Abstract
Introduction
Tuberculosis (TB) is still a major contributor to the global health burden. Pulmonary TB can lead to life-threatening respiratory failure necessitating extracorporeal membrane oxygenation (ECMO) therapy. However, data on ECMO experience in the management of TB patients are scarce.
Methods
We conducted a systematic review of the literature using the search terms ECMO, extracorporeal membrane oxygenation, TB and tuberculosis in three databases (Medline, Web of Science and EMBASE). Clinical data were extracted by two independent investigators. Clinical parameters, such as mode of ECMO therapy, duration of treatment and clinical outcomes, were assessed.
Results
Overall, 43 patients from 15 countries were included in the analysis. The age ranged from 0 to 65 years, 39.5% were male, and 60.5% were female. The majority of patients suffered from ARDS (83.4%), with a mean Horovitz quotient of 68.1 (range 30.0–131.0). 83.7% received VV-ECMO, and 24.3% received VA-ECMO. Coinfections and complications were frequently observed (45.5% and 48.6% respectively). At the end of the respective observation period, the overall outcome was excellent, with 81.4% survival.
Discussion
ECMO therapy in TB patients appears to be a feasible therapeutic option, providing a bridge until antimycobacterial therapy takes effect. As the underlying cause is reversible, we advocate for the evaluation of ECMO usage in these patients with acute cardiac or respiratory failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Tuberculosis (TB) is still a major contributor to the global health burden. After COVID-19, TB is the deadliest infectious disease from a single agent [1]. In 2021, there was an upsurge in the estimated number of deaths from 1.4 million in 2019 to 1.6 million [1]. The gap in access to TB diagnosis and treatment during the COVID-19 pandemic will continue to negatively impact the WHO’s END TB strategy for years to come [2].
Nevertheless, the COVID pandemic also brought a variety of new insights, for example into the treatment of acute respiratory distress syndrome (ARDS) [3]. According to the Berlin definition “ARDS is a type of acute diffuse, inflammatory lung injury, leading to increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue” [4]. With the New Global Definition of Acute Respiratory Distress Syndrome, the Berlin Definition has been expanded, “including the use of high nasal oxygen (HFNO), expanding the use of pulse oximetry in place of arterial blood gases, use of ultrasound for chest imaging, and the need for applicability in resource-limited settings” [5]. Pulmonary TB, if left untreated, can also lead to life-threatening respiratory failure [6]. Nevertheless, ARDS secondary to tuberculosis remains a rarity: studies from South Africa and India estimated that 2 to 4.8% of ARDS cases admitted to the ICU were caused by tuberculosis [7]. The numbers for tuberculosis-related ARDS are probably lower in countries with a lower prevalence of TB.
Therefore, practical experience in the management of ARDS due to tuberculosis is still limited. For non-TB patients with severe ARDS, treatment by extracorporeal membrane oxygenation (ECMO) is a well-established treatment option. ECMO is a form of extracorporeal life support that utilizes a heart–lung machine to facilitate gas exchange and minimize ventilator-associated lung injury [8]. The number of ECMO runs and survival rates have been increasing within the last decade, with a worldwide overall survival rate of 67% in 2022, and for adult patients receiving ECMO for respiratory failure the survival rate was 66% [9]. Moreover, it has recently been shown that 90-day mortality for patients, fulfilling the American–European Consensus Conference definition or the Berlin definition for ARDS, is significantly lowered by venovenous ECMO compared with conventional ventilator management [10]. However, ECMO therapy is still associated with a variety of complications such as bleeding, embolisms or circuit failure [11]. However, mortality for TB patients who required intubation or ARDS treatment other than ECMO ranged from 62 to 69% [12, 13]. The use of ECMO treatment can lead to significantly improved survival rates in non-TB patients, but is rarely conducted in patients with TB [8].
Herein, we present the available data on ECMO treatment in TB patients to evaluate whether ECMO therapy is a viable option for these patients.
Methods
This systematic review was performed in accordance with the current Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and checklist (PRISMA) [14]. A prespecified protocol for this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42022357405) on September 23rd 2022. We conducted a systematic review to identify studies about ECMO usage in the management of tuberculosis patients to investigate the efficacy of ECMO therapy within this cohort.
Search strategy and selection criteria
The databases Medline (PubMed), Web of Science, and EMBASE were searched without any restrictions on year of publication and language on September 1st, 2022, February 14th, 2023, and September 20th, 2023 for eligible studies. The search strategy was developed by using a combination of Medical Subject Heading terms (MeSH) with the keywords “extracorporeal membrane oxygenation “, “ECMO”, “tuberculosis” or “TB”. As the same operators were applicable for all three databases, the final search term ("tuberculosis" OR "Tb" OR "TBC" OR "Tuberc*") AND ("extracorporeal membrane oxygenation" OR "ECMO") was applied. Duplicates were removed, and the remaining references were independently screened by two reviewers (RI and ASZ). For each study, titles and abstracts were screened for eligibility. Afterwards, all relevant full-text manuscripts were reviewed by the same two authors in regard to the inclusion and exclusion criteria. In case of multiple articles reporting on the same patient, the information from all of them was merged into one data set. Differences were resolved through discussion, and final decisions were made by vote after consulting with NW.
Inclusion and exclusion criteria
Included were all studies reporting on a minimum of one patient with active/current tuberculosis caused by Mycobacterium tuberculosis complex and the use of ECMO therapy regardless of the patients’ age. The exclusion criteria were a history of previously treated and not currently active tuberculosis or mycobacterial infections due to non-tuberculous mycobacteria (NTM).
Statistical analysis
Clinical data were independently extracted by two reviewers (RI and ASZ) and checked by a third investigator for plausibility (NW). We extracted demographic parameters, such as age, sex, country of treatment and origin, clinical manifestations of TB and comorbidities as well as microbiological parameters including drug sensitivity, antimycobacterial therapy, and coinfections. For the evaluation of ECMO treatment variables such as the Horovitz quotient, type of ECMO (venovenous (VV V-V) / venoarterial (VA V-A) / (venovenoarterial (VVA V-VA)), duration of ECMO therapy, the possible advent of complications, and whether the patients expired on ECMO were collected. For subsequent mortality analysis, the status of survival was evaluated at the respective time of publication. All data were collected into an Excel database, and missing data were handled as “NA” (not available) in the resulting data table.
All statistical analyses were performed in R V.4.2. (“Vigorous Calisthenics”) using the tidyverse [15]. Categorical data are depicted as nominators with denominators and percentages, and continuous data are depicted as the mean with range for normally distributed data and median with interquartile range for non-normally distributed data. Normality was tested using the Shapiro–Wilk-Test. Geographical data were depicted using the ggmap package within R [16]. The overall survival of the included patients was assessed by generating an unadjusted Kaplan–Meier-curve with time-to-event analysis in R’s survival and survminer packages [17, 18]. For all statistical tests a significance level of alpha = 0.05 was used.
Results
Included patients and general characteristics
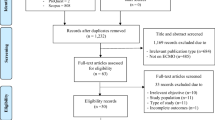
Overall, 40 publications from 1975 to 2022 were included resulting in a patient cohort of 43 patients (37 case reports, whereby four case reports reported on the same two patients, one case series, one retrospective cohort study and one prospective cohort study) (Fig. 1). The country with the highest number of published articles was the United States with 8 cases, followed by China with 5 cases and Germany with 4 cases (Fig. 2A). The mean age of the patients was 29.5 years (range 0–65 years), 39.5% (15/38) were male, and 60.5% (23/38) were female (Table 1). The majority of patients did not have any comorbidities. However, six patients received immunosuppressive therapy, four suffered from pre-existing diabetes, one from an HIV- infection, and in five cases, the patients were pregnant. In 41 articles, the clinical manifestation of tuberculosis was described: 68.3% (28/41) of the patients had isolated pulmonary tuberculosis, 29.3% (12/41) suffered from a disseminated infection, and one patient had isolated extrapulmonary tuberculosis (pericardial manifestation). Thirty-six of the 43 patients (83.7%) had pulmonary involvement and eight (21.1%) had additional abdominal involvement. Nine patients (23.7%) suffered from additional other manifestations such as bone marrow or muscle abscesses. Most patients (63.3%, 19/30) presented with fever, followed by weight loss (36.7%, 11/30) and night sweats (13.3%, 4/30). In X-ray or computer tomography (CT) examinations, 29 out of 33 patients (87.9%) showed bilobal infiltration, and 39.4% (13/33) showed cavities. During the observation period, 15 of 33 patients (45.5%) developed a coinfection, 10 of whom (66.7%) had bacterial pneumonia, 2 (13.3%) had a bloodstream infection, and 3 (20%) had a fungal infection (one candidiasis, one mucor-infection and one aspergillosis) (Table 1).
Susceptibility testing of the mycobacteria was reported in 29 cases (67.4%). A total of 90.0% (27/29) of these patients had fully drug-sensitive tuberculosis (Table 2), while three patients had mono-resistant isolates (one isoniazid resistance, one rifampicin resistance and one streptomycin resistance). No cases of multidrug-resistant tuberculosis (MDR) or extensively drug-resistant tuberculosis (XDR) were reported. The use of tuberculostatic therapy was described in 34 of 38 cases (89.5%). Patients mainly received tuberculostatic standard therapy consisting of isoniazide, a rifamycin, ethambutol and pyrazinamide. Additional steroid therapy was administered in 15 of 23 cases (65.2%).
Course of ECMO therapy
The majority of patients were described to suffer from ARDS (83.4%, 30/36) with a mean Horovitz quotient of 68.1 (IQR 30.0–131.0), while cardiac failure was described in 9/34 patients (26.1%) (Table 3). Twenty-seven out of 37 patients were exclusively on venovenous ECMO (VV V-V) (73%), while five patients were exclusively on venoarterial ECMO (VA V-A) (10.8%). In 4 additional patients, the type of ECMO was changed during the treatment process. All four of these patients were started on VA V-A due to respiratory failure coupled with hemodynamic instability.
If mentioned, ECMO was implanted with a median delay of 9 days after initiation of TB treatment. ECMO treatment was continued for a median duration of 10 days, ranging from 3 to 89 days. The median time of ventilation was 33 days, ranging from 0 to 130 days (n = 19). Time in the ICU ranged from 12 to 114 days with a median of 45.1 days (n = 10). Patients were observed for up to 27 months.
Complications during ECMO therapy occurred in 17 of 35 cases (48.6%). The most common complications were bleeding complications and thrombocytopenia (29.4% (5/17) and 23.5% (4/17)). Alveolar haemorrhage (4/17; 23.5%) and intracranial haemorrhage (2/17; 11.7%) were also mentioned. Pneumothoraxes occurred in 11.7% (2/17) and disconnection of failure of the ECMO pump was mentioned in 17.6% (3/17). Two patients needed long term ventilation after ECMO weaning (7.1%). Eight patients died during the total observation time (18.6%), seven of whom died during ECMO therapy (16.3%) with a mean time to death of 21.1 days (Fig. 2B). Causes of death were intracranial haemorrhage, cardiac asystole or multiorgan failure. One patient died during ECMO installation due to a vascular complication. Individual patient characteristics are summarized in Table 4.
Discussion
This meta-analysis demonstrates the feasibility of ECMO therapy in patients suffering from tuberculosis and respiratory or cardiac failure over a time period of 47 years. It appears that ECMO therapy in patients with TB could have a positive effect on overcoming ARDS or bridging the time until response to tuberculostatic therapy.
This study has two major limitations: first, although all available case reports in the medical literature have been included, the sample size remains comparatively low while reporting on a varied patient cohort, including newborns, children and adults with different courses of disease. Second, due to the relatively small sample size, the data presented here could be prone to publication bias and mortality could be underestimated.
The long observation period (1975 – present) was chosen to comprise the entire period of ECMO therapy. In this time period, the technology and experience of ECMO therapy have completely changed, and implementation and survival rates have been increasing especially over the last decade. Therefore, the early reported cases might not be comparable to the present data. However, there are only two studies prior to 2000, which does not result in any significant impact in our analysis.
Despite these limitations, this study comprises prior experience with the use of ECMO therapy in this vulnerable population. A similar small cohort with limited data on ECMO application are HIV patients with severe ARDS caused by Pneumocystis jirovecii pneumonia (PJP). Rilinger et al. recommended that patients with HIV-associated PJP should not be withhold from ECMO therapy, while showing comparable results to those of our patient cohort [19]. Therefore, ECMO as a therapeutic tool in severe TB-associated ARDS should be considered and further implemented in the clinical routine. In particular, young TB patients without any other comorbidities have good potential for rehabilitation and therefore a very good chance of complete recovery. The average age of our cohort was lower than that of comparable ECMO cohorts. In 2018 Friedrichson et. al. found that half of the German cohort’s patients were over 45 years old [20]. Only 22.5% of patients in our cohort was older than 45 years.
In addition to improving gas exchange, ECMO is initiated to reduce ventilator-induced lung injury (VILI) and allows the facilitation of ultralow volume lung ventilation. ECMO therapy can be utilized to buy time and preserve organ function until TB medication can lessen the mycobacterial load. Due to the slow growth of mycobacteria, it can take weeks to observe any effects of tuberculostatic treatment. Additional coinfections such as bacterial pneumonia and fungal infections growing in the affected lung can further prolong the healing process. As in our cohort the rate of coinfection was relatively high, and their role as the etiology of ARDS in these patients cannot be excluded.
We did not identify any case reports of patients with MDR tuberculosis receiving ECMO therapy. The lack of published cases may be due to the lower share of MDR TB (4.2% in the EU in 2020) or even be partially caused by the fact that in many countries it is still common practice that patients with MDR-TB lung disease undergo early lobectomy or pneumectomy [21, 22]. In our view, multidrug-resistant tuberculosis should not be a contraindication for ECMO. MDR therapy might pose some additional issues since many medications are available only as oral formulations, and adequate absorption from the gastrointestinal tract might be impeded in the critically ill. Inadequate drug levels were also a problem for the first-line drug regimens as highlighted in three of the case reports [23,24,25]. In two cases rifampicin was switched from oral to intravenous administration to circumvent the issues with gastrointestinal absorption, but therapeutic levels were still not reached using the standard dose [24, 25]. In one case, rifampicin levels only increased after ECMO weaning leading the authors to indicate that the subtherapeutic levels might be caused by the larger volume of distribution during the ECMO circuit [24]. These findings suggest that TB patients need higher doses of standard medications during ECMO therapy even in the absence of dialysis, but more studies are needed to support this hypothesis.
Conclusion
The practice of extracorporeal life support (ECLS) is becoming a crucial component of contemporary intensive therapy. Our systematic review and meta-analysis indicate that ECMO therapy is a feasible option for tuberculosis patients with respiratory or cardiac failure, bridging the time for pulmonary recovery. As the underlying disease is treatable, we advocate not to deprive this patient cohort of an evaluation for ECMO therapy. Additional prospective multicenter analyses are required to evaluate evidence-based guidance for clinical practice.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ECC02R:
-
Extracorporeal carbon dioxide removal
- ECMO:
-
Extracorporeal membrane oxygenation
- LFPPV-ECCO2R:
-
Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal
- MDR TB:
-
Multidrug resistant tuberculosis
- NTM:
-
Non-tuberculous mycobacteria
- TB:
-
Tuberculosis
- VA V-A:
-
Venoarterial ECMO
- VV V-V:
-
Venoveno ECMO
- VVA V-VA:
-
Venovenoarterial ECMO
- VVA V-AV:
-
Venoarterialveno ECMO
References
WHO. Global tuberculosis report 2021. Geneva: World Health Organization; 2021. (Licence: CC BY-NC-SA 3.0 IGO).
WHO. Global tuberculosis report 2021. Geneva: World Health Organization; 2022. (Licence: CC BY-NC-SA 3.0 IGO).
Benedetto M, Piccone G, Baiocchi M, Cerchierini E, Adversi M, Rossi A, et al. Increased duration and similar outcomes of V-V ECLS in patients with COVID-19 ARDS compared to non-COVID ARDS: Single center experience. Artif Organs. 2023;47(4):731–9.
Acute Respiratory Distress Syndrome. The Berlin Definition. JAMA. 2012;307(23). Cited 2023 May 12. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2012.5669.
Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2023;rccm:202303-0558WS.
Luies L, du Preez I. The Echo of Pulmonary Tuberculosis: Mechanisms of Clinical Symptoms and Other Disease-Induced Systemic Complications. Clin Microbiol Rev. 2020;33(4):e00036-e120.
Dyer RA, Chappell WA, Potgieter PD. Adult respiratory distress syndrome associated with miliary tuberculosis. Crit Care Med. 1985;13(1):12–5.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet Lond Engl. 2009;374(9698):1351–63.
ECLS Registry Report International Summary April, 2023 Report data through 2022. Extracorporeal Life Support Organization. 2023. Cited 2023 Sep 23. Available from: https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx.
Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46(11):2048–57.
The Risks & Complications of ECMO/ECLS. Extracorporeal Life Support Organization. Cited 2023 Sep 23. Available from: https://www.elso.org/ecmo-resources/ecmo-risks-and-complications.aspx.
Kim JY, Park YB, Kim YS, Kang SB, Shin JW, Park IW, et al. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2003;7(4):359–64.
Penner C, Roberts D, Kunimoto D, Manfreda J, Long R. Tuberculosis as a Primary Cause of Respiratory Failure Requiring Mechanical Ventilation. Am J Respir Crit Care Med. 1995;151(3_pt_1):867–72.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29:n71.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686.
Kahle D, Wickham H. ggmap: Spatial Visualization with ggplot2. R J. 2013;5(1):144.
Kassambara A. survminer: Drawing Survival Curves using ‚ggplot2‘. 2021. Cited 2023 May 12. Available from: https://cran.r-project.org/web/packages/survminer/index.html.
Therneau TM. survival: Survival Analysis. 2023. Cited 2023 May 12. Available from: https://cran.r-project.org/web/packages/survival/index.html.
Rilinger J, Staudacher DL, Rieg S, Duerschmied D, Bode C, Wengenmayer T. Extracorporeal membrane oxygenation in Pneumocystis jirovecii pneumonia: outcome in HIV and non-HIV patients. Crit Care. 2019;23(1):356.
Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. 2021;25(1):38.
European Centre for Disease Prevention and Control. Tuberculosis. In: ECDC. Annual epidemiological report for 2020. Stockholm: ECDC; 2022.
Vashakidze SA, Gogishvili SG, Nikolaishvili KG, Avaliani ZR, Chandrakumaran A, Gogishvili GS, et al. Adjunctive surgery versus medical treatment among patients with cavitary multidrug-resistant tuberculosis. Eur J Cardiothorac Surg. 2021;60(6):1279–85.
Vesteinsdottir E, Myrdal G, Sverrisson KO, Skarphedinsdottir SJ, Gudlaugsson O, Karason S. ARDS from miliary tuberculosis successfully treated with ECMO. Respir Med Case Rep. 2019;26:165–7.
Kim HS, Lee ES, Cho YJ. Insufficient Serum Levels of Antituberculosis Agents During Venovenous Extracorporeal Membrane Oxygenation Therapy for Acute Respiratory Distress Syndrome in a Patient with Miliary Tuberculosis. ASAIO J. 2014;60(4):484–6.
Strunk AK, Ciesek S, Schmidt JJ, Kühn C, Hoeper MM, Welte T, et al. Single- and multiple-dose pharmacokinetics of ethambutol and rifampicin in a tuberculosis patient with acute respiratory distress syndrome undergoing extended daily dialysis and ECMO treatment. Int J Infect Dis. 2016;42:1–3.
Acknowledgements
We thank Victor Næstholt Dahl for assistance with the database research. In addition, we appreciate the constructive discussions on our research question with our colleagues at the University Hospital Frankfurt am Main.
Funding
Open Access funding enabled and organized by Projekt DEAL. This is an investigator-initiated study that did not receive any specific funding. No external source of funding was used for this project.
Author information
Authors and Affiliations
Contributions
RI, Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing, AZ, Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing, JaK Writing – review & editing, JuK Writing – review & editing, NW Project administration, Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this was a literature review and meta-analysis no ethical approval or consent to participate was required.
Consent for publication
Not applicable.
Competing interests
Raja Idris received speaker fees from Tillotts Pharma GmbH.
Ann-Sophie Zielbauer received speaker fees from Tillotts Pharma GmbH.
Julia Koepsell received speaker fees from Tillotts Pharma GmbH.
Jan Kloka has nothing to declare.
Nils Wetzstein has nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Idris, R., Zielbauer, AS., Koepsell, J. et al. Extracorporeal membrane oxygenation (ECMO) in patients with tuberculosis: systematic review and meta-analysis of 43 cases. BMC Pulm Med 24, 47 (2024). https://doi.org/10.1186/s12890-023-02715-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02715-x