Abstract
Background
Anemia is relatively common in cancer patients, and baseline anemia is associated with poor survival in patients with non-small cell lung cancer (NSCLC). However, there is a lack of large-sample studies of patients with NSCLC with epidermal growth factor receptor (EGFR) mutations.
Methods
We retrospectively analyzed anemia‑related data for patients with NSCLC and EGFR mutations who were admitted to Zhejiang Cancer Hospital from January 2013 to June 2019 and treated with targeted therapy. The patients’ clinicopathological features were evaluated by χ2 tests and the relationships between clinical characteristics and prognosis were investigated using Kaplan–Meier and multivariate Cox regression analyses.
Results
A total of 2,029 patients treated with EGFR-tyrosine kinase inhibitors (TKIs) were finally enrolled in this study, of whom 24.6% had baseline anemia. Patients without baseline anemia had longer median overall survival (OS) than patients with baseline anemia (36.10 vs. 29.10 months, P = 0.001), and patients with grade < 2 anemia had longer median OS than those with grade ≥ 2 anemia (35.00 vs. 25.10 months, P < 0.001). Multivariate analyses identified baseline anemia as a factor predicting a poor prognosis in terms of OS in patients with EGFR mutations.
Conclusions
Baseline anemia is a significant factor predicting a poor prognosis in terms of OS in patients with NSCLC and EGFR mutations treated with targeted therapy. A higher grade of baseline anemia may also be related to shorter OS. And a higher risk of EGFR-mutated patients who had received targeted therapy could also be observed.
Similar content being viewed by others
Background
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related death [1, 2]. There are two main histological forms of lung cancer: non-small cell lung cancer (NSCLC), accounting for 85% of patients, and small cell lung cancer, accounting for the remaining 15% of patients [3, 4]. The World Health Organization estimates that lung cancer death rates will continue to rise worldwide, particularly in Asia, in line with increasing tobacco use [5].
Epidermal growth factor receptor (EGFR) gene mutations are common in patients with NSCLC. About one-third of patients with NSCLC carry EGFR mutations, with higher incidences in Asians, women, non-smokers, and adenocarcinoma patients [6, 7]. EGFR-targeted therapy has been an important step forward in the treatment of patients with EGFR mutations. However, although EGFR mutations provide a promising biomarker for patients with lung cancer treated with EGFR-tyrosine kinase inhibitors (TKIs), around 20% of patients with EGFR mutations fail to respond to these agents [8, 9]. Clinicians thus need to find effective factors to identify patients likely to benefit from treatment with EGFR-TKIs.
Cancer-related anemia (CRA) is relatively common in cancer patients and represents a multifactorial problem, with its severity potentially affected by immune, nutritional, and metabolic components [10]. Although the underlying mechanisms of CRA are unclear, it is thought to be caused directly by tumor suppression of hematopoiesis through bone marrow infiltration or via the production of cytokines leading to iron sequestration [11]. Previous studies have shown that CRA may be related to the activation of cytokines such as interferon-γ, interleukin-1, and tumor necrosis factor, which may inhibit the production of endogenous erythropoietin, impair iron use, and reduce the proliferation of erythrocyte precursors [12, 13]. CRA also exacerbates tumor hypoxia, which in turn increases tumor-cell tolerance and resistance to radiotherapy and chemotherapy, thus affecting clinical treatment efficacy and patient survival [14,15,16]. However, the impact of anemia on targeted therapy is still unclear.
Pretreatment anemia is associated with a poor prognosis in various malignant tumors, and this relationship has been a focus of clinical attention. A study of 147 patients with early-stage NSCLC treated with stereotactic body radiation therapy found that pretreatment anemia was a predictive factor of poor overall survival (OS), which is likely to reflect disease progression [17]. Tomita et al. [18] measured preoperative white blood cell counts, hemoglobin (Hb) levels, and platelet counts in 289 consecutive patients with NSCLC who had undergone surgical resection and found that the 5-year survival rates of patients with leukocytosis, anemia, and thrombocytopenia were only 25.0%, which was significantly worse than that of patients with normal blood cell counts (78.23%). Pre-treatment anemia is thus associated with a poor prognosis in patients with lung cancer. However, few large-sample studies have been conducted in patients with NSCLC and EGFR mutations treated with EGFR-TKIs. We therefore conducted a retrospective study of 2,029 patients with stage IV EGFR-mutated NSCLC who received EGFR-TKI therapy to investigate the prognostic value of baseline anemia and anemia grade in these patients. This represents the largest study of the importance of anemia in EGFR-mutated NSCLC.
Methods
Clinical data
We retrospectively analyzed anemia‑related data for patients with stage IV EGFR-mutated NSCLC who were admitted to Zhejiang Cancer Hospital, Hangzhou, China, from January 2013 to June 2019. A total of 2,029 patients were eligible for the study. The inclusion criteria were: i) pathologically confirmed stage IV NSCLC with EGFR mutations; ii) age > 18 years; iii) complete clinical data and follow‑up information; and iv) subsequent receipt of EGFR-TKIs. The exclusion criteria were: i) basic hematological diseases and ii) chronic nephropathy. Clinical staging was based on the 8th edition of the Tumor, Node, Metastasis staging system [19]. We extracted the following data from the patients’ medical records: patient demographics, smoking history, stage of lung cancer, pathological type of tumor, EGFR mutation subtype, brain metastasis, bone metastasis, antitumor treatment options, C-Reactive protein (CRP), baseline Hb content, and grading. EGFR mutation subtypes were detected by next-generation sequencing or polymerase chain reaction. In the study, EGFR-TKIs used were gefitinib (250 mg per day orally), erlotinib (150 mg per day orally), icotinib (125 mg three times per day orally), afatinib (40 mg per day orally) or osimertinib (80 mg per day orally). The follow‑up period lasted until October 2019.The protocol was approved by the institutional review board of Zhejiang Cancer Hospital. The study was carried out in accordance with the guidelines of the Helsinki Declaration (as revised in 2013) and the need for individual consent for this retrospective analysis was waived.
Grading of anemia
Baseline anemia was defined as a Hb level < 120.0 g/l for males and < 110.0 g/l for females at their first visit to the hospital. No patients had received radiotherapy, chemotherapy, targeted therapy, or immunotherapy. According to the National Cancer Institute criteria, anemia was categorized into five grades according to Hb level: grade 0, normal Hb level; grade 1 (mild anemia), males 100–120 g/l, females 100–110 g/l; grade 2 (moderate anemia), 80–100 g/l; grade 3 (severe anemia), 65–80 g/l; and grade 4 (life-threatening anemia), < 65 g/l [20]. In this study, patients were divided into non-anemic (n = 1,529) and anemic (n = 500) groups based on their baseline Hb levels.
Statistical analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 25.0 software. Quantitative variables were presented as mean ± standard deviation. Differences in demographic and clinical characteristics between the anemic and non-anemic groups were analyzed by Pearson’s χ2 test. Survival analysis was conducted using the Kaplan–Meier method and comparisons were made using the log-rank test. Hazard ratio (HR) and 95% confidence interval (CI) were calculated by Cox proportional hazard regression analysis. A P value < 0.05 was regarded as statistically significant.
Results
Patient characteristics
A total of 2,029 eligible patients with EGFR mutation-positive stage IV NSCLC were included in the study, all of whom received subsequent targeted therapy. Based on their baseline Hb levels, 1,529 (75.4%) and 500 (24.6%) patients were included in the non-anemic and anemic groups, respectively. The average Hb level in the 500 anemic patients was 102.45 ± 11.81 g/l. According to the National Cancer Institute scoring system, 328 patients (65.6%) had mild anemia, 148 (29.6%) had moderate anemia, 22 (4.4%) had severe anemia, and two (0.4%) had life-threatening anemia (Table 1). The demographic and clinical characteristics of the patients are summarized in Table 2. Baseline anemia was significantly less common in male than female patients (49.4% vs. 50.6%, P = 0.024). EGFR-mutation type also differed significantly between anemic and non-anemic patients (P = 0.014): the proportions of exon 19 deletions (44.6% vs. 45.3%) and L858R point mutations (41.0% vs. 44.9%) were lower while the rate of other mutation subtypes (14.4% vs. 9.8%) was higher in the anemic group compared with the non-anemic group. The incidence of baseline anemia was significantly lower in patients without bone metastasis than in patients with bone metastasis (22.5% vs. 26.6%, P = 0.034). There were no significant differences in age, ECOG PS, smoking history, pathological type, brain metastasis, CRP or EGFR-TKI between the two groups (P > 0.05).
Effects of baseline anemia and grade on patient survival
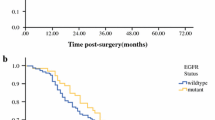
Patients with anemia had a shorter median OS than patients without anemia [29.10 (95% CI, 25.05–33.15) months vs. 36.10 (95% CI, 33.07–39.13) months, P = 0.001] (Fig. 1A). Patients were divided into two groups according to the grade of anemia (grade < 2 and grade ≥ 2). Higher baseline anemia grade was associated with a shorter OS and poorer prognosis: patients with grade < 2 anemia had significantly longer median OS than those with grade ≥ 2 anemia [35.00 (95% CI 32.45–37.55) months vs. 25.10 (95% CI, 17.03–33.14) months, P < 0.001] (Fig. 1B). In addition, among anemic patients with EGFR mutations, the median OS durations were similar in patients with exon 19 deletions and those with L858R point mutations [29.10 (95% CI, 24.45–33.76) vs. 29.20 (95% CI, 21.12–37.28) months, respectively, P = 0.950] (Fig. 1C).
Univariate and multivariate analyses of clinical features and prognosis
Univariate analysis showed that OS was significantly correlated with age, ECOG PS, pathological type, EGFR-mutation subtype, brain metastasis, bone metastasis, surgical history, chemotherapy, and baseline anemia (Table 3). In contrast, there was no significant correlation with sex, smoking history, or radiotherapy. Factors that were significant in univariate analysis were included in Cox multivariate regression analysis, which identified ECOG PS, brain metastasis, bone metastasis, surgical history, chemotherapy, and baseline anemia as independent prognostic factors for OS in patients with stage IV EGFR-mutated NSCLC who received targeted therapy. These results indicated that baseline anemia was associated with a poor prognosis in patients with NSCLC with EGFR mutations (Fig. 2).
Discussion
We compared the clinical outcomes of patients with NSCLC harboring activating EGFR mutations who received targeted therapy. Baseline anemia was significantly correlated with prognosis in these patients, with OS decreasing in line with increasing anemia grade in patients with EGFR mutations treated with targeted therapy. To the best of our knowledge, the current study including 2,029 patients was the largest study of anemia in patients with EGFR-mutated NSCLC treated with targeted therapy. The aim of the study was to investigate the prognostic value of baseline anemia and anemia grade in these patients.
In the 2004 European Cancer Anaemia Survey report, 39% of cancer patients had pre-treatment anemia at the start of the survey [21]. In our study, 1,529 (75.4%) and 500 (24.6%) patients were classified as non-anemic and anemic, respectively. The mean Hb level among the 500 patients with baseline anemia was 102.45 ± 11.81 g/l, and most patients with baseline anemia had mild to moderate anemia, including 328 patients (65.6%) with mild anemia and 148 (29.6%) with moderate anemia, compared with only 22 (4.4%) with severe anemia and two (0.4%) with life-threatening anemia.
The present analysis identified baseline anemia as a factor associated with a poor prognosis in patients with NSCLC with EGFR mutations. Previous studies also showed that baseline anemia could affect patients’ clinical outcomes. An analysis [22] of 186 NSCLC patients with EGFR mutations treated with first-line tyrosine kinase inhibitors found that anemic patients had shorter median OS than non-anemic patients [24.83 (95% CI, 17.49–32.17) months vs. 42.10 (95% CI, 31.87–52.34) months, P = 0.031], and anemia [HR = 2.573 (95% CI, 1.12–5.90), P = 0.026] was the only independent factor predicting poor OS. Chen et al. [23] reported that baseline anemia and anemia grade were independent prognostic factors in patients with stage IV NSCLC. They found that patients without baseline anemia had longer OS than patients with baseline anemia (28.0 vs. 17.4 months, P < 0.001) and OS decreased with increasing anemia grade, with patients with grade 0 anemia having the longest OS (28.0 months) and patients with grade 3 and 4 anemia having the shortest OS (8.6 months). A meta-analysis of 23 studies by Liu et al. showed that preoperative anemia was associated with poor OS in lung cancer patients, and the risk of death was about 1.58 times higher in patients with preoperative anemia compared with patients without anemia [summarized HR = 1.58 (95% CI, 1.44–1.75)] [24]. Tanaka et al. [25] evaluated the prognostic significance of anemia in patients with NSCLC undergoing stereotactic body radiation therapy, and anemia was confirmed as the only significant factor in multivariate analysis (P = 0.025). Another study showed that anemia was not an independent predictor of short-term outcomes but was independently associated with significantly reduced survival in patients undergoing resection for lung cancer [26]. These studies demonstrated that anemia was associated with a poor prognosis in patients with NSCLC. However, the sample sizes of previous studies have been small, and few studies have been limited to patients with EGFR mutations who received targeted therapy.
In this study, we focused on the relationship between baseline anemia and prognosis in patients with EGFR-mutated NSCLC treated with targeted therapy. We found that male sex, and bone metastasis were significantly associated with a higher incidence of baseline anemia. In addition, the mutation subtypes varied between patients with and without anemia: the proportions of exon 19 deletions and L858R point mutations were lower in the anemia group compared with the non-anemic group, while the rate of other mutation subtypes was conversely higher in the anemic group. The rates of baseline anemia were also higher in patients > 65 years old, smokers, and patients with brain metastasis compared with the corresponding population, but the differences were not significant. Patients without baseline anemia had longer OS than patients with baseline anemia [36.10 (95% CI, 33.07–39.13) months vs. 29.10 (95% CI, 25.05–33.15) months, P = 0.001], and OS decreased with increasing baseline anemia grade, with OS being longer in patients with grade < 2 anemia compared with those with grade ≥ 2 anemia [35.00 (95% CI 32.45–37.55) months vs. 25.10 (95% CI, 17.03–33.14) months, P < 0.001]. Moreover, the median OS in patients with exon 19 deletions was similar to that in patients with L858R point mutations [29.10 (95% CI, 24.45–33.76) vs. 29.20 (95% CI, 21.12–37.28) months, P = 0.950]. Univariate and multivariate analyses found that ECOG PS, brain metastasis, bone metastasis, surgical history, chemotherapy, and baseline anemia were independent prognostic factors for OS in patients with stage IV EGFR-mutated NSCLC who received targeted therapy. These results indicate the prognostic value of baseline anemia in patients with NSCLC with EGFR mutations, thus highlighting the importance of the early treatment of anemia and the control of Hb levels in cancer patients, and suggesting that improving the management of anemia may also prolong patient survival.
This study had some limitations. Importantly, the primary data were obtained retrospectively, which may have affected the results, and this was also a single‑center study. In addition, control of other comorbid conditions may have been lacking, and baseline anemia could be a surrogate for other medical comorbidities, which may confound our results. However, as the largest study of anemia in patients with NSCLC with EGFR mutations, we believe that the results are of great significance and may help to guide the treatment of NSCLC in patients with EGFR mutations. However, more multicenter prospective studies are needed to confirm our results.
Conclusions
This study demonstrated that baseline anemia and anemia grade were significantly correlated with prognosis in patients with NSCLC with EGFR mutations who received targeted therapy. These data suggest that routine measurement of Hb levels during cancer work-up may help to predict the prognosis in NSCLC patients with EGFR mutations.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.
Abbreviations
- CRA:
-
Cancer-related anemia
- EGFR:
-
Epidermal growth factor receptor
- TKI:
-
Tyrosine kinase inhibitor
- Hb:
-
Hemoglobin
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- SD:
-
Standard deviation
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;6:394–424.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;2:87–108.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;9:1240–2.
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;4:605–44.
Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;2:154–62.
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;3:169–81.
Hirsch FR, Varella-Garcia M, Cappuzzo F, McCoy J, Bemis L, Xavier AC, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2007;4:752–60.
Zucali PA, Ruiz MG, Giovannetti E, Destro A, Varella-Garcia M, Floor K, et al. Role of cMET expression in non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Ann Oncol. 2008;9:1605–12.
Maccio A, Madeddu C, Gramignano G, Mulas C, Tanca L, Cherchi MC, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica. 2015;1:124–32.
Dicato M, Plawny L, Diederich M. Anemia in cancer. Ann Oncol. 2010;21(Suppl 7):vii167-72.
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;9:1271–6.
Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol. 2020;145:102837.
Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;6:492–508.
Lazzari G, Silvano G. From anemia to erythropoietin resistance in head and neck squamous cell carcinoma treatment: a carousel driven by hypoxia. Onco Targets Ther. 2020;13:841–51.
Vaupel P, Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Clin Biol. 2005;1:5–10.
Shaverdian N, Veruttipong D, Wang J, Kupelian P, Steinberg M, Lee P. Pretreatment anemia portends poor survival and nonlocal disease progression in patients with Stage I non-small cell lung cancer treated with Stereotactic body radiation therapy. J Thorac Oncol. 2016;8:1319–25.
Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;7:2687–90.
Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the tumor, node, metastasis staging of lung cancer (8(th) edition): rationale, radiologic findings and clinical implications. World J Radiol. 2017;6:269–79.
Experts Committee on Cancer -Related Anemia; Chinese Society of Clinical Oncology (CSCO). Clinical practice guidelines on cancer-related anemia (2012–2013 Edition). Chin Clin Oncol. 2012;2:18.
Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European cancer anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;15:2293–306.
Kang HS, Shin AY, Yeo CD, Park CK, Kim JS, Kim JW, et al. Clinical significance of anemia as a prognostic factor in non-small cell lung cancer carcinoma with activating epidermal growth factor receptor mutations. J Thorac Dis. 2020;5:1895–902.
Chen C, Song Z, Wang W, Zhou J. Baseline anemia and anemia grade are independent prognostic factors for stage IV non-small cell lung cancer. Mol Clin Oncol. 2021;3:59.
Liu Y, Bai YP, Zhou ZF, Jiang CR, Xu Z, Fan XX. Preoperative anemia as a prognostic factor in patients with lung cancer: a systematic review and meta-analysis of epidemiological studies. J Cancer. 2019;9:2047–56.
Tanaka H, Ono T, Manabe Y, Kajima M, Fujimoto K, Yuasa Y, et al. Anemia is a prognostic factor for overall survival rate in patients with non-small cell lung cancer treated with stereotactic body radiation therapy. Cancer Manag Res. 2021;13:7447–53.
Taylor M, Abah U, Hayes T, Eadington T, Smith M, Shackcloth M, et al. Preoperative anemia is associated with worse long-term survival after lung cancer resection: a multicenter cohort study of 5,029 patients. J Cardiothorac Vasc Anesth. 2021. https://doi.org/10.1053/j.jvca.2021.08.029.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing a draft of this manuscript.
Funding
The study was funded by the Medical Scientific Research Foundation of Zhejiang Province (No. 2022KY653). The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
LF, ZS and JW developed the study protocol. JW, YH, and JX performed data collection. WW and JS performed data analysis. JW, JX and YH wrote the manuscript. LF, ZS and WW revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study and protocol were approved by the Institutional Review Board of the Zhejiang Cancer Hospital (IRB-2022-63). The study was conducted in accordance with the Declaration of Helsinki. All procedures were performed in accordance with the relevant guidelines and regulations. The need for informed consent was waived by the Institutional Review Board of the Zhejiang Cancer Hospital, because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, J., Xiang, J., Hao, Y. et al. Baseline anemia predicts a poor prognosis in patients with non-small cell lung cancer with epidermal growth factor receptor mutations: a retrospective study. BMC Pulm Med 22, 381 (2022). https://doi.org/10.1186/s12890-022-02158-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02158-w