Abstract
Background
Although asthma does not appear to be a risk factor for severe coronavirus disease 2019 (COVID-19), outcomes could vary for patients with different asthma subtypes. The objective of this analysis was to compare COVID-19 outcomes in real-world cohorts in the United States among patients with asthma, with or without evidence of allergy.
Methods
In a retrospective analysis of the COVID-19 Optum electronic health record dataset (February 20, 2020–January 28, 2021), patients diagnosed with COVID-19 with a history of moderate-to-severe asthma were divided into 2 cohorts: those with evidence of allergic asthma and those without (nonallergic asthma). After 1:1 propensity score matching, in which covariates were balanced and potential bias was removed, COVID-19 outcomes were compared between cohorts.
Results
From a COVID-19 population of 591,198 patients, 1595 patients with allergic asthma and 8204 patients with nonallergic asthma were identified. After propensity score matching (n = 1578 per cohort), risk of death from any cause after COVID-19 diagnosis was significantly lower for patients with allergic vs nonallergic asthma (hazard ratio, 0.48; 95% CI 0.28–0.83; P = 0.0087), and a smaller proportion of patients with allergic vs nonallergic asthma was hospitalized within − 7 to + 30 days of COVID-19 diagnosis (13.8% [n = 217] vs 18.3% [n = 289]; P = 0.0005). Among hospitalized patients, there were no significant differences between patients with allergic or nonallergic asthma in need for intensive care unit admission, respiratory support, or COVID-19 treatment.
Conclusions
Asthma subtype may influence outcomes after COVID-19; patients with allergic asthma are at lower risk for hospitalization/death than those with nonallergic asthma.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can result in life-threatening respiratory symptoms [1, 2]. Pre-existing chronic respiratory diseases, such as chronic obstructive pulmonary disease or interstitial lung disease [3,4,5,6], have been shown to be associated with poorer outcomes after SARS-CoV-2 infection, but evidence that asthma is also a risk factor is less clear. Studies investigating the relationship between asthma and COVID-19 have reported variable findings, although the general consensus is that asthma is not associated with a greater risk of severe outcomes such as hospitalization [3, 6,7,8,9,10,11,12], although this may be complicated by the use of biologics [13].
Interestingly, studies have reported that COVID-19 outcomes may be better for patients with allergic asthma than for patients with nonallergic asthma. For example, analysis of UK Biobank data found that nonallergic asthma was significantly associated with severe COVID-19, whereas allergic asthma had no association with severe COVID-19 [8]. In addition, a South Korean nationwide cohort analysis found that patients with nonallergic asthma had a greater risk of SARS-CoV-2 infection and poorer clinical outcomes than patients with allergic asthma [14]. Similarly, a recent cohort study in the United States found that, compared with patients with nonallergic asthma, patients with allergic asthma were half as likely to be hospitalized with COVID-19 [15].
Because outcomes associated with COVID-19 may differ by data source (e.g., insurance database vs hospital records), and by country (e.g., patients’ demographic characteristics, comorbidities, and background asthma medications), we conducted a real-world retrospective electronic health record (EHR) database analysis in the United States. To assess potential differences between asthma subtypes, COVID-19 outcomes, including deaths, hospitalizations, and interventions and treatments, were evaluated and compared in patients with and without evidence of allergic asthma.
Methods
Database
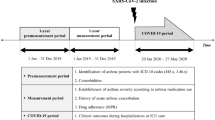
This was a retrospective analysis of data from the COVID-19 Optum EHR database (Optum Inc., Eden Prairie, MN, USA), which includes medical records sourced from high proportions of ambulatory, hospital, and integrated delivery networks throughout the United States, regardless of insurance provider. Data for patients of any age (capped at birth year of 1930 and earlier) with confirmed COVID-19 between February 20, 2020 and January 28, 2021, were used.
The index date for COVID-19 diagnosis was the earliest date of presumed diagnosis or laboratory-confirmed SARS-CoV-2 infection, defined according to the US Centers for Disease Control and Prevention (CDC) guidelines, which include an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code of U07.1 (COVID-19, virus identified) or U07.2 (COVID-19, virus not identified) [2]; a positive polymerase chain reaction diagnostic test for SARS-CoV-2; or an ICD-10 diagnosis code of B97.29 (other coronavirus as the cause of diseases classified elsewhere) without a negative diagnostic test within a 14-day window (± 7 days) [16].
To protect patient anonymity and confidentiality in compliance with the Health Insurance Portability and Accountability Act of 1996, all data used in this analysis were deidentified. The study was conducted according to the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki 2013. The study did not require institutional review board approval because only deidentified data were included. Administrative permissions were not required to access the data.
Cohort identification
Patient records were included in the analysis if they had evidence of persistent moderate-to-severe asthma, defined as ICD-10-CM J45.4X or J45.5X [17], at any time since October 2015. The cohort of patients with asthma with evidence of allergy (referred to as allergic asthma) was defined by a positive specific immunoglobulin E (IgE) serum test (IgE ≥ 0.35 kU/L), or skin prick test (Current Procedural Terminology code 95004) ordered by a relevant specialist (e.g., allergist, pulmonologist, dermatologist), or by a record of omalizumab use (Xolair®, Genentech, Inc., and Novartis Pharmaceuticals Corporation; Healthcare Common Procedure Coding System J code J2357). The cohort of patients without evidence of allergic asthma (referred to as nonallergic asthma) was defined as failing to meet the criteria for allergic asthma plus absence of any allergic comorbidities within 360 days before the index date. Patient demographics, clinical characteristics, and asthma medication use in the 12 months before the COVID-19 index date were extracted from the database. Patients were followed up from the index date through the end of data follow-up (date of the last record) or death, whichever occurred first.
Propensity score matching
After identification of cohorts, 1:1 propensity score matching was undertaken to obtain 2 comparable populations for evaluation in which specified key demographic and clinical covariates were balanced and potential bias in between-cohort comparisons from measured confounding factors was removed [18]. The propensity score was defined as the probability of being in the allergic asthma cohort vs being in the nonallergic asthma cohort, conditional on the demographic and clinical variables in a logistic regression model. The variables used for matching included demographic characteristics (age [mean and category], sex, race, ethnicity, geographical region, index month [to account for temporal changes in diagnosis and management of COVID-19]), and asthma status (severity and medication categories [excluding biologics]). Clinical risk factors for severe COVID-19 illness were selected according to assignment of “strong and most consistent” or “mixed” evidence levels assigned based on published literature, CDC evidence, and practicing medical doctors (authors). Charlson Comorbidity Index and CDC definitions [19] were aligned to give a consolidated list of clinical variables, and ICD-10-CM codes assigned to these to facilitate identification in the EHR database. These included cancer, cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, hypertension, obesity, pregnancy, smoking status, and type 2 diabetes. Patients with allergic asthma were matched 1:1 to patients with nonallergic asthma on the propensity score using greedy nearest neighbor algorithm with a caliper width of 0.1.
Outcomes
The outcomes evaluated included numbers of patient deaths from any cause after COVID-19 index date; number of deaths within 30 days after COVID-19 index date; number of deaths from any cause after COVID-19 index date among hospitalized patients; hospitalizations and emergency department (ED) visits from 7 days before to 30 days after COVID-19 index date; intensive care unit (ICU) admission; length of stay (days); interventions, such as invasive or noninvasive ventilation; and treatments for COVID-19. Health care resource use was assessed as hospital resource use (hospitalizations and ED visits) and outpatient visits (visits to a health care practitioner office, nonspecified, COVID-19–related, or asthma-related). Selection of the COVID-19 treatments evaluated was based on medical opinion and published treatments (to date of analysis). COVID-19 outcomes not requiring hospitalization, such as changes in senses of smell and taste, outpatient visits (all-cause, asthma-related, COVID-19–related visits to a health care practitioner office), and hospital resource use rates (as resource rate ratios) were also evaluated.
Statistical analysis
Descriptive analyses stratified by allergic asthma vs nonallergic asthma were performed for continuous variables using means, medians, and SDs, and for categorical variables using counts and percentages. Unadjusted differences in baseline characteristics between the 2 asthma cohorts were tested using nonparametric Mann–Whitney U tests for continuous variables, and χ2 tests and Fisher’s exact texts (with small cell counts) for categorical variables [20]. Poisson regression models were used to estimate hospital and outpatient care resource use counts per 1000 days and rate ratios, with 95% CIs and χ2 testing of regression coefficients [21]. The relative hazard of death comparing asthma groups and hazard ratios with 95% CIs was estimated using a Cox proportional hazards model. All analyses were performed using SAS Studio Release 3.7 (Enterprise Edition; ©2012–2017; SAS Institute Inc., Cary, NC, USA). Statistical significance threshold was set a priori using 2-sided tests at P < 0.05.
Results
Population characteristics
A total of 591,198 records were identified for patients who had been diagnosed with COVID-19 during the study period. Among patients with an asthma diagnosis, considerably fewer patients (n = 1595 [0.3%]) had a diagnosis of allergic asthma than nonallergic asthma (n = 8204 [1.4%]; Fig. 1). Patients with allergic asthma were slightly younger (mean [SD] age, 46.1 [18.8] years vs 49.4 [19.7] years; P < 0.0001) and more likely to have severe asthma (26.5% [n = 422/1595] vs 6.5% [n = 533/8204]).
Study flow chart. a Cutoff value for positive immunoglobulin E (IgE) test was 0.35 kU/L or greater. b Not mutually exclusive. c Allergic comorbid conditions include allergic rhinitis, acute sinusitis, chronic sinusitis, anaphylaxis, conjunctivitis, dermatitis, food allergy, and urticaria. COVID-19 coronavirus disease 2019
Propensity score matching yielded study cohorts for allergic and nonallergic asthma that both included 1578 patients (Table 1). Patients with allergic or nonallergic asthma were broadly similar in terms of clinical characteristics and clinical risk factors for severe COVID-19, and there were more females than males in both cohorts. There were differences before COVID-19 diagnosis in asthma medication (such as use of biologics) due to differences in the treatment of allergic and nonallergic asthma. A higher proportion of patients with allergic asthma than nonallergic asthma had a history of severe asthma within the previous 360 days (25.8% [n = 407] vs 20.3% [n = 321]; P < 0.0001), although the overall Charlson Comorbidity Index scores were similar for the matched cohorts.
Deaths
More patients with nonallergic asthma than allergic asthma died (from any cause) after a diagnosis of COVID-19 (2.4% [n = 38] vs 1.2% [n = 19]; P = 0.0111; Table 2). Similarly, death from any cause within 30 days of COVID-19 diagnosis also occurred more frequently in patients with nonallergic asthma than allergic asthma (2.0% [n = 32] vs 0.9% [n = 14]; P = 0.0266). Among patients who visited the ED or who were hospitalized between 7 days before and 30 days after the index date, a higher proportion of patients with nonallergic asthma than allergic asthma died (from any cause).
Cox proportional hazards modeling showed that risk of death after the COVID-19 index date was significantly lower for patients with allergic asthma than nonallergic asthma (hazard ratio, 0.48; 95% CI 0.28–0.83; P = 0.0087). However, among the individuals who died, the number of days between index date and death was not statistically significant according to allergic asthma history (Table 2).
Hospitalization, hospitalization outcomes, and treatment
Patients with nonallergic asthma were more likely to have visited the ED or to have at least 1 hospital admission within 7 days before or 30 days after their index date than patients with allergic asthma. The proportion of patients hospitalized with COVID-19 was greater for patients with nonallergic asthma than allergic asthma (18.3% [n = 289] vs 13.8% [n = 217]; P = 0.0006; Table 3). A similar proportion of those hospitalized in the 2 cohorts were admitted because of their asthma (nonallergic asthma, 73.0% [n = 211]; allergic asthma, 75.1% [n = 163]). Once patients were hospitalized, there were no significant differences between patients with allergic or nonallergic asthma in terms of need for ICU admission, ventilation, or oxygen therapy support, or in the proportion of patients readmitted (after release) within 30 days.
The proportion of hospitalized patients treated with medications for COVID-19, and the number of medications administered, were similar between the 2 cohorts (Table 4). The exception was dexamethasone, which was administered in 21.2% (n = 334) of patients with nonallergic asthma vs 17.4% (n = 274; P = 0.0068) of patients with allergic asthma, a finding not observed with other glucocorticoids.
Outpatient visits, and outcomes not requiring hospitalization
The number of outpatient visits for any reason was similar between the cohorts (nonallergic asthma, 48.0% [n = 758] vs allergic asthma, 45.9% [n = 724]; P = 0.2253) The number of outpatient visits for asthma was also similar between patients with nonallergic asthma (36.1% [n = 569]) or allergic asthma (38.2% [n = 603]; P = 0.2253). In addition, COVID-19–related outcomes that did not require hospitalization, including loss or distortion of sense of smell, change in sense of taste, or disturbances of senses of both taste and smell, were reported for less than 2.5% of patients (data not shown), and there were no significant differences between patients with allergic or nonallergic asthma.
Health care resource use
Patients with nonallergic asthma had a greater rate of hospital resource use (inpatient days and ED visits), a lower rate of outpatient visits for any reason or for asthma, and higher outpatient visits for COVID-19, compared with patients with allergic asthma (Table 5). The overall outpatient resource use rates related to any changes in senses of taste and smell were low, but slightly higher for patients with allergic asthma.
Discussion
Our real-world analysis used a large database (including EHR from health care facilities across most of the United States) to evaluate COVID-19 outcomes in patients with different asthma subtypes. We found that patients with evidence of allergic asthma had a lower risk of hospitalization or death from any cause after diagnosis, compared with patients with nonallergic asthma. However, once hospitalized, the rates of ICU admissions, need for respiratory support, and treatment were similar in both cohorts. In addition, we found that only around 11% of patients with COVID-19 and asthma had allergic asthma, which was a lower proportion than might be expected from epidemiological studies of asthma (at least half and up to 78% of patients with asthma have allergic asthma [22,23,24,25]). Although we were unable to estimate the rate of SARS-CoV-2 infection among asthma groups due to the nature of the available data, this may suggest that patients with allergic asthma have a lower risk of SARS-CoV-2 infection than those with nonallergic asthma. Nevertheless, we found that overall, nonallergic asthma was associated with worse outcomes than allergic asthma, which may be important to consider during treatment.
Our findings are in line with previous studies and support that allergic asthma is associated with lower risk of severe COVID-19 outcomes than nonallergic asthma [8, 11, 14]. Our findings also align with a study that found that patients with asthma and rhinosinusitis or allergic rhinitis (i.e., markers of allergic asthma) were less likely to be hospitalized than patients with COVID-19 without these concomitant conditions [26]. More broadly, a number of studies have shown that atopic diseases are associated with milder COVID-19 [27], or less risk of hospitalization due to COVID-19 [28].
The potentially lower risk of SARS-CoV-2 infection in patients with allergic asthma compared with nonallergic asthma could theoretically be explained by differences in expression of the key SARS-CoV-2 cellular receptor, angiotensin-converting enzyme 2 (ACE2). ACE2 expression is upregulated in individuals who are at higher risk of severe COVID-19, such as people with diabetes and obesity, and in those who smoke [29,30,31]. Jackson et al. evaluated expression of ACE2 in the airways of in adults and children with asthma and found that ACE2 expression was lower in the airway of patients with allergic sensitization and asthma compared with patients with nonallergic asthma [29]. Reduced ACE2 expression combined with expression of immunomodulators could contribute to lower COVID-19 susceptibility for patients with nonallergic asthma, and this hypothesis is supported by a growing body of preclinical and clinical evidence [29, 32,33,34,35,36,37].
This study had a number of strengths, including a large sample size of patients from across the United States. The data also allowed tracking of patients with COVID-19 from diagnosis through to treatment, and distinguishing of patient symptomology between patients with a positive COVID-19 test and those with a negative COVID-19 test. However, there are a number of limitations that could hinder the applicability and generalizability of the findings, which are mostly related to the database. The major limitation is that ICD-CM-10 codes do not exist for allergic asthma; as a result, the case definitions used for the 2 cohorts were based on current literature, clinical opinion, and available data in the database, which included a positive IgE test, omalizumab use, or a skin prick test ordered by a specialist. Therefore, it is possible some patients may have been misclassified as not having allergic asthma if these data were not available. However, a positive skin prick test is thought to be likely if it has been ordered by a specialist, and omalizumab is indicated for use in allergic asthma and not in nonallergic asthma. Although it is possible that omalizumab could be prescribed for chronic spontaneous urticaria in patients with nonallergic asthma, and these patients could have been misclassified as having allergic asthma, the presence of asthma as a comorbid condition in patients with chronic spontaneous urticaria is low [38]. In addition, the database does not cover all health care facilities in the United States and may not be nationally representative of patients who attend smaller ambulatory practices. The outcomes assessed in this study are also limited because causes of death were not available in the database and the analysis of deaths could only be reported as deaths due to any cause and not specifically attributed to COVID-19. Finally, our understanding of COVID-19 is dynamic and rapidly advancing, and the current analysis therefore may not include any newly identified risks or other factors, such as the impact of treatments, vaccines, or new variants.
Conclusions
In summary, this real-world retrospective analysis of a large EHR database from the United States found that allergic asthma was associated with a lower risk of poor outcomes, including a lower risk of death, from COVID-19 compared with nonallergic asthma. Among patients with asthma and SARS-CoV-2 infection, a lower-than-expected proportion had allergic asthma, which may suggest a lower infection rate. These findings contribute to the growing body of evidence on SARS-CoV-2 infections in patients with asthma, including differences in outcomes for asthma subtypes.
Availability of data and materials
The data that support the findings of this study are available from Optum Inc, but restrictions apply to the availability of these data. For this study, the data were used under license and so are not publicly available. Data are available from the authors upon reasonable request and with permission from Optum Inc.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- CDC:
-
Centers for Disease Control and Prevention
- COVID-19:
-
Coronavirus disease 2019
- ED:
-
Emergency department
- EHR:
-
Electronic health record
- ICD-10-CM:
-
International Classification of Diseases, Tenth Revision, Clinical Modification
- ICU:
-
Intensive care unit
- IgE:
-
Immunoglobulin E
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, Carson G, Dunning J, Harrison EM, Baillie JK, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9(7):699–711.
Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56.
Drake TM, Docherty AB, Harrison EM, Quint JK, Adamali H, Agnew S, Babu S, Barber CM, Barratt S, Bendstrup E, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. an international multicenter study. Am J Respir Crit Care Med. 2020;202(12):1656–65.
Aveyard P, Gao M, Lindson N, Hartmann-Boyce J, Watkinson P, Young D, Coupland CAC, Tan PS, Clift AK, Harrison D, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9(8):909–23.
Hartmann-Boyce J, Gunnell J, Drake J, Otunla A, Suklan J, Schofield E, Kinton J, Inada-Kim M, Hobbs FDR, Dennison P. Asthma and COVID-19: review of evidence on risks and management considerations. BMJ Evid Based Med. 2020;26:195.
Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146(2):327–9.e4.
Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, Rentsch CT, Williamson E, Drysdale H, Croker R, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–20.
Sitek AN, Ade JM, Chiarella SE, Divekar RD, Pitlick MM, Iyer VN, Wang Z, Joshi AY. Outcomes among patients with COVID-19 and asthma: a systematic review and meta-analysis. Allergy Asthma Proc. 2021;42(4):267–73.
Gao YD, Agache I, Akdis M, Nadeau K, Klimek L, Jutel M, Akdis CA. The effect of allergy and asthma as a comorbidity on the susceptibility and outcomes of COVID-19. Int Immunol. 2022;34(4):177–88.
Caminati M, Vultaggio A, Matucci A, Senna G, Almerigogna F, Bagnasco D, et al. Asthma in a large COVID-19 cohort: prevalence, features, and determinants of COVID-19 disease severity. Respir Med. 2021;176:106261.
Eger K, Hashimoto S, Braunstahl GJ, Brinke AT, Patberg KW, Beukert A, et al. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med. 2020;177:106287.
Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, Kim SY, Yon DK, Lee SW. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790–8.
Eggert LE, He Z, Collins W, Lee AS, Dhondalay G, Jiang SY, Fitzpatrick J, Snow TT, Pinsky BA, Artandi M, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy. 2022;77(1):173–85.
Emergency use ICD codes for COVID-19 disease outbreak [https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak].
Asthma codes for ICD-10 [https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20Management/finances-coding/Asthma-Codes-ICD-10.pdf].
Rosenbaum PR, Rubin DM. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e1.
SW L. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3.
Arbes SJ Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120(5):1139–45.
Backman H, Raisanen P, Hedman L, Stridsman C, Andersson M, Lindberg A, Lundback B, Ronmark E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016—results from three population surveys. Clin Exp Allergy. 2017;47(11):1426–35.
Chen M, Shepard K 2nd, Yang M, Raut P, Pazwash H, Holweg CTJ, Choo E. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin Exp Allergy. 2021;51(4):546–55.
Pakkasela J, Ilmarinen P, Honkamaki J, Tuomisto LE, Andersén H, Piirilä P, Hisinger-Molkanen H, Sovijarvi A, Backman H, Lundback B, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20:9.
Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, Kudlaty E, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–14.e4.
Naziroglu T, Aksu K. Rare atopy in COVID-19 patients or COVID-19 famine in atopic patients? Dermatol Ther. 2021;34(1): e14581.
Keswani A, Dhana K, Rosenthal JA, Moore D, Mahdavinia M. Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(4):479–81.
Jackson DJ, Busse WW, Bacharier LB, Kattan M, O’Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–6.e3.
Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):841.
Herman-Edelstein M, Guetta T, Barnea A, Waldman M, Ben-Dor N, Barak Y, Kornowski R, Arad M, Hochhauser E, Aravot D. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system. Cardiovasc Diabetol. 2021;20:90.
Branco ACCC, Sato MN, Alberca RW. The possible dual role of the ACE2 receptor in asthma and coronavirus (SARS-CoV2) infection. Front Cell Infect Microbiol. 2020;10:550571.
Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80–8.e8.
Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, Rios CL, Pruesse E, Nolin JD, Plender EG, et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11(1):5139.
Liu S, Zhi Y, Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78–88.
Camiolo M, Gauthier M, Kaminski N, Ray A, Wenzel SE. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146(2):315–24.e7.
Bonser LR, Eckalbar WL, Rodriguez L, Shen J, Koh KD, Ghias K, Zlock LT, Christenson S, Woodruff PG, Finkbeiner WE, et al. The type 2 asthma mediator IL-13 inhibits SARS-CoV-2 infection of bronchial epithelium. Am J Respir Cell Mol Biol. 2022;66(4):391–401.
Wong MM, Keith PK. Presence of positive skin prick tests to inhalant allergens and markers of T2 inflammation in subjects with chronic spontaneous urticaria (CSU): a systematic literature review. Allergy Asthma Clin Immunol. 2020;16:72.
Acknowledgements
Medical writing assistance was provided by Janelle Keys, PhD, CMPP, of Envision Pharma Group, and was funded by Genentech, Inc., a member of the Roche Group.
Funding
This analysis was funded by Genentech, Inc., a member of the Roche Group, and Novartis Pharma AG. Genentech, Inc., a member of the Roche Group, was involved in study design, data collection, data analysis and interpretation, and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
CTJH, YR, KR, SG, and AI designed the study and interpreted the results. TRM, WB, and RJK interpreted the study results. CSM and AS completed the statistical analysis and interpreted the study results. All authors critically reviewed the manuscript and read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
To protect patient anonymity and confidentiality in compliance with the Health Insurance Portability and Accountability Act of 1996, all data used in this analysis were deidentified. The study was conducted according to the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki 2013. Administrative permissions were not required to access the data. The study did not require institutional review board approval as this research falls under the HIPPA Privacy Rule for Research, Limited Data Sets with a Data Use Agreement. A data use agreement was entered into by both the covered entity (Optum Inc COVID-19 database) and the researcher (Genentech Inc), pursuant to which the covered entity may disclose a limited data set to the researcher for research, public health, or health care operations. See 45 CFR 164.514(e).
Information on Optum.® de-identified COVID-19 Electronic Health Record dataset (2007–2021), August 2021
Given the urgent need to clinically understand the novel virus of COVID 19, Optum developed a low latency data pipeline that enables minimal data lag, while preserving as much clinical data as possible. The data is sourced from Optum’s longitudinal EHR repository, which is derived from dozens of healthcare provider organizations in the United States, including more than 700 Hospitals and 7000 Clinics. The data is certified as de-identified by an independent statistical expert following HIPAA statistical de-identification rules (Guidance Regarding Methods for De‐identification of Protected Health Information in Accordance with the Health Information Insurance Portability and Accountability Act (HIPAA) Privacy Rule (Dated as September 4, 2012, as first released on November 26, 2012) and managed according to Optum® customer data use agreements.
Consent for publication
The study did not require consent for publication approval because only deidentified data were included. See information on the Optum Inc. database above.
Competing interests
TRM has been part of speaker bureaus for ALK, AstraZeneca, Boehringer Ingelheim, Genentech, Inc., and Optinose, and an advisory board member for Stallergenes Greer. WB has received grant support from the National Institutes of Health (NIH)-National Institute of Allergy and Infectious Diseases, and consultant fees from AstraZeneca, Genentech, Inc., GlaxoSmithKline, Novartis, and Sanofi/Regeneron. CTJH, YR, and CSM are former employees of Genentech, Inc. AS holds stock in F. Hoffmann-La Roche Ltd. KR, AS, SG, and AI are employees of Genentech, Inc. RJK has consulted for Boehringer Ingelheim, Genentech, Inc., and United Therapeutics; has received research support from Boehringer Ingelheim and the NIH; was a clinical investigator for Bellerophon, the NIH, Respivant, and Toray; and was on the data and safety monitoring board for Pliant and PureTech.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Murphy, T.R., Busse, W., Holweg, C.T.J. et al. Patients with allergic asthma have lower risk of severe COVID-19 outcomes than patients with nonallergic asthma. BMC Pulm Med 22, 418 (2022). https://doi.org/10.1186/s12890-022-02230-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02230-5