SB, Magaziner J, Koval KJ, Siu AL. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J Orthop Trauma. 2004;18:369–74.
Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg (Br). 2006;88:1053–9.
Swain DG, Nightingale PG, Patel JV. Blood transfusion requirements in femoral neck fracture. Injury. 2000;31:7–10.
Zhang L, Bail H, Mittlmeier T, Haas NP, Schaser K-D. Immediate microcirculatory derangements in skeletal muscle and periosteum after closed tibial fracture. J Trauma. 2003;54:979–85.
Kazmi SSH, Stranden E, Kroese AJ, Slagsvold C-E, Diep LM, Stromsoe K, Jorgensen JJ. Edema in the lower limb of patients operated on for proximal femoral fractures. J Trauma. 2007;62:701–7.
Kristensen MT, Bandholm T, Bencke J, Ekdahl C, Kehlet H. Knee-extension strength, postural control and function are related to fracture type and thigh edema in patients with hip fracture. Clin Biomech. 2009;24:218–24.
Hornberg I, Bengtsson A, Bergman B. Compression dressing after hip joint replacement reduces the need of allogeneic blood transfusion. Lakartidningen. 2002;99:397–9.
Johansson T, Engquist M, Pettersson L-G, Lisander B. Blood loss after total hip replacement: a prospective randomized study between wound compression and drainage. J Arthroplasty. 2005;20:967–71.
Schröder D, Pässler HH. Combination of cold and compression after knee surgery. A prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 1994;2:158–65.
Deal DN, Tipton J, Rosencrance E, Curl WW, Smith TL. Ice reduces edema. A study of microvascular permeability in rats. J Bone Joint Surg Am. 2002;84-A:1573–8.
Knobloch K, Grasemann R, Jagodzinski M, Richter M, Zeichen J, Krettek C. Changes of Achilles midportion tendon microcirculation after repetitive simultaneous cryotherapy and compression using a Cryo/Cuff. Am J Sports Med. 2006;34:1953–9.
Nadler SF, Weingand K, Kruse RJ. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician. 2004;7:395–9.
Schaser K-D, Disch AC, Stover JF, Lauffer A, Bail HJ, Mittlmeier T. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007;35:93–102.
Schaser K-D, Stover JF, Melcher I, Lauffer A, Haas NP, Bail HJ, Stöckle U, Puhl G, Mittlmeier TW. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61:642–9.
Scumpia PO, Sarcia PJ, Kelly KM, DeMarco VG, Skimming JW. Hypothermia induces anti-inflammatory cytokines and inhibits nitric oxide and myeloperoxidase-mediated damage in the hearts of endotoxemic rats. Chest. 2004;125:1483–91.
Leegwater NC, Willems JH, Brohet R, Nolte PA. Cryocompression therapy after elective arthroplasty of the hip. Hip Int. 2012;22:527–33.
Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19:334–7.
Albrecht S, Le Blond R, Köhler V, Cordis R, Gill C, Kleihues H, Schlüter S, Noack W. Cryotherapy as analgesic technique in direct, postoperative treatment following elective joint replacement. Z Orthop Ihre Grenzgeb. 1997;135:45–51.
Scarcella JB, Cohn BT. The effect of cold therapy on the postoperative course of total hip and knee arthroplasty patients. Am J Orthop. 1995;24:847–52.
Leegwater NC, Sierevelt I, Nolte PA. The efficacy of continuous-flow cryotherapy machines in the acute recovery phase after lower extremity surgery: a systematic review with meta-analysis of randomized controlled trials. OA Orthop. 2014;2:9.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55.
Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
NVvH. Guideline: Treatment of Proximal Femur Fractures in the Elderly Patient (Dutch: richtlijn: Behandeling van de proximale femurfractuur bij de oudere mens). 2010. http://www.kwaliteitskoepel.nl/assets/structuredfiles/NOV/Betrokkenbij/Behandeling%20van%20de%20proximale%20femurfractuur%20bij%20de%20oudere%20mens%202008.pdf.
Kristensen MT, Bandholm T, Foss NB, Ekdahl C, Kehlet H. High inter-tester reliability of the new mobility score in patients with hip fracture. J Rehabil Med. 2008;40:589–91.
Kristensen MT, Foss NB, Ekdahl C, Kehlet H. Prefracture functional level evaluated by the New Mobility Score predicts in-hospital outcome after hip fracture surgery. Acta Orthop. 2010;81:296–302.
Kristensen MT, Kehlet H. Most patients regain prefracture basic mobility after hip fracture surgery in a fast-track programme. Dan Med J. 2012;59:A4447.
Vreeswijk R, Kalisvaart KJ, Kalisvaart I, Van Gool WA, Eikelenboom P. Development and Validation of the Delirium Risk Assessment Score (DRAS). Age Ageing. in press.
Van Gemert LA, Schuurmans MJ. The Neecham Confusion Scale and the Delirium Observation Screening Scale: capacity to discriminate and ease of use in clinical practice. BMC Nurs. 2007;6:3.
Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–42.
Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O’Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer’s disease in ethnically diverse highly educated individuals: an analysis of the NACC database. J Gerontol A Biol Sci Med Sci. 2012;67:890–6.
Sallam K, Amr M. The use of the mini-mental state examination and the clock-drawing test for dementia in a tertiary hospital. J Clin Diagn Res. 2013;7:484–8.
De Boer C, Mattace-Raso F, Van der Steen J, Pel JJM. Mini-mental state examination subscores indicate visuomotor deficits in Alzheimer’s disease patients: a cross-sectional study in a Dutch population. Geriatr Gerontol Int. 2014;14:880–5.
Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–2.
Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–8.
Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–7.
Ware LJ, Epps CD, Herr K, Packard A. Evaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale, and Iowa pain thermometer in older minority adults. Pain Manag Nurs. 2006;7:117–25.
Pautex S, Herrmann F, Le Lous P, Fabjan M, Michel J-P, Gold G. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalized elderly demented patients. J Gerontol Ser A Biol Sci Med Sci. 2005;60:524–9.
Kaasalainen S, Crook J. A comparison of pain-assessment tools for use with elderly long-term-care residents. Can J Nurs Res. 2003;35:58–71.
Chung KC, Barlev A, Braun AH, Qian Y, Zagari M. Assessing analgesic use in patients with advanced cancer: development of a new scale--the Analgesic Quantification Algorithm. Pain Med. 2014;15:225–32.
Vissers KCP, Besse K, Hans G, Devulder J, Morlion B. Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10:85–93.
De Vries R, Haas F. English translation of the Dutch Blood Transfusion guideline 2011. Vox Sanguinis. 2012;103:363.
De Rooij SE, Van Munster BC, Korevaar JC, Casteelen G, Schuurmans MJ, Van der Mast RC, Levi M. Delirium subtype identification and the validation of the Delirium Rating Scale—Revised-98 (Dutch version) in hospitalized elderly patients. Int J Geriatr Psychiatry. 2006;21:876–82.
Smith R. Validation and reliability of the Elderly Mobility Scale. Physiotherapy. 1994;11:744–7.
Prosser L, Canby A. Further validation of the Elderly Mobility Scale for measurement of mobility of hospitalized elderly people. Clin Rehabil. 1997;11:338–43.
Kristensen MT, Henriksen S, Stie SB, Bandholm T. Relative and absolute intertester reliability of the timed up and go test to quantify functional mobility in patients with hip fracture. Journal of the American Geriatrics Society. 2011;59:565–7.
Kristensen MT, Foss NB, Kehlet H. Timed “up & go” test as a predictor of falls within 6 months after hip fracture surgery. Phys Ther. 2007;87:24–30.
Laflamme GY, Rouleau DM, Leduc S, Roy L, Beaumont E. The Timed Up and Go test is an early predictor of functional outcome after hemiarthroplasty for femoral neck fracture. J Bone Joint Surg Am. 2012;94:1175–9.
Kristensen MT, Foss NB, Kehlet H. Factors with independent influence on the “Timed Up and Go” test in patients with hip fracture. Physiother Res Int. 2009;14:30–41.
De Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2012;35:1–9.
De Morton NA, Davidson M, Keating JL. The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes. 2008;6:63.
De Morton NA, Davidson M, Keating JL. Validity, responsiveness and the minimal clinically important difference for the de Morton Mobility Index (DEMMI) in an older acute medical population. BMC Geriatr. 2010;10:72.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31.
Penninx BWJH, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7.
Volpato S, Cavalieri M, Guerra G, Sioulis F, Ranzini M, Maraldi C, Fellin R, Guralnik JM. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008;63:1393–8.
Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health. 2012;24:863–78.
Hoeymans N, Van Lindert H, Westert GP. The health status of the Dutch population as assessed by the EQ-6D. Qual Life Res. 2005;14:655–63.
Gosling CM, Gabbe BJ, Williamson OD, Sutherland AM, Cameron PA. Validity of outcome measures used to assess one and six month outcomes in orthopaedic trauma patients. Injury. 2011;42:1443–8.
Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg (Br). 2004;86:561–5.
Acknowledgements
None.
Funding
The cryocompression devices and prerequisites are sponsored by Best Medical Recovery systems (Abcoude, The Netherlands). During the enrollment of the trial freezers, ice cubes and clean wraps are supplied at the investigators’ request. Upon completion of the trial all material are returned to Best Medical recovery systems. ‘Achmea Zorgverzekeringen’ (grant number: Z557) and ‘DSW Zorgverzekeringen’ (grant identifier: GRAPES) funded the study. The funding covers meetings, central organizational costs and CFC treatment of patients only. The design, management, analysis and reporting of the study are conducted entirely independent of the aforementioned sponsors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NL, PN, FB, NK, AP and AM made substantial contributions to conception and design of the study. NL was responsible for drafting this manuscript. The other authors (including the aforementioned) have given valuable advice and comments on this manuscript. All of the authors will encourage their residents to include patients and NL will oversee the appropriateness of included patients and data collection. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Leegwater, N.C., Nolte, P.A., de Korte, N. et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord 17, 153 (2016). https://doi.org/10.1186/s12891-016-1000-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-016-1000-4