Abstract
Background
Type 2 diabetes (T2D) leads to serious respiratory problems. This study investigated the effectiveness of high-intensity interval training (HIIT) on T2D-induced lung injuries at histopathological and molecular levels.
Methods
Forty-eight male Wistar rats were randomly allocated into control (CTL), Diabetes (Db), exercise (Ex), and Diabetes + exercise (Db + Ex) groups. T2D was induced by a high-fat diet plus (35 mg/kg) of streptozotocin (STZ) administration. Rats in Ex and Db + Ex performed HIIT for eight weeks. Tumor necrosis factor-alpha (TNFα), Interleukin 10 (IL-10), BAX, Bcl2, Lecithin, Sphingomyelin (SPM) and Surfactant protein D (SPD) levels were measured in the bronchoalveolar lavage fluid (BALF) and malondialdehyde (MDA) and total antioxidant capacity (TAC) levels were measured in lung tissue. Lung histopathological alterations were assessed by using H&E and trichrome mason staining.
Results
Diabetes was significantly associated with imbalance in pro/anti-inflammatory, pro/anti-apoptosis and redox systems, and reduced the SPD, lecithin sphingomyelin and alveolar number. Performing HIIT by diabetic animals increased Bcl2 (P < 0.05) and IL10 (P < 0.01) levels as well as surfactants components and TAC (P < 0.05) but decreased fasting blood glucose (P < 0.001), TNFα (P < 0.05), BAX (P < 0.05) and BAX/Bcl2 (P < 0.001) levels as well as MDA (P < 0.01) and MDA/TAC (P < 0.01) compared to the diabetic group. Furthermore, lung injury and fibrosis scores were increased by T2D and recovered in presence of HIIT.
Conclusion
These findings suggested that the attenuating effect of HIIT on diabetic lung injury mediated by reducing blood sugar, inflammation, oxidative stress, and apoptosis as well as improving pulmonary surfactants components.
Graphical Abstract
Type 2 diabetes increased inflammation, oxidative stress and apoptosis and reduced pulmonary surfactants , while high intensity training improved these negative effects

Similar content being viewed by others
Introduction
Diabetes is a metabolic disorder that is associated with continuous hyperglycemia and abnormal metabolism of carbohydrates, proteins, and lipids, caused by insufficient insulin secretion, and decreased tissue sensitivity to insulin [1]. According to the World Health Organization (WHO) in 2016, the prevalence of diabetes was 171 million people in 2000, and it is projected to reach 366 million people by 2030. Type 2 diabetes (T2D) accounts for 90–95% of all cases, where the body becomes resistant to insulin, and insulin cannot enter cells [2]. Hyperglycemia disrupts the oxygen balance and cellular regeneration, increasing the gene expression of cytokines, and inflammation, and inducing apoptosis [3].
The lung is one of the organs that are damaged in T2D. NADH/NAD imbalance increases the production of reactive oxygen species (ROS), oxidative stress, and cell death in diabetic lungs [4]. Mechanisms that cause lung dysfunction in diabetics include oxidative stress [5], microangiopathy of capillaries of alveoli and lung arteries [6], changes in lung elastin and collagen [7], and surfactant dysfunction [8]. Surfactant, secreted in the alveolar space, is a lipoprotein complex produced by type 2 alveolar cells [9]. Hyperglycemia disrupts the expression of surfactant proteins genes [8]. Diabetes and insulin resistance decrease the levels of antioxidants such as catalase (CAT), glutathione (GSH), and superoxide dismutase (SOD) [10]. T2D leads to an increase in inflammatory cytokines such as TNF-α and IL-6 and a decrease in some anti-inflammatory cytokines such as IL-10 [5, 11].
Exercise training has been accepted as a non-pharmacological therapy to improve health conditions [12,13,14]. It has been proven that exercise training protects diabetic patients against oxidative stress by increasing the amount of antioxidant enzymes [15, 16]. An experimental study demonstrated that moderate and regular exercise training reduces CRP, IL-6, TNF-α and increases adiponectin levels and IL-10 [17].In general, exercise has anti-inflammatory effects especially in diabetes [18]. Another study has shown that aerobic exercise plays a protective role in lipopolysaccharide (LPS)-induced acute lung injury [19].In addition, it has been shown that exercise can protect lungs against methotrexate-induced lung injury [20]. Furthermore, the beneficial effects of aerobic exercise on blood sugar control and diabetes complications increase with exercise intensity, and more adaptation is achieved with high-intensity interval training (HIIT) [21].
In this study, we used of rats as an animal model for T2D induction. The rat as an experimental animal model of human disease offers various favorable circumstances and advantages over the mouse and different species [22, 23]. Rat is extensively used as a suitable animal model for understanding the metabolic profile and pathology involved in different stages of type 2 diabetes [24].
Studies related to the effects of exercise on the diabetic lung are incomplete and, we are facing to lack of information regarding the impact of HIIT exercise on the diabetic lung. Therefore, considering the negative effect of T2D on lung tissue and the positive effects of appropriate exercise on health stability, in the present study the impact of a specific type of exercise i.e., HIIT was investigated on some inflammatory, oxidative, apoptotic, surfactant, and histopathological indices of the lungs of diabetic rats.
Method
Animal care
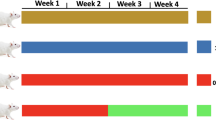
Twenty-eight eight-week-old male Wistar rats with an average weight of 200 g were purchased from the animal farm of Kerman University of Medical Sciences (KUMS) and kept them in special polycarbonate cages at 23 ± 2 °C and a 12:12 dark–light cycle. Throughout the experiment, all animals had ad libitum access to food and water. All experimental protocols were approved by ethics committee of Kerman university of medical sciences (Ethics approval code: IR.KMU.AH.REC.1400.173). For reducing suffering and distress of animals, their cages were cleaned every day. During the study to reduce the pain of the animals, we did not use electric shocks during exercise, and at the end of the study, the animals were sacrificed with a high dose of ketamine and xylazine, and we tried to do this process without pain. Also, Ethics approval all methods are reported in accordance with ARRIVE guidelines. After being acclimatized to the laboratory environment, the animals were randomly assigned to one of four groups (n = 7 in each group): control (CTL), type 2 diabetes (Db), exercise (Ex), and type 2 diabetes + exercise (Db + Ex). The Ex and Db + Ex groups performed 8 weeks of HIIT.A schematic representation for method stages was illustrated in Fig. 1.
Induction of diabetes
Db + Ex and Db groups were fed a high-fat diet (HFD) for 2 months, as shown in Table 1. After this period, the animals fasted for 12 h and then received a single intraperitoneal injection of 35 mg/kg streptozotocin (STZ). Three days later, their fasting blood glucose (FBG) levels were measured using a glucometer. FBG levels were measured at three time points: before starting the intervention (month 0), after diabetes induction (2 months of HFD and STZ injection), and 48 h after the training period using an Accu-Chek glucometer (USA) [25, 26].
Exercise protocol
All of the animals were familiarized with a motorized treadmill prior to the experiments. They ran on the treadmill at a speed of 8 m/min with an incline of 0% for 10 min per day over the course of 5 consecutive days. Both the Ex and T2D + Ex groups performed an incremental running test to determine their maximum speed (Vmax). They ran for 2 min at a speed of 6 m/min speed, and every 2 min the speed was increased by 2 m/min until the rats were exhausted. The last min tolerated speed was considered Vmax. The HIIT protocol was carried out five times a week for 8 weeks by rats in Ex and T2D + Ex groups [30]. The rats’ Vmax was measured every 2 weeks, and the new Vmax was used to calculate relative speed for the next 2 weeks. This protocol was designed in our lab, and is referred to as the K1 protocol (Table 2) [25]. All ethical principles of exercise in animals were considered in this study. For example, the design of the treadmill was such that it was not possible to fall off the animal from treadmill and also, the rats did not breathe hard and did not get exhausted because at the beginning of the study based on VO2max, an exercise protocol designed for animals. In addition, the VO2max was measured during study period.
Serum and tissue sampling
After 48 h since the last training session, the animals were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Blood samples were collected from the animal’s heart after 12 h of fasting, and right lung tissues were harvested and stored in freezer for molecular investigations. The blood samples were left at room temperature for 30 min and then centrifuged at 1000 g for 20 min at 4 °C was to measure BGF. The resulting serum samples were stored at a temperature of – 80 °C [27].
BALF collection
After the sacrifice of the animal, bronchoalveolar lavage fluid (BALF) was collected from the left lung. Briefly, a median sternotomy was performed, the trachea was isolated. The right main bronchus was clamped and a catheter was inserted to the left main bronchus of the animal. Then 2.5 mL of normal saline was instilled into the bronchoalveolar space of the left lung and the instilled fluid was then harvested by aspiration into the syringe. The right lung was not washed to preserve it for histopathology and molecular evaluations. The BALF was immediately centrifuged (4 ◦C, 10 min, 1000 g), and the supernatant was used to measure the levels of cytokines and surfactant proteins and apoptotic factors [28, 29].
ELISA
The levels of TNFα (Bio-techne Co, USA, Catalog No: DY510-05), IL-10 (Bio-techne Co, USA, Catalog No : R1000), SP-D (Elabscience Co, USA, Catalog No : E-EL-R0831), Lecithin (Sigma-Aldrich Co, USA, Catalog No : MAK049 ), SPM (Sigma-Aldrich Co, USA, Catalog No : MAK154), BAX (LSBio Co, USA, Catalog No : LS-F5064 ) and Bcl2 (LSBio Co, USA, Catalog No : LS-F4135) were assessed using ELISA in the BALF according to the manufacturer’s instructions of relevant kits protocol. Oxidative stress factors were measured in lung tissue. For this purpose, the lung tissues were homogenate through sonication and total proteins were assessed by Bradford method in homogenate samples. Malondialdehyde (MDA), as an index of lipid peroxidation, was estimated using the concentration of thiobarbituric acid reactive substances (TBARS) at 550 nm and Total antioxidant capacity (TAC) was determined by the ferric reducing ability of plasma (FRAP) assay at 593 nm [30] .
Histopathology
The right lungs of animals were harvested and immersed in 10% formalin. After the paraffin molding of the tissues, 5 mm thick sections which were stained with hematoxylin and eosin (H&E) were prepared, and later examined microscopically by a pathologist who was blind to the animal groupings. Six fields of same area of the lung for each rat were analyzed [31, 32]. Also, Mason trichrome staining was done to measure tissue fibrosis score according to the Hübner et al. scoring system [33]. The slides were examined by a pathologist, who was blinded to the group of animals. H&E-stained slides were scored as: absent (0), minimal (1), mild (2), moderate (3), and severe (4) lesions for peribronchial inflammation, inflammatory cell infiltration, expansion of the alveolar interstitial space, enlargement of airway, destruction of septum of alveoli, congestion, and fibrosis. The total lung score was expressed as the sum of the scores for each parameter. Mean alveolar number (MAN) as an indicator for density of alveoli was calculated by MAN = AN/surface area (SA) (mm2) formula according to AN in each field of view and SA of the field [14, 34].
Statistical analysis
The data are reported as a mean ± standard error of the mean (SEM). The Shapiro Wilk test was used for examining the normality of the data. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used for analysis of parametric data. The Kruskal-Wallis and Mann–Whitney test was utilized for analysis of lung injury and fibrosis data. The significance level was considered at P < 0.05.
Results
The effects of HIIT and T2D on FBG and body weight
The FBG assessed to confirm diabetes induction method and the effect of HIIT on its level. Fasting blood glucose was significantly increased after diabetes induction (2 months of high-fat diet and STZ injection) (month 2) compared with baseline (month 0) in Db and Db + Ex group (P < 0.001), with no significant difference between these groups. Performing HIIT significantly reduced FBG in Db + Ex group vs. Db group (P < 0.001) (Table 3). In other words, exercise was able to bring blood glucose levels closer to normal levels.
On the other hand, animals’ body weight significantly increased in Db and Db + Ex groups after diabetes induction (month 2) in rats. In addition, the weight was decreased in Db and Db + Ex groups, with more decrease in the Db group (P < 0.05) in post-test (month 4). (Table 3).
The effects of T2D and HIIT on BALF cytokines and lung tissue oxidative stress
BALF cytokines were evaluated to investigate the effects of HIIT on inflammation due to T2D. In the rats with T2D, the levels of TNF-α increased compared to the control group (P < 0.001). However, in Db + Ex group the levels of TNF-α were reduced (P < 0.05 vs. Db group) (Fig. 2A). The levels of IL-10 in the rats with T2D were decreased compared to the control group (P < 0.05), however in the Db + Ex group, the value of this cytokine elevated in comparison with the T2D group (P < 0.01) (Fig. 2B). Results showed that there was no significant difference between the exercise and control groups in relation to these cytokines.
The effects of HIIT on BALF TNFα and IL-10 and lung tissue MDA, TAC and MDA/TAC ratio following T2D. The results are presented as mean ± SEM. CTL: control, Db: type 2 diabetic (STZ injected), Ex: exercise only, and Db + Ex: type 2 diabetic exercise. A The levels of tumor necrosis factor (TNFα). B The levels of interleukine-10 (IL-10). C Malondialdehyde (MDA), (D) Total anti-oxidant capacity (TAC), (E) MDA/TAC ratio. * P < 0.05 & *** P < 0.001 compared to the CTL and # P < 0.05 &## P < 0.01 compared to the Db
T2D significantly increased the MDA level (P < 0.001) (Fig. 2C) and diminished the TAC level in lung tissue (P < 0.05) (Fig. 2D) and increased the MDA/TAC ratio (P < 0.001) (Fig. 2E) in comparison with the CTL group. Performing HIIT by Db + Ex group for 8 weeks significantly restored these alterations and the MDA/TAC ratio decreased in this group (P < 0.01 vs. Db group) so that it had not significant difference with CTL group (Fig. 2E). Despite the reduction of TAC in exercise group vs. CTL group (P < 0.001), MDA/TAC ratio didn’t significant difference between these groups.
The effects of T2D and HIIT on apoptosis indices and surfactant proteins in BALF
Our results revealed that the levels of Bcl2 in BALF of diabetic rats were decreased (P < 0.05) (Fig. 3A) while the levels of BAX were increased (P < 0.01) (Fig. 3B). Also, the BAX to Bcl2 ratio as an index of apoptosis/survival was higher in Db group than CTL group (P < 0.001) (Fig. 3C). Furthermore, 8 weeks of HIIT could improve apoptosis/survival index through increasing Bcl2 and reducing BAX compared to the Db group (P < 0.001). Our results showed that the status of apoptotic factors in the exercise group had not difference in comparison with the control group.
The effects of HIIT on BALF BAX, Bcl2 and their ratio and surfactant proteins following T2D. The results are presented as mean ± SEM. CTL: control, Db: type 2 diabetic (STZ injected), Ex: exercise only, and Db + Ex: type 2 diabetic + exercise. A Bcl2, (B) BAX, (C) BAX/Bcl2 ratio. D Sphingomyelin (SPM), (E) Lecithin, (F) Surfactant protein D (SPD). * P < 0.05 & ** P < 0.01& *** P < 0.001 compared to the CTL and # P < 0.05 & ## P < 0.01 & ### P < 0.001 compared to the Db
Our findings disclosed that the levels of surfactant components (SP-D, SPM and Lecithin) in Db group was lower than CTL group (P < 0.001 for SPM and lecithin & P < 0.05 for SPD) while following HIIT, the levels of these proteins were increased compared to the Db group significantly (P < 0.01 for SPM & P < 0.05 for lecithin and SPD) (Fig. 3D, E, F). There was no significant difference between exercise and control groups regarding pulmonary surfactants.
The effects of T2D and HIIT and on lung fibrosis
Fibrotic tissue was significantly increased in the lung of diabetic rats (P < 0.001), which mitigated following HIIT (P < 0.001) (Fig. 4A (micrographs) and Fig. 4B).
Micrographs of the lung (A) in CTL (a), Db (b); Ex (c); Db + Ex (d) groups. Comparison of fibrosis score in the lung tissue of different groups illustrated in figure (B). (Magnifcation:×40, scale bar: 40 μm).Black arrows indicate fibrotic areas. Data are means ± SEM .*** P < 0.001 compared to the CTL.### P < 0.001 compared to the Db
The effects of T2D and HIIT on histological score and mean alveolar number
Semi-quantitative histological scoring of the lungs demonstrated moderate to severe inflammation, interstitial leukocytes infiltration, congestion, destruction of septum of alveoli, expansion of the alveolar interstitial space, and fibrosis in diabetes group in comparison with CTL and Ex groups (P < 0.001). Combination of exercise with diabetes significantly decreased the negative effect of diabetes (P < 0.01); however, there was still some degree of damage in Db + Ex group (Fig. 5A(c) and B). Diabetes was associated with reduction in AN/SA (mm2) when compared with CTL and Ex groups (Fig. 5A (d) and C) (P < 0.001) and exercise training attenuated this effect (P < 0.05) (Fig. 5A (c) and C).
Micrographs of the lung (A) in CTL (a), Db (b); Ex (c); Db + Ex (d) groups. Comparison of histological score (B) and mean alveolar number (MAN) (C) in the lung tissue of different groups illustrated in this figure. (Magnifcation:×40, scale bar: 40 μm).Blue arrows indicate inflammation and orange arrows indicate congestion. Data are means ± SEM .*** P < 0.001 compared to the CTL. # P < 0.05 & ## P < 0.01 compared to the Db
Discussion
The aim of this study was the examination of the effects of 8 weeks of HIIT on tissue injury, some pro/anti-inflammatory cytokines, survival/apoptotic proteins, redox balance and surfactant components in lung of diabetic rats.
Results showed that following T2D, inflammation, oxidative stress, apoptosis, and injury increased in lung tissue and BALF. Also, the levels of pulmonary surfactants components in BALF were reduced due to T2D.On the other hand, 8 weeks of HIIT could reverse all mentioned alterations toward normal levels.
The trends of FBG and BW changes (Table 3) approved the T2D induction and were in line with other previous studies [25, 35, 36] .Also, we showed the serum insulin reduction in T2D rats in our previous research [35].In addition, as reported in our previous publications, food intake was increased owing to T2D (polyphagia) and we demonstrated that HIIT may modulate appetite regulation in rats with T2D through leptin signaling [35].
The lung has a complex alveolar-capillary network which may be targeted by diabetic damage [37]. Diabetic patients frequently report respiratory symptoms [38] and are at increased risk of several pulmonary diseases [39]. It has been shown that T2D is associated with an increased prevalence of respiratory symptoms as compared to the general population [38].
Present study revealed that diabetes increased TNFα as a pro-inflammatory cytokine and reduced IL-10 as an anti-inflammatory cytokine in BALF (Fig. 2A & B). Consistent with our findings, Talakatta et al. showed increasing levels of TNFα and decreasing levels of IL10 in the serum of diabetic animals [40]. Also, Dennis et al. reported that increased TNFα levels are associated with inadequate glucose control in T2D and impaired lung function [41]. In another study, it has been shown that the levels of IL-6 and IL-17 were increased in diabetic lungs [42]. Diabetes is a pro-inflammatory state associated with airway inflammation [43]. Hyperglycemia increases chronic inflammation, inflammatory cytokine release, and oxidative stress through activation of the nuclear factor kappa B (NFκB) pathway and NADPH oxidase (NOX) as well as the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [44]. Our findings showed that HIIT could ameliorate inflammation through increasing IL10 and decreasing TNFα in diabetic rats. Consistent with this, some investigations have disclosed the important role of HIIT in reducing inflammation [45,46,47]. Mohammadi zadeh et al. showed that HIIT reduces pro-inflammatory markers and increases anti-inflammatory markers in T2D patients [48].In addition, Azizi et al. demonstrated that swimming training reduced inflammation in pulmonary tissue through diminishing IL1β in type 1 diabetic mice [49]. Thus, it seems that HIIT has anti-inflammatory effects in diabetic lung.
The other findings of the present study were decreasing levels of TAC and increasing levels of MDA and MDA/TAC as an index of redox imbalance in favor of oxidant components in lung tissue following T2D (Fig. 2C-E). Performing 8 weeks HIIT by diabetic rats improved the redox balance in our study. In line with these findings, evidence indicates that in the diabetic lung, the activity of superoxide dismutase (SOD) was decreased, while the contents of nitric oxide (NO) and malondialdehyde (MDA) were significantly increased [50]. MDA has been documented as a primary biomarker of free radical mediated lipid damage and oxidative stress [51]. Increased level of MDA in diabetics suggests that peroxidative injury may be involved in the development of diabetic complications. The increase in lipid peroxidation is also an indication of decline in defense mechanisms of enzymatic and nonenzymatic antioxidants [52].On the other hand, it has been shown that the TAC level was reduced following T2D [53]. The positive effect of HIIT on reducing oxidative stress has been shown in various tissues by decreasing lipid peroxidation and enhancing antioxidants defenses [54,55,56]. Also, Machado et al. revealed that treadmill running (5 days a week for 9 weeks) increased SOD and catalase in lung of newborn diabetic rats [57].The reduction of MDA/TAC ratio as an index of oxidant/anti-oxidant index following HIIT confirms the positive effect of this type of training and is in line with previous findings.
Our findings demonstrated the apoptosis activity as decreasing level of Bcl2 and increasing level of BAX and BAX/Bcl2 ratio in lung of diabetic rats and HIIT reversed this process (Fig. 3A-C). It has been shown that apoptosis of lung epithelial cells was increased in diabetic animals compared to non-diabetic. This was associated with increased inflammation and oxidative stress in the lungs of diabetic rats [58]. High blood sugar levels in diabetic rats leads to increased apoptosis of lung cells and decreased lung function [59]. BAX and Bcl2 may play a role in the pathogenesis of diabetic lung disease. For example, one study found that BAX expression was increased in the lung tissue of diabetic rats while the Bcl2 expression was decreased and that this was associated with increased apoptosis and lung injury [60]. Consistent with our finding, previous studies indicated that HIIT reduced apoptosis via increasing Bcl2 and decreasing BAX expression in heart following diabetes [61, 62]. Also, it has been shown that swimming exercise has anti-apoptotic impacts through reducing BAX in lungs of diabetic mice [49].
Our results revealed that the levels of SP-D, SPM and lecithin diminished in BALF of diabetic rats while HIIT could improve these components (Fig. 3D-F). One study disclosed that diabetic rats had decreased levels of SP-A and SP-B, which are important components of the pulmonary surfactant system [63]. SP-D is an important regulatory protein that may aid in controlling chronic inflammation, reducing oxidative radical formation, facilitating phagocytosis and agglutination, reducing cell death, and enhancing apoptotic and necrotic cell clearance and it has been shown that SP-D reduce due to T2D [64]. Lopez et al. demonstrated that Serum SP-D concentration can be a useful biomarker for detecting lung impairment in obese patients with T2D [65]. The effect of exercise on the level of pulmonary surfactants following diabetes has not been well investigated. However, Increment of pulmonary surfactant (surfactant protein A) of young male rats after six weeks interval training showed by Mirdar et al. [66]. Another study revealed that exercise can improve pulmonary surfactants due to lung injury [67]. The present study suggests that HIIT may have a beneficial role in maintaining pulmonary function by restoring lung surfactant components in diabetes.
We observed increased fibrotic tissue and obvious pathoβlogical changes in lung tissue due to T2D, and HIIT significantly improved these malformations (Figs. 4 and 5). In line with this finding, it has been shown that hyperglycemia in diabetes accelerates fibrotic changes in the lung through the activation of TGF-β signaling pathways [40]. Also, Machado et al [57] showed pathological changes such as increased bronchoconstriction index and polymorphonuclear cells in the lungs of diabetic rats that recovered by moderate exercise training. Before all the effects, we indicated that HIIT was able to lower blood sugar in diabetic rats (Table 3). It has been shown that HIIT as a time-efficient exercise option can be safe and effective for reducing blood glucose levels in individuals with, or at risk for, T2D [68]. Little et al. showed that Low-volume HIIT reduces hyperglycemia in patients with T2D [69]. Therefore, HIIT can be useful in improving pulmonary lesions following T2D by reducing blood sugar levels.
Limitations and future perspectives
It is mentioned that in this study, we examined and measured some anti-inflammatory cytokines, total anti-oxidant capacity, two important proteins involved in apoptosis, and surfactant proteins.However, due to finance limitations, we were not able to measure other factors such as other pro-inflammatory interleukines such as IL-6, IL-4, IL1β and IL-17 and the activity of MPO, GPX, SOD and caspases involved in apoptosis, which complement and strengthen the present findings of this study.The measurment of the above mentioned items will be considered in future studies.
Conclusion
The findings of the present study showed that, 8 weeks of HIIT as a non-pharmacological intervention can improve inflammation, oxidative stress, apoptosis, and histopathological changes in the lungs of diabetic rats. Also, HIIT can increase the level of surfactant components in the BALF of diabetic rats and hence may contribute to the improvement of pulmonary function. It seems that these positive effects of HIIT are caused by its anti-hyperglycemic effects thereby maintaining the balance of redox, apoptosis, and inflammatory/anti-inflammatory systems. Exercise is a non-pharmacological intervention and has few side effects compared to drug treatments. Considering the promising results of this study and similar animal studies on the protective effects of exercise against diabetic lung, more clinical studies are suggested to generalize these findings to humans.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rajizadeh MA, Aminizadeh AH, Esmaeilpour K, Bejeshk MA, Sadeghi A, Salimi F. Investigating the effects of Citrullus colocynthis on cognitive performance and anxiety-like behaviors in STZ-induced diabetic rats. Int J Neurosci. 2023;133(4):343–55.
Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol. 2012;4(4):90–102.
Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34(31):2436–43.
Wu J, Jin Z, Yan L-J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–9.
Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45.
Kuziemski K, Specjalski K, Jassem E. Diabetic pulmonary microangiopathy—fact or fiction? Endokrynol Polska. 2011;62(2):171–6.
Südy R, Schranc Á, Fodor GH, Tolnai J, Babik B, Peták F. Lung volume dependence of respiratory function in rodent models of diabetes mellitus. Respir Res. 2020;21(1):1–12.
Foster DJ, Ravikumar P, Bellotto DJ, Unger RH, Hsia CC. Fatty diabetic lung: altered alveolar structure and surfactant protein expression. Am J Physiology-Lung Cell Mol Physiol. 2010;298(3):L392–L403.
Castillo-Sánchez JC, Cruz A, Pérez-Gil J. Structural hallmarks of lung surfactant: lipid-protein interactions, membrane structure and future challenges. Arch Biochem Biophys. 2021;703:108850.
Amjadi A, Mirmiranpour H, Sobhani SO, Moazami Goudarzi N. Intravenous laser wavelength radiation effect on LCAT, PON1, catalase, and FRAP in diabetic rats. Lasers Med Sci. 2020;35:131–8.
Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13(2):1165–72.
Bejeshk M-A, Joukar S, Shahouzehi B, Asadi-shekari M, Rajizadeh M, Raji-amirhasani A, Naderi-boldaji V. Combinatorial effect of lower extremity blood flow restriction and low intensity endurance exercise on aorta of old male rats: Histomorphological and molecular approach. Artery Res. 2018;24:22–31.
Rajizadeh MA, Sheibani V, Bejeshk MA, Mohtashami Borzadaran F, Saghari H, Esmaeilpour K. The effects of high intensity exercise on learning and memory impairments followed by combination of sleep deprivation and demyelination induced by etidium bromide. Int J Neurosci. 2019;129(12):1166–78.
Alavi SS, Joukar S, Rostamzadeh F, Najafipour H, Darvishzadeh-Mahani F, Mortezaeizade A. Involvement of sirtuins and klotho in cardioprotective effects of exercise training against waterpipe tobacco smoking-induced heart dysfunction. Front Physiol. 2021;12:680005.
Poblete-Aro C, Russell-Guzman J, Parra P, Soto-Munoz M, Villegas-Gonzalez B, Cofre-Bolados C, Herrera-Valenzuela T. Exercise and oxidative stress in type 2 diabetes mellitus. Rev Méd De Chile. 2018;146(3):362–72.
Amirazodi M, Mehrabi A, Rajizadeh MA, Bejeshk MA, Esmaeilpour K, Daryanoosh F, Gaeini A. The effects of combined resveratrol and high intensity interval training on the hippocampus in aged male rats: an investigation into some signaling pathways related to mitochondria. Iran J Basic Med Sci. 2022;25(2):254.
Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere C, Dunn SR, McCue P, Mizisin A, Sharma K. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296(4):F700-8.
Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):600–11.
Reis Goncalves CT, Reis Goncalves CG, de Almeida FM, dos Santos Lopes FDTQ, dos Santos Durão ACC, dos Santos FA, da Silva LFF, Marcourakis T, Castro-Faria-Neto HC. Vieira RdP: protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit Care. 2012;16(5):1–11.
Rajizadeh MA, Hosseini MH, Bahrami M, Hosseini NS, Rostamabadi F, Bagheri F, Khoramipour K, Najafipour H, Bejeshk MA. Comparison of preventive and therapeutic effects of continuous exercise on acute lung injury induced with methotrexate. Exp Physiol. 2023;108(9):1215–27.
Savikj M, Zierath JR. Train like an athlete: applying exercise interventions to manage type 2 diabetes. Diabetologia. 2020;63:1491–9.
Bryda EC. The mighty mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207.
Kottaisamy CPD, Raj DS, Prasanth Kumar V, Sankaran U. Experimental animal models for diabetes and its related complications-a review. Lab Anim Res. 2021;37(1):23.
Sharma P, Garg A, Garg S, Singh V. Animal model used for experimental study of diabetes Mellitus: an overview. Asian J Biomat Res. 2016;2(4):99–110.
Khoramipour K, Bejeshk MA, Rajizadeh MA, Najafipour H, Dehghan P, Farahmand F. High-intensity interval training ameliorates molecular changes in the Hippocampus of male rats with the Diabetic Brain: the role of Adiponectin. Mol Neurobiol. 2023:60(6):3486–95.
Ebrahimi MN, Khaksari M, Sepehri G, Karam GA, Raji-Amirhasani A, Azizian H. The effects of alone and combination tamoxifen, raloxifene and estrogen on lipid profile and atherogenic index of ovariectomized type 2 diabetic rats. Life Sci. 2020;263:118573.
Rajizadeh MA, Nematollahi MH, Jafari E, Bejeshk MA, Mehrabani M, Razeghinia MS, Najafipour H. Niosome nanocarrier enhances the ameliorating effects of myrtenol in the lungs of rats with experimental asthma. OpenNano. 2023;11:100129.
Bejeshk MA, Beik A, Aminizadeh AH, Salimi F, Bagheri F, Sahebazzamani M, Najafipour H, Rajizadeh MA. Perillyl alcohol (PA) mitigates inflammatory, oxidative, and histopathological consequences of allergic asthma in rats. Naunyn Schmiedebergs Arch Pharmacol. 2023:1–11.
Bejeshk MA, Pourghadamyari H, Najafipour H, Eftekhari M, Mottaghipisheh J, Omidifar N, Azizi M, Rajizadeh MA, Doustimotlagh AH. The hydroalcoholic extract of nasturtium officinale reduces lung inflammation and oxidative stress in an ovalbumin-induced rat model of asthma. Evid Based Complement Alternat Med. 2022;2022:5319237.
Bejeshk MA, Aminizadeh AH, Jafari E, Motamedi S, Zangiabadi I, Ghasemi A, Fathi M, Nezhadi A, Akhgarandouz F, Bejeshk F. Myrtenol Ameliorates Recognition Memories’ impairment and anxiety-like Behaviors Induced by Asthma by mitigating hippocampal inflammation and oxidative stress in rats. Neuroimmunomodulation. 2023:30(1):42–54.
Joukar S, Asadipour H, Sheibani M, Najafipour H, Dabiri S. The effects of Melissa officinalis (lemon balm) pretreatment on the resistance of the heart to myocardial injury. Pharm Biol. 2016;54(6):1005–13.
Mahdavi N, Joukar S, Najafipour H, Asadi-Shekaari M. The promising effect of barberry (Zereshk) extract against experimental pulmonary microvascular remodeling and hypertension: a comparison with sildenafil. Pharm Biol. 2016;54(3):509–15.
Hübner R-H, Gitter W, Eddine El Mokhtari N, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44(4):507–17.
Nakhaee MR, Zolfaghari MR, Joukar S, Nakhaee N, Masoumi-Ardakani Y, Iranpour M, Nazari M. Swimming exercise training attenuates the lung inflammatory response and injury induced by exposing to waterpipe tobacco smoke. Addict Health. 2020;12(2):109.
Khoramipour K, Rezaei MH, Madadizadeh E, Hosseini MS, Soltani Z, Schierbauer J, Moser O. High intensity interval training can ameliorate hypothalamic appetite regulation in male rats with type 2 diabetes: the role of Leptin. Cell Mol Neurobiol. 2023:43(8):4295–307.
Rajizadeh MA, Moslemizadeh A, Hosseini MS, Rafiei F, Soltani Z, Khoramipour K. Adiponectin receptor 1 could explain the sex differences in molecular basis of cognitive improvements induced by exercise training in type 2 diabetic rats. Sci Rep. 2023;13(1):16267.
Khateeb J, Fuchs E, Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev Diabet Stud. 2019;15:1–15.
De Santi F, Zoppini G, Locatelli F, Finocchio E, Cappa V, Dauriz M, Verlato G. Type 2 diabetes is associated with an increased prevalence of respiratory symptoms as compared to the general population. BMC Pulm Med. 2017;17(1):1–8.
Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60.
Talakatta G, Sarikhani M, Muhamed J, Dhanya K, Somashekar BS, Mahesh PA, Sundaresan N, Ravindra PV. Diabetes induces fibrotic changes in the lung through the activation of TGF-β signaling pathways. Sci Rep. 2018;8(1):11920.
Dennis RJ, Maldonado D, Rojas MX, Aschner P, Rondón M, Charry L, Casas A. Inadequate glucose control in type 2 diabetes is associated with impaired lung function and systemic inflammation: a cross-sectional study. BMC Pulm Med. 2010;10(1):1–7.
Bejeshk MA, Bagheri F, Salimi F, Rajizadeh MA. The diabetic lung can be ameliorated by citrullus colocynthis by reducing inflammation and oxidative stress in rats with type 1 diabetes. Evid Based Complement Alternat Med. 2023;2023:5176645.
Mulrennan S, Baltic S, Aggarwal S, Wood J, Miranda A, Frost F, Kaye J, Thompson PJ. The role of receptor for advanced glycation end products in airway inflammation in CF and CF related diabetes. Sci Rep. 2015;5(1):8931.
Kolahian S, Leiss V, Nürnberg B. Diabetic lung disease: fact or fiction? Rev Endocr Metab Disord. 2019;20(3):303–19.
Munk PS, Breland UM, Aukrust P, Ueland T, Kvaløy JT, Larsen AI. High intensity interval training reduces systemic inflammation in post-PCI patients. Eur J Cardiovasc Prev Rehabil. 2011;18(6):850–7.
Steckling F, Farinha J, Santos D, Bresciani G, Mortari J, Stefanello S, Courtes A, Duarte T, Duarte M, Moresco R. High intensity interval training reduces the levels of serum inflammatory cytokine on women with metabolic syndrome. Exp Clin Endocrinol Diabetes. 2016;124(10):597–601.
Leite AB, Lima HN, de Oliveira Flores C, Oliveira CA, Cunha LEC, Neves JL, Correia TML, de Melo FF, Oliveira MV, de Magalhães ACM. High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sci. 2021;266:118880.
Asle Mohammadi Zadeh M, Kargarfard M, Marandi SM, Habibi A. Diets along with interval training regimes improves inflammatory & anti-inflammatory condition in obesity with type 2 diabetes subjects. J Diabetes Metab Disord. 2018;17:253–67.
Azizi N, Rahbarghazi A, Bavil FM, Rahbarghazi R, Ghaffari-Nasab A, Rezaie J, Delkhosh A, Ahmadi M. Swimming training reduced inflammation and apoptotic changes in pulmonary tissue in type 1 diabetic mice. J Diabetes Metab Disord. 2023:1–8.
Zheng H, Wu J, Jin Z, Yan L-J. Potential biochemical mechanisms of lung injury in diabetes. Aging Dis. 2017;8(1):7.
Shodehinde SA, Oboh G. Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac J Trop Biomed. 2013;3(6):449–57.
Saddala RR, Thopireddy L, Ganapathi N, Kesireddy SR. Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root. Exp Toxicol Pathol. 2013;65(1–2):15–9.
Dordević G, Durić S, Apostolskit S, Dordević V, Zivković M. Total antioxidant blood capacity in patients with type 2 diabetes mellitus and distal symmetrical polyneuropathy. Vojnosanit Pregl. 2008;65(9):663–9.
Freitas DA, Rocha-Vieira E, Soares BA, Nonato LF, Fonseca SR, Martins JB, Mendonça VA, Lacerda AC, Massensini AR, Poortamns JR, et al. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol Behav. 2018;184:6–11.
Lu Y, Wiltshire HD, Baker JS, Wang Q. Effects of high intensity exercise on oxidative stress and antioxidant status in untrained humans: a systematic review. Biology. 2021;10(12):1272.
Groussard C, Maillard F, Vazeille E, Barnich N, Sirvent P, Otero YF, Combaret L, Madeuf E, Sourdrille A, Delcros G, et al. Tissue-specific oxidative stress modulation by exercise: a comparison between MICT and HIIT in an obese rat model. Oxid Med Cell Longev. 2019;2019:1965364.
Machado LMQ, Serra DS, Neves TG, Cavalcante FSÁ, Ceccatto VM, Leal-Cardoso JH, Zin WA, Moreira‐Gomes MD. Pulmonary impairment in type 2 diabetic rats and its improvement by exercise. Acta Physiol. 2022;234(1):e13708.
Li H, Liu J, Wang Y, Fu Z, Hüttemann M, Monks TJ, Chen AF, Wang J-M. MiR-27b augments bone marrow progenitor cell survival via suppressing the mitochondrial apoptotic pathway in type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313(4):E391–401.
Bolat D, ÜLGER M, BARAN M, Turan IT, Arzu Y. Lung injury aggravated in streptozotocin-induced diabetes: an experimental study. Cukurova Med J. 2022;47(1):175–82.
Chen Y, Zhang F, Wang D, Li L, Si H, Wang C, Liu J, Chen Y, Cheng J, Lu Y. Mesenchymal stem cells attenuate diabetic lung fibrosis via adjusting Sirt3-mediated stress responses in rats. Oxid Med Cell Longev. 2020;2020:8076105.
Delfan M, Delphan M, Kordi MR, Ravasi AA, Safa M, Gorgani-Firuzjaee S, Fatemi A, Bandarian F, Nasli-Esfahani E. High intensity interval training improves diabetic cardiomyopathy via miR-1 dependent suppression of cardiomyocyte apoptosis in diabetic rats. J Diabetes Metab Disord. 2020;19:145–52.
Ramezani N, Vanaky B, Shakeri N, Soltanian Z, Fakhari Rad F, Shams Z. Evaluation of Bcl-2 and Bax expression in the heart of Diabetic rats after four weeks of high intensity interval training. Med Lab J. 2019;13(1):15–20.
Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–69.
Ghanayem N, Abou Elnour E, El Wahsh R, El-Shazlya R, Abou Elenin M. Surfactant protein D in chronic obstructive pulmonary disease and type 2 diabetes mellitus. Menoufia Med J. 2017;30(1):297–304.
López-Cano C, Lecube A, García-Ramírez M, Muñoz X, Sánchez E, Seminario A, Hernández M, Ciudin A, Gutiérrez L, Hernández C, et al. Serum surfactant protein D as a biomarker for measuring lung involvement in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2017;102(11):4109–16.
Mirdar S, Naiestany F, Hamidian G, Hedayati M. Increment of alveolar macrophages and pulmonary surfactant of young male rats after six weeks interval training. Sport Physiol. 2018;9(36):59–72.
Da Cunha MJ, Da Cunha AA, Scherer EB, Machado FR, Loureiro SO, Jaenisch RB, Guma F, Lago PD, Wyse AT. Experimental lung injury promotes alterations in energy metabolism and respiratory mechanics in the lungs of rats: prevention by exercise. Mol Cell Biochem. 2014;389:229–38.
Francois ME, Little JP. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015;28(1):39.
Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–60.
Acknowledgements
We would like to thank the Kerman university of medical sciences for its support.
Funding
This work was supported by Kerman University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Mohammad Amin Rajizadeh: molecular analysis Kayvan Khoramipour: recording heart function Siyavash Joukar: main idea and editing the manuscript Fatemeh Darvishzadeh: histopathological analysis Maryam Iranpour: reviewed the manuscript Mohammad Abbas Bejeshk : reviewed the manuscript Maryam Doostaki : reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods are reported in accordance with ARRIVE guidelines. All stages of keeping and scarifying the animals were performed according to the rules of the ethics committee of Kerman University of Medical Sciences ; (Ethics approval code: IR.KMU.AH.REC.1400.173).
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rajizadeh, M.A., Khoramipour, K., Joukar, S. et al. Lung molecular and histological changes in type 2 diabetic rats and its improvement by high-intensity interval training. BMC Pulm Med 24, 37 (2024). https://doi.org/10.1186/s12890-024-02840-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02840-1