Abstract
Background
Mortality rates in patients with COVID-19 undergoing mechanical ventilation in the intensive care unit are high. The causes of this mortality have been rigorously investigated. The aim of the present study is to establish mortality risk factors related to lung mechanics measured at days 1 and 5 in patients with covid-19 ARDS managed with invasive mechanical ventilation in the intensive care unit.
Methods
A retrospective observational multicenter study including consecutive patients with a confirmed diagnosis of COVID-19-induced ARDS, admitted to three institutions and seven intensive care units in the city of Bogota between May 20, 2020 and May 30, 2022 who required mechanical ventilation for at least five days. Data were collected from the medical records of patients who met the inclusion criteria on day 1 and day 5 of mechanical ventilation. The primary outcome assessed was mortality at day 30.
Results
A total of 533 consecutive patients admitted with ARDS with COVID-19 were included. Ventilatory ratio, plateau pressure and driving pressure measured on day 5 were significantly higher in non-survivors (p < 0.05). Overall, 30-day follow-up mortality was 48.8%. The increases between day 1 and day 5 in the ventilatory ratio (OR 1.42, 95%CI 1.03–2.01, p = 0.04), driving pressure (OR 1.56, 95%CI 1.10–2.22, p = 0.01); and finally plateau pressure (OR 1.9, 95%CI 1.34–2.69, p = 0.001) were associated with an increased risk of death. There was no association between deterioration of PaO2/FIO2 index and mortality (OR 1.34, 95%CI 0.96–1.56, p = 0.053).
Conclusions
Ventilatory ratio, plateau pressure, driving pressure, and age were identified as independent risk factors for 30-day mortality in patients with ARDS due to COVID-19 on day 5 of invasive mechanical ventilation.
Similar content being viewed by others
Background
In most patients, the COVID-19 infection had a mild course with symptoms characterized by fever, loss of smell and malaise. However, 10–20% of patients developed severe disease requiring oxygen therapy and admission to the intensive care unit (ICU), progressing to acute respiratory distress syndrome (ARDS) and requiring mechanical ventilation (MV) [1]. Mortality in patients with critical COVID-19 was high, ranging from 15 to 74% when invasive MV was required [2].
Male patients who are active smokers and aged over 60 years face a higher risk of hospital death. Comorbidities including diabetes, arterial hypertension, cerebrovascular disease, respiratory diseases and chronic kidney disease also influence the prognosis of COVID-19 [3].
The ventilatory ratio (VR), calculated as [ventilation per minute (ml/min) × PaCO2 (mm Hg)]/(predicted body weight (kg) × 100 × 37.5), is a recently defined bedside measurement, which acts as a surrogate for the dead space fraction. It is easily obtained at the bedside with arterial blood gasometry and minute ventilation assessment [4]. It has been suggested that physiologic dead space is a stronger predictor of non-COVID 19 ARDS outcomes than oxygenation [5].
Monteiro et al. and Sinha et al. demonstrated that patients with ventilatory ratio > 2 (median) on day 1 had significantly lower 90-day survival than those with ventilatory ratio ≤ 2; they also found VR on day 1 to be significantly associated with 28-day mortality [6, 7].
A recent study in patients who received MV at ICU admission and for a further three days found that a high VR and an increase in VR at day 3 were associated with mortality in those with COVID-19 [8].
The measurement of VR has generated great interest thanks to the ease of application of its formula and the importance of its measurement. It provides relevant information on the dead space fraction (Vd/Vt) and can help to prepare corrective measures to counteract the harmful effects associated with the increase in this parameter.
The aim of the present study was the main objective is to establish that values of pulmonary mechanics, including the ventilatory ratio, are risk factors for 30-day mortality in mechanically ventilated with ARDS due covid-19.
Materials and methods
A multicenter, observational, retrospective study that included patients with ARDS due to COVID-19 infection admitted to three institutions in the city of Bogota. Consecutive patients from these institutions were included retrospectively by reviewing medical records. Three investigators collected and stored the data independently on a controlled form. The results were presented in accordance with the STROBE guidelines for reporting observational studies in epidemiology [9]. The present study was approved by the Ethics and Research Committee of the Fundación Universitaria Sanitas - CEIFUS 3347-22.
Patients
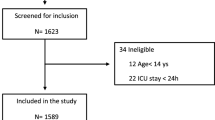
Consecutive patients admitted to three intensive care units in the city of Bogota with a confirmed diagnosis of COVID-19 between May 2020 and May 2022 were eligible for inclusion in the study. Specific inclusion criteria were: age over 18 years, requirement of MV and an ICU stay of at least 5 days at the Clínica Reina Sofía, Clínica Universitaria Colombia and Clínica Santa María del Lago in the city of Bogotá Colombia. Exclusion criteria were noninvasive MV and/or high-flow nasal cannula, Sequential Organ Failure Assessment Score (SOFA) score above 12 in the first 24 h of ICU admission, requirement of extracorporeal membrane oxygenation (ECMO) in the first 5 days of MV, and incomplete data in the clinical history records (Fig. 1).
Definitions
ARDS was diagnosed according to the Berlin definition guidelines. Tidal volume was reported in mL/kg of predicted body weight (PBW), the formula used to calculate the predicted or ideal weight was: men height (cm) − 152.4 × 0.91 + 50 women height (cm) − 152.4 × 0.91 + 45.5. Crs was calculated as tidal volume/ (plateau pressure − PEEP). Driving pressure was defined as plateau pressure minus PEEP. Ventilatory ratio was defined as (minute ventilation x PaCO2) / (PBW × 100 × 37.5). Delta measurements were calculated using the difference between the day 3 MV value and the day 1 MV value. Pulmonary mechanics was measured in VCV mechanical ventilation mode.
Outcome
The primary outcome is mortality at day 30. We also collected data on the duration of the MV.
Data collection
The following information was recorded: age, sex, predicted weight, severity of the disease assessed with the SOFA scale on ICU admission, comorbidities including diabetes, arterial hypertension, chronic kidney disease, obesity, cardiovascular disease, hypothyroidism, and chronic obstructive pulmonary disease (COPD). The values of the mechanical ventilator programming parameters at day 1 and day 5 were recorded, including PEEP, FIO2, tidal volume, respiratory frequency, and values of pulmonary mechanics, driving pressure, static compliance and plateau pressure, arterial blood gas values pH, arterial CO2 pressure, oxygenation evaluated by the PaO2/FIO2 index, patient position and ventilation efficiency assessed by the VR. The time spent on MV, length of ICU stay, total length of hospital stay and death at 30 days were also included.
Statistical analysis
Statistical analysis was performed with the statistical software program STATA version 15 licensed to Unisanitas. Categorical variables were described using absolute and relative frequencies, and quantitative variables using measures of central tendency and dispersion, depending on the distribution of the data evaluated by the Shapiro-Wilk test (p < 0.05). Categorical variables were compared by the Chi-square test or Fisher’s exact test, while continuous variables were compared by the nonparametric Wilcoxon rank sum test.
A logistic regression model was carried out after determining a priori a list of possible factors based on their clinical relevance and the results of the bivariate analyses. The backwards method was used to enter the cofactors, and for the final model a p value < 0.05 was considered statistically significant. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated. To evaluate the relevant cofactors, the model’s goodness-of-fit was evaluated with the Hosmer-Lemeshow test. The relative quality of the model was evaluated by calculating the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The difference in absolute values between days 1 and 5 of the variables associated with mortality was calculated.
Simple collinearity was assessed using Pearson’s correlation coefficient (r). Correlation between the VR and the other study variables was performed using the Spearman correlation coefficient, multicollinearity was assessed by analysing variance inflation factors.
Results
Study population
A total of 533 patients were included. Most were male (68.5% n = 365) and the median age was 63 years (53–72). The most frequent comorbidity was arterial hypertension, present in 215 (40.3%), and only 40 (7.5%) had chronic kidney disease. The 30-day mortality rate was 48.8% (260 patients). Table 1 summarizes the main demographic and baseline clinical characteristics of the study population.
Clinical features at the start of mechanical ventilation
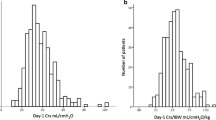
At the start of MV the median PaO2/FIO2 index was 122 (89–160) mmHg; pH value 7.31 (7.24–7.36); lactate level 1.5 (1.3–1.85) mg/dl; PaCO2 49.8 (42–59) mmHg; with a respiratory rate of 18 [18,19,20] per minute; tidal volume 7.3 (6.8–7.9) ml/kg ideal weight. Three hundred and forty-four patients (64.5%) required pronation on the first day; driving pressure was 12 [10,11,12,13,14,15] mmHg, plateau pressure 24 [22,23,24,25,26,27] mmHg; pulmonary compliance 37 (30–45) ml/cmH2O and VR 1.83 (1.48–2.2); median duration of MV was 13 days; in non-survivors median duration of MV was 13 days [8,9,10,11,12,13,14,15,16,17,18,19,20] vs. 15 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] in survivors (p 0.001); median FiO2 in non-survivors was 70% (50–90) vs. 60% (40–80) in survivors p 0.02; SOFA score was 6.0 (5.0–7.0).
Clinical evolution at day 5 of mechanical ventilation
The main findings found at day 5 are reported in Table 2. There was evidence of an increase in the PaO2/FIO2 ratio to 148 mmHg compared with the start of MV. Non-survivors obtained a lower PaO2/FIO2 index than survivors: 130 (90–159) vs. 169 (140–197) p 0.015, and a lower pH (7.35, range 7.26-7. 41) vs. 7.40 (range 7.37–7.44) p 0.001; plateau pressure was higher in non-survivors (26, range 22–28) vs. 23 [21,22,23,24,25] p 0.001, driving pressure was significantly higher in non-survivors (14, range 11–16) vs. 12 [10,11,12,13,14] p 0.02, and finally VR was significantly higher in non-survivors, 2.1 (1.8–2.5) vs. 1.8 (1.6–2.1) p 0.01 (see Fig. 2).
Association between the different variables measured at day 5 and mortality and changes between the start of mechanical ventilation and day 5. Association between ventilatory ratio at day 5 (A), plateau pressure day 5 (B) and driving pressure day 5 (C) and mortality at day 30. Figure (D) shows the change in the value of driving pressure, (E) plateau pressure and (F) ventilatory ratio between day 1 and day 5 with statistically significant differences. The horizontal lines of the box-and-whisker plots refer to the median, while the upper and lower lines represent the interquartile range
Predictors of 30-day mortality
The 30-day mortality rate was 48.8%. The highest mortality was found in the 71–80 years age group in which 85 patients died (70.2% of the total for the age group). The mortality rate was 68.6% in men vs. 31.4% in women. The pronation was a protective factor for mortality (OR crude 0.77. 95% CI 0.66–0.90 p 0.001) after including possible confounding factors such as days of MV, initial lactate, PaO2/FIO2 index at day 5, ventilatory ratio at day 5, plateau pressure at day 5, driving pressure at day 5 and age, the logistic regression model showed that the VR was independently associated with mortality (OR 2.1, 95% CI 1.35–3.3 p 0.001). As secondary findings, driving pressure (OR 2.9, 95% CI 1.7-5.0 p 0.001), plateau pressure (OR 2.0, 95% CI 1.02-4.0 p 0.04) and age range 71–80 (OR 5.2, 95% IC 2.76-10.0 p 0.001). Were independently associated with mortality. (see Table 3).
The increases in VR at day 5 above 2.0 (OR 1.42 95% CI 1.03–2.01, p 0.04), in driving pressure at day 5 (OR 1.56, 95% CI 1.10–2.22, p 0.001) and in plateau pressure at day 5 (OR 1.9 95% CI 1.34–2.69, p 0.001) significantly raised the risk of mortality, but the deterioration of the PaO2/FIO2 index was not associated with an increased mortality risk (Table 4).
Discussion
The aim of the present study was the main objective is to establish that values of pulmonary mechanics, including the ventilatory ratio, are risk factors for 30-day mortality in mechanically ventilated with ARDS due covid-19. The findings show that the VR, driving pressure, plateau pressure measured at day 5 and the change in these variables between days 1 and 5, age and heart failure were associated with mortality at 30 days of follow-up. The 30-day mortality rate in patients who remained on MV for at least 5 days was 48.8%.
The demographic variables associated with higher mortality were age, in agreement with several recent studies [10,11,12], heart failure and male sex [11, 13, 14].
In agreement with previous studies [8, 15,16,17], we did not find an association between the PaO2/FIO2 index and mortality at the beginning of MV, and nor did oxygenation impairment from day 1 to day 5 appear to be associated with mortality. In this cohort of patients the median compliance was 36 ml/cmH2O, also in agreement with several other studies in patients with ARDS (18, 19, 20, 21); however, compliance was not significantly associated with prognosis. It has been proposed that ARDS patients with COVID-19 may have two different phenotypes related to pulmonary compliance and that this distinction could be used to guide a rigorous, personalized titration of the PEEP value [22]. In our study, however, the PEEP value did not differ significantly between survivors and non-survivors measured at the different time points.
Among the complementary treatments for refractory hypoxemia, prone position was used in 56.9% of the patients considered to present relevant values. The pronation reduced the risk of mortality, as has been reported elsewhere [23]. We did not obtain data on the specific causes of nonpronation. In this cohort driving pressure was shown to be a relevant variable and its increase was associated with mortality, as other investigations have shown [24,25,26]. Also in agreement with other studies [23, 27], the increase in plateau pressure meant a higher risk of mortality.
The VR is a validated index in controlled modes of MV. It is frequently used, given the ease of its calculation at the patient’s bedside by recording the PaCO2 and minute ventilation, and it can be used as a surrogate for the dead space fraction vd/vt; a value close to 1 means that pulmonary ventilation is normal [4]. Deficient ventilation is frequent in patients with ARDS, as previous studies have reported [28,29,30]. High VR values in patients with ARDS without COVID-19 were associated with mortality [15, 31]; additionally, it has been demonstrated that patients with ARDS and COVID-19 presented a high VR associated with increased vd/vt [21, 32], as we found in our study.
We did not find an association between VR at day 1 and 30-day mortality. In previous work, this association was found to be statistically significant at the beginning of MV [8, 15]. In contrast to our results, in a cohort of 927 consecutive ARDS patients with COVID-19 it was reported that a rising VR at day 3 was not independently associated with 28-day mortality after adjustment for a baseline risk model that included chronic comorbidities and ventilatory and oxygenation parameters [16]. A major difference between that study and ours is that we evaluated the change between day 1 and day 5, finding that the increase of this value above 2.0 on day 5 was associated with a greater risk of mortality at 30 days, this finding can be explained by the deterioration of pulmonary mechanics during the course of the disease.
In our cohort there were no significant differences in the SOFA scale measured on day 1 between survivors and non-survivors; this may probably be due to the performance of the scale used early in the ICU, Another aspect to take into account and which may justify this finding is that the clinical condition of the patients at the beginning of the MV did not show high severity and later the function of the organs could deteriorate rapidly in this group of patients, considering the moment of evaluation of this severity scale in the ICU as a relevant topic for future research.
Among the limitations of the study, we should note that measurements on day 1 and day 5 are only two time points in the course of ARDS due to COVID-19 and may represent the moments when the disease was at its worst in the ICU. Nor was information on metabolism and CO2 production available, two phenomena that may intervene in ventilatory efficiency, is a topic that has been previously researched; finally, the retrospective nature of the study may have introduced biases.
In addition, we were unable to calculate the sample size objectively, although the number of participants we obtained sufficient power to detect significant differences in the primary outcome. Strengths of the study include the multicenter design that allows the collection of information from other institutions, the multivariate analysis at day 1 and day 5 and the evaluation of the change produced at these two time points that substantiate to the results obtained.
The results of this study have implications for clinical practice, since they show that the measurement of the VR is a tool that can be added to driving pressure and plateau pressure for the prognosis of ARDS patients with COVID-19, thanks to its simple formula that can be applied at the patient’s bedside to estimate the dead space fraction. The findings presented here may help to guide decision making for ARDS patients on MV in the ICU.
Conclusions
Ventilatory ratio, plateau pressure, driving pressure, and age were identified as independent risk factors for 30-day mortality in patients with ARDS due to COVID-19 on day 5 of invasive mechanical ventilation.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive care unit
- ARDS:
-
Acute Respiratory Distress Syndrome
- COVID 19:
-
Coronavirus disease 2019
- PEEP:
-
Positive end-expiratory pressure
- VR:
-
Ventilatory ratio
- FIO2 :
-
Fraction of inspired oxygen
- PaCO2 :
-
Partial pressure of carbon dioxide in arterial blood
- VD/VT:
-
Dead space fraction
- SOFA:
-
Sequential Organ Failure Assessment score
- PaO2/FIO2 :
-
Arterial oxygen pressure/inspired oxygen fraction index
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- ECMO:
-
Extracorporeal membrane oxygenation
- Crs:
-
Static compliance
References
Elsayed HH, Hassaballa AS, Ahmed TA, Gumaa M, Sharkawy HY, Moharram AA. Variation in outcome of invasive mechanical ventilation between different countries for patients with severe COVID-19: a systematic review and meta-analysis. PLoS ONE June. 2021;4(6):e0252760.
Grasselli G, Cattaneo E, Florio G, Ippolito M, Zanella A, Cortegiani A, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care March. 2021;20(1):115.
Xiang G, Xie L, Chen Z, Hao S, Fu C, Wu Q, et al. Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Ann Palliat Med March. 2021;10(3):2723–35.
Sinha P, Fauvel NJ, Singh S, Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth May. 2009;102(5):692–7.
Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med January. 2014;15(2):149–58.
Monteiro ACC, Vangala S, Wick KD, Delucchi KL, Siegel ER, Thompson BT, et al. The prognostic value of early measures of the ventilatory ratio in the ARDS ROSE trial. Crit Care September. 2022;29:26:297.
Sinha P, Fauvel NJ, Singh P, Soni N. Analysis of ventilatory ratio as a novel method to monitor ventilatory adequacy at the bedside. Crit Care Lond Engl February. 2013;27(1):R34.
Torres A, Motos A, Riera J, Fernández-Barat L, Ceccato A, Pérez-Arnal R, et al. The evolution of the ventilatory ratio is a prognostic factor in mechanically ventilated COVID-19 ARDS patients. Crit Care Lond Engl September. 2021;13(1):331.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of Observational studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med October. 2007;16(10):e297.
Tian T, Zhang J, Hu L, Jiang Y, Duan C, Li Z, et al. Risk factors associated with mortality of COVID-19 in 3125 counties of the United States. Infect Dis Poverty January. 2021;4(1):3.
Sepandi M, Taghdir M, Alimohamadi Y, Afrashteh S, Hosamirudsari H. Factors Associated with Mortality in COVID-19 patients: a systematic review and Meta-analysis. Iran J Public Health July. 2020;49(7):1211–21.
Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. January 2021;1(1):17–26.
Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors Associated with Mortality among patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. October 2020;1(10):1345–55.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis August. 2021;21(1):855.
Morales-Quinteros L, Schultz MJ, Bringué J, Calfee CS, Camprubí M, Cremer OL, et al. Estimated dead space fraction and the ventilatory ratio are associated with mortality in early ARDS. Ann Intensive Care November. 2019;21:9:128.
Morales-Quinteros L, Neto AS, Artigas A, Blanch L, Botta M, Kaufman DA, et al. Dead space estimates may not be independently associated with 28-day mortality in COVID-19 ARDS. Crit Care May. 2021;17:25:171.
Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med February. 2021;9(2):139–48.
Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med. May 26, 2020;10.1097/CCM.0000000000004457.
Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically Ill patients in the Seattle Region — Case Series. N Engl J Med March. 2020;30:NEJMoa2004500.
Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a Cohort Study. Am J Respir Crit Care Med June. 2020;15(12):1560–4.
Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, et al. Respiratory mechanics and Gas Exchange in COVID-19–associated Respiratory Failure. Ann Am Thorac Soc September. 2020;17(9):1158–61.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 Pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–102.
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe Acute Respiratory Distress Syndrome. N Engl J Med June. 2013;6(23):2159–68.
Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the Acute Respiratory Distress Syndrome. N Engl J Med February. 2015;19(8):747–55.
Guérin C, Papazian L, Reignier J, Ayzac L, Loundou A, Forel JM, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care Lond Engl November. 2016;29(1):384.
Aoyama H, Pettenuzzo T, Aoyama K, Pinto R, Englesakis M, Fan E. Association of driving pressure with mortality among ventilated patients with Acute Respiratory Distress Syndrome: a systematic review and Meta-Analysis*. Crit Care Med February. 2018;1(2):300–6.
Yasuda H, Sanui M, Nishimura T, Kamo T, Nango E, Abe T, et al. Optimal Upper limits of Plateau pressure for patients with Acute Respiratory Distress Syndrome during the First Seven days: a Meta-regression analysis. J Clin Med Res January. 2021;13(1):48–63.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the Acute Respiratory Distress Syndrome. N Engl J Med April. 2002;25(17):1281–6.
Kallet RH, Alonso JA, Pittet JF, Matthay MA. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care September. 2004;49(9):1008–14.
Kallet RH, Zhuo H, Ho K, Lipnick MS, Gomez A, Matthay MA. Lung Injury etiology and other factors influencing the Relationship between Dead-Space Fraction and Mortality in ARDS. Respir Care October 1. 2017;62(10):1241–8.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med February. 2019;1(3):333–41.
Beitler JR, Thompson BT, Matthay MA, Talmor D, Liu KD, Zhuo H, et al. Estimating dead-space fraction for secondary analyses of ARDS clinical trials. Crit Care Med May. 2015;43(5):1026–35.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
HMP, JMA, JEM participated in protocol development, study design and collection of information from medical records; CIR, MI, participated in study management, HMP, RM, JM, DMF, DRC, JRM contributed to statistical analysis, interpretation of data, analysis of results and writing of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Ethics and Research Committee of the Fundación Universitaria Sanitas - CEIFUS 3347-22 and conducted in accordance with the principles of the Declaration of Helsinki. All subjects and their legal guardians who participated in the study signed informed consent forms for participation approved by the institutional ethics committee.
Consent for publication
All subjects and their legal guardians who participated in the study signed an informed consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Parada-Gereda, H.M., Avendaño, J.M., Melo, J. et al. Association between ventilatory ratio and mortality in patients with acute respiratory distress syndrome and COVID 19: A multicenter, retrospective cohort study. BMC Pulm Med 23, 425 (2023). https://doi.org/10.1186/s12890-023-02733-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02733-9