Abstract
Objective
The purpose of this study was to evaluate the usefulness of intraprocedural CT and prior PET/CT fusion imaging in improving the diagnostic yield of CT-guided transthoracic core-needle biopsy (CNB) in lung masses.
Methods
In total, 145 subjects with lung masses suspicious for malignancy underwent image-guided transthoracic CNB. According to imaging modality the subjects were divided into two groups. PET/CT images obtained no more than 14 days before the biopsy were integrated with intraprocedural CT images. The integrated or fused images were then used to plan the puncture sites. The clinical characteristics, diagnostic yield of CNB, diagnostic accuracy rate, procedure-related complications and procedure duration were recorded and compared between the two groups. Final clinical diagnosis was determined by surgical pathology or at least 6-months follow-up. The diagnostic accuracy of CNB was obtained by comparing with final clinical diagnosis.
Results
145 subjects underwent CNB with adequate samples, including 76 in fusion imaging group and 69 in routine group. The overall diagnostic yield and diagnostic accuracy rate were 80.3% (53/66), 82.9% (63/76) for fusion imaging group, 70.7% (41/58), 75.4% (52/69) for routine group, respectively. In addition, the diagnostic yield for malignancy in fusion imaging group (98.1%, 52/53) was higher than that in routine group (81.3%, 39/48). No serious procedure-related complications occurred in both two groups.
Conclusion
CNB with prior PET/CT fusion imaging is particularly helpful in improving diagnostic yield and accurate rate of biopsy in lung masses, especially in heterogeneous ones, thus providing greater potential benefit for patients.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Currently, CT-guided transthoracic core-needle biopsy (CNB) has emerged as a reliable and safe procedure for the diagnosis of indeterminate lung lesions, especially for the ones that are ineligible for surgical resection [1]. Excellent diagnostic accuracy and a relative low complication rate for this procedure have been confirmed in previous studies [2,3,4]. The diagnostic accuracy of CNB for malignant lung tumors varies between 92.7 and 99% [5,6,7,8]. However, false-negative rate may be substantially higher in large heterogeneous lesions or those with cystic and necrotic areas since only a small part of the lesion is obtained by CNB. In addition, adjacent atelectasis or obstructive pneumonia may also affect final biopsy results. For this reason, functional/metabolic methods, such as 18F-FDG PET/CT, which are helpful for identifying the hypermetabolic regions that represent actual biological behavior of the lesions for biopsy, are promising supplementary imaging tool for CNB [9,10,11,12].
The integration of intraprocedural CT and prior PET/CT images have been used in percutaneous biopsy of bone lesions, abdominal masses, mediastinal tumors and lung lesions [9,10,11, 13], PET/CT guided biopsy of suspected lung lesions was investigated in a previous study, but CNB under the guidance of PET/CT posed a certain radiation risk to the operator [14], moreover no consensus for its usefulness has been reached yet. However, to the best of our knowledge, there is no published information on prior PET/CT and CT fused images used in biopsy for lung masses. Also, we hypothesized that intraprocedural CT and prior PET/CT fused images are helpful in yielding a positive and accurate biopsy result. Thus, the purpose of this study was to establish the usefulness of intraprocedural CT and prior PET/CT fusion imaging in improving diagnostic yield of CNB in lung lesions by comparing to that of traditional CNB.
Materials and methods
The institutional review board of the Ethical and Scientific Committees of China-Japan Friendship Hospital approved the present retrospective study and waived the requirement for informed consent for collecting data from the related patients. Written informed consent for CT-guided transthoracic biopsy had been obtained from all patients prior to performing the procedure.
Study subjects
From January 2017 to December 2019, 145 patients with lung lesions that were suspicious for malignancy based on clinical and imaging findings were referred for CT-guided biopsy in our institution. The inclusion criteria were as follows: (1) lung mass or consolidation greater than 30 mm in short diameter; (2) 18 years or older; (3) definite clinical diagnosis by surgery or long-term follow-up (at least 6 months). In addition, the intra-procedural CT and prior PET/CT image fusions subgroup must have had their PET/CT examinations in our hospital as integration of two imaging modalities can not be performed in PACS otherwise, and the interval between PET/CT examination and CT-guided transthoracic CNB was no more than two weeks. The clinical characteristics of the patients were summarized in Table 1.
18F-FDG PET/CT
18F-FDG PET-CT was performed using an integrated PET-CT scanner (GE Discovery ST; GE Healthcare Life Sciences, Chalfont, UK). All patients were fasted for at least 6 h before PET/CT scanning, and then 7.4 MBq/kg 18F-FDG was administered intravenously. (Atom Hi-Tech Co., Ltd., Beijing, China). One hour after 18F-FDG injection, the patient was supine and a PET scan was performed from the head to the mid-thigh. The attenuation correction and anatomical coregistration were derived from concomitant CT data without oral or intravenous contrast agents. The regional concentration of 18F-FDG was determined and expressed as the standardized uptake value (SUV). By using standard software tools provided with the PET/CT scanner, the SUV was adjusted for the injected dose of 18F-FDG and patient's weight. To minimize variation and ensure repeatability, the maximum SUV (SUVmax) was defined as the peak SUV of the pixel with the highest count in consecutive trans-axial scans. All PET/CT images will be automatically transferred to PACS after acquisition. During CNB procedure (detailed information described below), PET/CT images will be automatically matched with intraprocedural CT images using a built-in calibration software in PACS. The fusion images can also be adjusted manually by setting at least three points of common anatomical reference.
Patient preparation and CT-guided transthoracic CNB procedure
Screening coagulation tests were ordered routinely before biopsy. Patients on antiplatelet or anticoagulant agents (e.g. aspirin, clopidogrel, warfarin etc.) were risk assessed independently by the interventional radiologists (with 24 and 13 years of experience respectively) and their referring physicians on the patients’ risk–benefit of medication cessation. The international normalized ratio needs to be maintained below 1.5, and the minimum platelet count maintained at 70,000/μL. Impaired coagulation status and platelet counts need to be corrected as much as possible if the above thresholds are not met [14]. In all cases, the interventional radiologists, thoracic surgeons and physicians jointly reviewed all available images including PET/CT and planned the biopsy approach.
Before CT-guided biopsy, patients were coached on slow breathing and held the breath at certain degree of inspiratory. All the planning and localization CT scans were carried out using the same 16-detector-row scanner (Aquilion 16; Canon Medical Systems). The following parameters were for the planning CT: scanning method = helical acquisition mode; tube currents = 50 mAs; tube voltage = 120 kVp; rotation time = 0.5 s; beam pitch = 1.0; imaging FOV = 400; slice thickness = 5 mm; the following parameters for the localization CT: scanning method = axial acquisition mode; tube currents = 50 mAs; tube voltage = 120 kVp; rotation time = 0.5 s; imaging FOV = 400; slice thickness = 4 mm. Scanning slices was limited to just cover the lesion in order to reduce radiation dose.
A planning CT scan (10-cm range) was performed before percutaneous needle insertion, and then the biopsy system including introducer needle (Cook Incorporated) and BioPince™ full core biopsy instrument (Argon medical devices, INC) was introduced in a stepwise manner under the guidance of images (CT images alone or CT and PET/CT fused images). An appropriate puncture point on a patient’s skin was marked to determine the shortest needle entry route while avoiding the inclusion of bullae and vascular structures. After local anesthesia with 2% lidocaine, a 10-cm-long 17-gauge percutaneous introducer needle was then advanced to puncture from the skin on the marked point without penetrating the parietal pleura. After confirming the direction of the tip of the puncture needle by the second CT scan (1.6 cm range), the needle was advanced further into the lesion (hypermetabolic area on fused images). Then the third CT scan was performed to confirm the final position of the tip of the needle before obtaining specimens coaxially. Two to three specimens were collected by BioPince™ full core biopsy instrument and subjected to histological evaluation for specific diagnosis (Figs. 1, 2). Finally, the fourth CT scan (the entire lung) was performed to rule out the presence of possible complications (e.g. pneumothorax, intrapulmonary hemorrhage, hemothorax, air embolism, etc.) after pulling out introducer needle. The CT-guided biopsy method was consisted with our previous studies. [15, 16] The severity of procedural complications was evaluated according to the Society of Interventional Radiology Standards of Practice Committee classification of complications [17], and procedure duration (defined as the interval time between the initial scout image and the end of the final CT scan) was also recorded. In addition, a repeat biopsy was considered when the patient’s clinical condition was obviously inconsistent with initial biopsy results (two patients) or when chemotherapy or targeted therapy regimens need to be adjusted (twenty patients). However, only the initial procedure result was recorded and analyzed in this study.
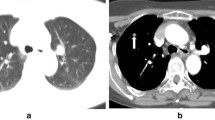
A 69-year-old man with suspected primary lung malignancy. a The intraprocedural non-contrast CT image (using mediastinal soft tissue window) showed a homogenous mass. b 18F-FDG PET/CT imaging showed a mass with uneven uptake (SUVmax = 12.8) (arrow) in the left lower lobe. c CT and prior PET/CT fused image showed FDG-avid peripheral region of the mass were targeted. Final surgery revealed lung large cell carcinoma with central necrosis
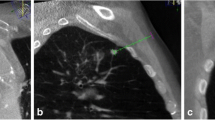
A 52-year-old man with suspected primary lung malignancy. a The intraprocedural non-contrast CT image (using mediastinal soft tissue window) showed a heterogenous mass in right upper lobe. b 18F-FDG PET/CT imaging showed heterogeneous uptake (SUVmax 10.2) in the mass with periphery hypometabolism. c CT and prior PET/CT fused image showed the biopsy needle punctured into hypermetabolic region of the mass. Surgical resection showed poorly differentiated lung adenocarcinoma with atelectasis
Pathology results of biopsy
According to histopathological and microbiological analysis, the results of biopsy specimens were categorized as follows: malignancy; specific benign disease (e.g., hamartoma, infection, vasculitis); non-specific benign (fibrosis, necrosis or inflammation without identification of a specific disease) and nondiagnostic (only normal parenchymal cells and no infectious agents were found) [2], The final diagnosis was established through surgical resection or clinical and radiological follow-up (Once a month for first three month. If the lesion keeps stable, the interval will be adjusted to three months, if not, keep the interval before.) for at least 6 months following biopsy (13 subjects with malignancy died 6 months after biopsy). Diagnostic yield was defined as the percentage of specimens from biopsy with the diagnosis of malignancy and specific benign disease (Nbiopsy/Nfinal diagnosis) [2]. In addition, all biopsy results mentioned above were classified as true or false diagnosis by whether biopsy specimen diagnoses correlated with the final diagnosis. Diagnostic accuracy rate was defined as the percentage of specimens with true diagnosis [3].
Statistical analysis
According to CT-guided biopsy with or without PET/CT fusion imaging, all the subjects were divided into two groups: fusion imaging group and routine group. Continuous variables were expressed as mean ± standard deviation (SD) or median ± interquartile range (IQR). Independent-sample t-tests and Pearson’s chi-square tests were used for group comparisons of continuous and categorical variables. All statistical analysis was completed using SPSS 20.0. Statistical significance was set at P < 0.05.
Results
Totally, 145 subjects (46 females, 99 males; mean age, 63.1 years; range, 22 ~ 84 years) who underwent CT-guided biopsy with adequate samples were enrolled, including 76 in fusion imaging group and 69 in routine group. Detailed clinical characteristics were summarized in Table 1. Of all subjects, 39 subjects had received surgery, 13 subjects died 6 months after biopsy for malignant disease progression, and 97 subjects had follow-up at least 6 months after initial biopsy.
The final diagnosis was obtained including 101 malignant lesions, 23 specific benign lesions, 21 non-specific benign lesions (Table 2). The overall diagnostic yield and diagnostic accuracy rate were 80.3% (53/66), 82.9% (63/76) for fusion imaging group, 70.7% (41/58), 75.4% (52/69) for routine group, respectively. In addition, the diagnostic yield for malignancy in fusion imaging group (98.1%, 52/53) was obviously higher than that in routine group (81.3%, 39/48).
According to the Society of Interventional Radiology Standards of Practice Committee Classification [18], there were only minor complications, including localized pneumothorax (39/145; 26.9%), intrapulmonary hemorrhage (24/145; 16.6%) and hemoptysis (10/145; 6.9%), However, none of these patients needed further treatment. Furthermore, the rate of overall procedure-related complications in fusion imaging group was slightly lower than that in routine group (35.5% vs 44.9%). The procedure duration between two groups showed no significant difference (P = 0.198).
Discussion
In this study, we found that 1) CNB was an effective and safe method in evaluating lung lesions with or without prior PET/CT fusion imaging, 2) intraprocedural CT and prior PET/CT fusion imaging can improve overall diagnostic yield and accuracy rate of CNB, especially in malignant lesions, 3) the overall complication rate in fusion imaging group was relatively lower, in contrast to routine group. The observations described above can be briefly summarized as follows: CNB with prior PET/CT fusion imaging is a feasible approach that improves diagnostic yield and accurate rate in lung masses (short diameter over 30 mm), thus providing greater potential benefit for patients.
The normal lung tissue is spongy and gas-filled, demonstrating periodic morphological changes during deflation and inflation, which is quite different from other solid organs (such as liver, bone). Large lung lesions (such as lung cancer) are prone to cause peripheral pneumonia, atelectasis, and even regional necrosis, which are hard to be distinguished from tumor on non-contrast CT images during biopsy. Furthermore, variation of breathing movement is another confounding factor. The interventional radiologist may have to repeatedly adjust the puncture site to avoid ribs or large vessels during biopsy. In addition, the pleural cavity and adjacent small airway are unavoidable structures during percutaneous lung biopsy, thus introducing a high risk of complications (such as pneumothorax, hemoptysis). In other words, percutaneous transthoracic biopsy is a relatively difficult procedure with high complication rate. Pneumothorax, intrapulmonary hemorrhage and hemoptysis were the most common complications in our sample, with an incidence of 26.9%, 16.6% and 6.9%, respectively. These results were similar to those of previous studies [6, 7].
As we all know, 18F-FDG PET/CT provides metabolic information related to disease and has shown a great advantage in targeting potential abnormal disease, especially malignant tumors [2,3,4, 9,10,11,12]. In the current study, only the subjects with lung mass or consolidation greater than 30 mm in short diameter were enrolled. Generally, the larger the lung lesion, the more likely it is to be heterogeneous (necrosis, fibrosis, atelectasis, etc.), resulting in an uneven FDG uptake [19]. Theoretically, intraprocedural CT and prior PET/CT fusion imaging can identify the lesion site and its extent better, thus reducing a preselection bias for biopsy-site and improving diagnostic performance. However, FDG avidity could also be observed in some benign tumors, infective and inflammatory conditions that are difficult to distinguish from each other [20]. It is no wonder that even though FDG-avid regions were targeted, some false negative biopsy results were obtained in the current study. Actually, similar conditions were also reported in previous studies [9,10,11,12]. This might be one of reasons that no consensus has been reached yet for its usefulness. A recent study reported that CNB under the guidance of PET/CT was superior to CT-guided biopsy, PET/CT guided biopsy of lung lesions led to fewer inconclusive biopsies in comparison with CT guided biopsy, with similar complication rates. However, PET/CT guided biopsy had a certain radiation risk to the operator. In addition, the size of the lesion in that study was not limited to mass or consolidation. In clinical work, the risk of necrosis and bleeding of smaller nodules was low. Generally, significant pathological results can be obtained according to CT guidance biopsy. PET/CT guided biopsy was not necessary in these cases. Their research results also showed that lesions that required rebiopsy in the PET/CT group had a greater average size than those on the CT group (9.6 × 4.6 cm, p = 0.04). In our study, we enrolled masses or consolidation larger than 3 cm. In these cases, PET/CT images were necessary to guide biopsy [17].
Interestingly, even though CNB of lung lesions is a high-risk procedure, but no severe complications developed. Furthermore, intraprocedural CT and prior PET/CT fused imaging allows for more accurate identification for FDG-avid lesions that more accessible to biopsy, reducing the likelihood of complications and procedure duration. So, the procedure duration of fusion imaging group was slightly shorter than that of routine group (8.7 min vs. 9.4 min), although no significant difference was observed.
There are several limitations in the current study. First, this is a retrospective study performed in a single center. Second, currently, PET/CT examination is not a routine item covered by health insurance, thus introducing a potential selection bias for the patients in fusion image group, in other words, fusion imaging group has significantly fewer benign lesions than routine group. Third, even though the follow-up duration was relatively limited compared to previous studies [9,10,11,12], 97(66.9%) subjects were followed up for more than 10 months, the follow-up duration of previous studies, and hence a definite clinical diagnosis could be obtained to some extent.
Conclusion
CNB with prior PET/CT fusion imaging is particularly helpful in improving diagnostic yield and accurate rate of biopsy in lung masses, especially in heterogeneous ones, thus providing greater potential benefit for patients.
Availability of data and materials
The imaging datasets used and/or analyzed during the current study are not publicly available due to the policies of the institutions. The research datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CNB:
-
CT-guided transthoracic core-needle biopsy
- PACS:
-
Picture archiving and communication system
- SUV:
-
Standardized uptake value
- FOV:
-
Field of view
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27:138–48.
Brioulet J, David A, Sagan C, Cellerin L, Frampas E, Morla O. Percutaneous CT-guided lung biopsy for the diagnosis of persistent pulmonary consolidation. Diagn Interv Imaging. 2020;101:727–32.
Kiranantawat N, McDermott S, Fintelmann FJ, Montesi SB, Price MC, Digumarthy SR, et al. Clinical role, safety and diagnostic accuracy of percutaneous transthoracic needle biopsy in the evaluation of pulmonary consolidation. Respir Res. 2019;20:23.
Padrão E, Rodrigues M, Guimarães S, Caetano Mota P, Melo N, Souto Moura C, et al. Diagnostic yield of computed tomography-guided transthoracic lung biopsy in diffuse lung diseases. Respiration. 2018;96:455–63.
Yeow KM, Tsay PK, Cheung YC, Lui KW, Pan KT, Chou AS. Factors affecting diagnostic accuracy of CTguided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol. 2003;14:581–8.
Laurent F, Latrabe V, Vergier B, Montaudon M, Vernejoux JM, Dubrez J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: results with an automated 20-gauge coaxial cutting needle. Clin Radiol. 2000;55:281–7.
Yang W, Sun W, Li Q, Yao Y, Lv T, Zeng J, et al. Diagnostic accuracy of CT-guided transthoracic needle biopsy for solitary pulmonary nodules. PLoS ONE. 2015;10: e131373.
Nath A, Prashanth A, Lal H, Kumar S, Barai S, Gambhir S. Robotic-assisted computed tomography-guided 18F-FDG PET/computed tomography-directed biopsy for diagnosis of intra thoracic lesions. Nucl Med Commun. 2020;41:246–51.
Tatli S, Gerbaudo VH, Mamede M, Tuncali K, Shyn PB, Silverman SG. Abdominal masses sampled at PET/CT-guided percutaneous biopsy: initial experience with registration of prior PET/CT images. Radiology. 2010;256:305–11.
Yokoyama K, Ikeda O, Kawanaka K, Nakasone Y, Tamura Y, Inoue S, et al. Comparison of CT-guided percutaneous biopsy with and without registration of prior PET/CT images to diagnose mediastinal tumors. Cardiovasc Intervent Radiol. 2014;37:1306–11.
Cerci JJ, Tabacchi E, Bogoni M, Delbeke D, Pereira CC, Cerci RJ, et al. Comparison of CT and PET/CT for biopsy guidance in oncological patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1269–74.
Guo W, Hao B, Chen HJ, Zhao L, Luo ZM, Wu H, et al. PET/CT-guided percutaneous biopsy of FDG-avid metastatic bone lesions in patients with advanced lung cancer: a safe and effective technique. Eur J Nucl Med Mol Imaging. 2017;44:25–32.
Fontana F, Piacentino F, Ierardi AM, Carrafiello G, Coppola A, Muollo A, et al. Comparison between CBCT and fusion PET/CT-CBCT guidance for lung biopsies. Cardiovasc Intervent Radiol. 2021;1:73–9.
Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1.
Xu Y, Ma L, Sun H, Huang Z, Zhang Z, Xiao F, Ma Q, Li C, Zhang X, Xie S. CT-guided microcoil localization for pulmonary nodules before VATS: a retrospective evaluation of risk factors for pleural marking failure. Eur Radiol. 2020;30(10):5674–83.
Huang ZG, Sun HL, Wang CL, Gao BX, Chen H, Yang MX, Chen XL. CT-guided transthoracic needle biopsy of pulmonary lesions: comparison between the cutting needle and aspiration needle. Br J Radiol. 2021;94(1118):20190930.
Cerci JJ, Bogoni M, Cerci RJ, Masukawa M, Neto CCP, Krauzer C, et al. PET/CT guided biopsy of suspected lung lesions requires less rebiopsy than CT guided biopsy due to inconclusive results. J Nucl Med. 2020;62:1057.
Lempel JK, Raymond DP, Ahmad U, O’Malley S, Bolen MA, Graham R, et al. Video-assisted thoracic surgery resection without intraoperative fluoroscopy after CT-guided microcoil localization of peripheral pulmonary nodules. J Vasc Interv Radiol. 2018;29:1423–8.
Guralnik L, Rozenberg R, Frenkel A, Israel O, Keidar Z. Metabolic PET/CT-guided lung lesion biopsies: impact on diagnostic accuracy and rate of sampling error. J Nucl Med. 2015;56:518–22.
Cerci JJ, Tabacchi E, Bogoni M. Fluorodeoxyglucose-PET/computed tomography-guided biopsy. PET Clin. 2016;11:57–64.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Conception and design: HS, YL. Acquisition of data, or analysis and interpretation of data: All authors. Drafting the article or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of China-Japan Friendship Institute of Clinical Medicine (IRB code: 2020–13). All methods were performed in accordance with the relevant guidelines and regulations. It was determined to be a retrospective analysis of de-identified data, and was determined to be exempt from requiring written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Y., Xu, Y., Lin, J. et al. Improving CT-guided transthoracic biopsy diagnostic yield of lung masses using intraprocedural CT and prior PET/CT fusion imaging. BMC Pulm Med 22, 311 (2022). https://doi.org/10.1186/s12890-022-02108-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02108-6