Abstract
Background
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection in intensive care units (ICUs). The present study aims to determine the prevalence of pathogenic bacteria, their biofilm formation, and molecular typing from patients with HAP in southwestern Iran.
Methods
Fifty-eight patients with HAP participated in this cross-sectional study. Sputum and endotracheal aspirate were collected from each patient for isolation and detection of bacteria. Biofilm formation was evaluated using Congo red agar or Microtiter plate assay. The antimicrobial susceptibility patterns of the isolates were investigated. The multiplex polymerase chain reaction (M-PCR) technique was used to determine the Staphylococcal Cassette Chromosome mec (SCCmec) types of methicillin-resistant Staphylococcus aureus (MRSA) strains. All S. aureus isolates were typed using the agr typing method. A repetitive element sequence-based PCR (rep-PCR) typing method was used for typing of Gram-negative bacteria. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software version 15 and the chi-square test.
Results
Bacteria were isolated in 52 (89.7%) of patients. Acinetobacter baumannii (A. baumannii) was the most prevalent organism (37%), followed by S. aureus, Pseudomonas aeruginosa (P. aeruginosa), and Escherichia coli (E. coli). Using the PCR method, 56 bacteria were detected. A. baumannii was the most prevalent (35.7%) organism. A. baumannii and P. aeruginosa were biofilm-producing. All Gram-negative isolates were colistin-sensitive, and most of the A. baumannii isolates were multidrug-resistant (MDR). MRSA was identified in 12 (80%) S. aureus isolates, and 91.6% of MRSA were SCCmec type III. The agr type III was the most predominant. The rep-PCR analysis showed seven different patterns in 20 A. baumannii, six patterns in 13 P. aeruginosa, and four patterns in 6 E. coli.
Conclusion
A. baumannii was more prevalent than S. aureus in ventilator-associated pneumonia (VAP), while S. aureus is a major pathogen in non-ventilator hospital-acquired pneumonia (NV-HAP), possibly due to the tendency of the former to aquatic environments. Based on the rep-PCR typing method, it was concluded that bacteria were transmitted from patients or healthcare workers among different wards. Colistin can be used as a treatment in Gram-negative MDR isolates.
Similar content being viewed by others
Background
Hospital-acquired pneumonia (HAP) is defined as a parenchymal lung infection not occurring at the time of hospitalization or during the incubation period but 48 h after hospital admission. HAP is the second most common nosocomial infection, leading to prolonged hospitalization, increasing costs, and high morbidity/mortality rates [1]. Common signs and symptoms include fever, leukocytosis, purulent secretions, increasing respiratory rates, abnormal chest examination, tachypnea, and impaired oxygenation [2]. HAP is divided into two subgroups: ventilator-associated pneumonia (VAP) and non-ventilator hospital-acquired pneumonia (NV-HAP) [3]. VAP, a subset of HAP, occurring 48 h or more after tracheal intubation and connected to the ventilator, is the most common infection in ICUs. NV-HAP occurs in patients hospitalized for at least 48 h and not connected to a ventilator [4, 5]. Bacterial infections are the leading cause of HAP. Among them, Acinetobacter spp, P.aeruginosa, E. coli, Klebsiella pneumonia (K. pneumoniae), and S. aureus (especially MRSA) were the most common agents [6,7,8]. Among the major sources of these bacteria are hospital environments, patients’ microbial flora, and primary hospitalized patients [9].
Biofilm formation is one of the most effective factors in the pathogenicity of these bacteria. Biofilm may cause bacteria to develop antimicrobial resistance and interfere with host defense mechanisms. Biofilm can also be a reservoir for the recurrence of these pathogens [10]. An endotracheal tube is a potential reservoir for microorganisms to infect the respiratory tract, a risk factor for VAP, since microorganisms can adhere to its surface, and some species form biofilm thereon. These microorganisms may be transmitted to the lungs during suctioning [11]. Therefore, biofilm-producing bacteria can persist in hospital environments and significantly contribute to nosocomial infections (especially in HAP), leading to treatment failure, rising costs of treatment, and mortality in patients.
Molecular typing methods are widely used to identify infectious species for epidemiological studies, determine the population structure of microbial communities and genetic diversity within a species. Molecular typing of bacteria can effectively prevent and control infections. Repetitive element sequence-based polymerase chain reaction (rep-PCR) is a molecular typing method used to determine clonal relationships, genotyping, and phylogenetic relationships between closely related species [12, 13]. It is extensively used to identify, track, and study diversity in microorganisms [14]. Many typing methods are applied for S. aureus (an important nosocomial pathogen), including multi locus sequence typing (MLST), S. aureus protein A (spa) typing, SCCmec typing, and accessory gene regulator (agr) typing. They can effectively describe epidemiologic trends and implement infection control strategies [15]. The agr locus is one of the major regulators of virulence factor production (hemolysins, enterotoxins, MSCRAMMs, etc.) in S. aureus [16]. Itis controlled by P2 and P3 promoters. The P2 operon consists of four genes: agrA, agrB, agrC, and agrD. The P3 operon transcribes a regulatory RNA molecule called RNAIII [17]. So far, four major agr groups (I–IV) have been identified based on the amino acid sequence polymorphism of the agr-encoded autoinducing peptide (agrD) and its corresponding receptor (agrC). The agr types are different in their properties and prevalence in various geographical areas. Thus, identifying predominant types in each region can serve epidemiological purposes. MRSA strains harbor the mecA gene and are located on the SCC. Based on complete sequence data, thirteen SCCmec types (I–XIII) have been defined in MRSA [18]. Among the various SCCmec types reported worldwide, types I-III are known as predominant HA-MRSA [19]. SCCmec typing can significantly contribute to the detection of strains associated with nosocomial infections [20].
Since microorganisms responsible for HAP differ between geographical regions and among hospitals or patients in one region and most empirically treated HAP, collecting information on bacterial pathogens and their antibiotic resistance patterns is necessary. Hence, the present study aimed to detect microorganisms from patients with HAP, evaluate biofilm production and antimicrobial susceptibility patterns of bacterial isolates, and conduct molecular typing (SCCmec typing, agr typing, and rep-PCR) of bacterial isolates collected from patients with HAP admitted in Shahid Beheshti and Imam Sajjad hospitals in southwestern Iran.
Method
Sampling method
This descriptive cross-sectional study was performed on 58 patients hospitalized in the Imam Sajjad and Shahi Beheshti hospitals affiliated to Yasuj University of Medical Sciences (YUMS) in southwest Iran from 2018 to 2019. The inclusion criteria are as follows: (1) should be above 15 years of age, (2) should have at least one or two of the following symptoms: fever, leukocytosis, leukopenia, purulent secretion of lungs, sputum, and cough, (3) should have been infected 48 h after hospitalization, and (4) for VAP, should have received mechanical ventilation 48 h or longer after intubation. Patients who were unwilling to enter into the study voluntarily, those suffering from immunodeficiency, and those showing signs of pneumonia within less than 48 h of hospitalization were excluded. The patients were diagnosed by a physician specializing in internal medicine and infectious diseases. The first morning sputum samples and endotracheal aspirate (ETA) were collected in sterile containers under aseptic conditions and delivered to the microbiology laboratory. The samples were then divided into two parts: the first part was inoculated on Mac Conkey agar (Condalab, Spain), blood agar (5% sheep), and chocolate agar (Condalab, Spain) media and incubated at 37 °C for 24–48 h. The bacteria were then identified based on Gram staining, colony morphology, and standard biochemical tests. The PCR method was applied for the final confirmation of isolated bacteria. The second part is stored at − 20 °C for molecular analyses. Demographic data, including age, sex, and clinical characteristics, were collected. Before sampling, informed consent was obtained from each patient. This study has been approved by the Research Ethics Committee of the Yasuj University of Medical Sciences (IR.YUMS.REC. 1396.194).
Biofilm formation assay
For Gram-negative bacteria, biofilm formation was evaluated using the microtiter plate method [21]. First, each isolate was inoculated in Trypticase Soy Broth (TSB) and incubated at 37 °C for 24 h. Then, a 1:100 dilution of this suspension was prepared and 200 μl of this dilution was inoculated in sterile 96-well flat-bottom polystyrene microtiter plates. The negative control wells only contained sterile TSB medium and were incubated for 24 h at 37 °C (each test was repeated three times). Afterward, the contents of the wells were emptied, and each well was gently washed three times with 200 μl phosphate-buffered saline (PBS). The plates were then dried at room temperature, and 150 μl of 99% methanol were added to each well to fix the bacteria inside the wells. After 15 min, the contents of the wells were drained, and the plates were allowed to dry at laboratory temperature. The wells were then stained with 200 μl of 1% crystal violet for 20 min. They were then rinsed gently with water, to each 200 μl of acetic acid 33% was added to remove crystal violet. The plates were then incubated at 37 °C for 15 min, and the light absorption of the painted wells at 620 nm was read by enzyme-linked immunosorbent assay (ELISA). They were classified into four groups: strong biofilm producers, moderate biofilm producers, weak biofilm producers, and non-biofilm producers based on optical density (O.D.). For S. aureus, biofilm formation was evaluated using the Congo red method according to Avila-Novoa et al.’s method [22]. Black colonies yielded positive results. Weak biofilm-producing isolates remained pink, while occasional darkening at the centers of colonies was also observed. Bright red colonies were considered negative.
Antimicrobial susceptibility testing
Antibiotic susceptibility pattern of isolates was performed using the disc diffusion method on Mueller Hinton Agar (Condalab, Spain) according to the Clinical and Laboratory Standards Institute (CLSI) guideline for the following antibiotics: penicillin (10 μg), cefepime (30 μg), cefoxitin (30 μg), sulfamethoxazole (23/75 μg), tazobactam (10 μg) + piperacillin (100 μg), cefotaxime (30 μg), ceftriaxone (30 μg), meropenem (10 µg), clindamycin (2 μg), clarithromycin (15 μg), levofloxacin (5 μg), azithromycin (15 μg) (BD-BBL Company, USA). The Minimum Inhibitory Concentration (MIC) of colistin and vancomycin was determined using the broth microdilution method. Bacterial isolates resistant to three or more different antimicrobial classes were identified as MDR isolates. S. aureus (ATCC 25923) and E. coli (ATCC 25922) were used as control strains.
Molecular detection of bacterial pathogens
DNA was extracted from clinical specimens including sputum and ETA by utilizing the phenol–chloroform method and then stored at − 20 °C as DNA. This study employed the boiling method for bacterial isolates by the culture method. Table 1 lists the primers used in this study. The PCR program was as follows: the PCR protocol was carried out in a thermocycler (Bio-Rad, T100, USA) with an initial denaturation at 94 °C for 4 min, followed by 34 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s for nucA, extension at 72 °C for 1 min, and a final cycle of extension at 72 °C for 5 min. For the detection of A. baumannii, P. aeruginosa, E. coli, and K. pneumoniae, the program was as follows: initial denaturation at 94 °C for 4 min, 30 cycles of denaturation at 94 °C for 45 s, annealing at 59 °C for 30 s for gltA and oprL, at 57 °C for 60 s for aroE and ureD, extension at 72 °C for 1 min, and a final cycle of extension at 72 °C for 5 min. The control strains included S. aureus ATCC25923, K. pneumoniae ATCC 1290, E. coli ATCC 25922, P. aeruginosa PAO1, A. baummanii ATCC 19606. The sequences of oligonucleotide primers for the detection of bacteria are shown in Table 1 [23,24,25].
Molecular typing (SCCmec typing and agr typing)
Multiplex PCR conditions were as follows: an initial denaturing at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 60 °C for 45 s for SCCmec types I, II, III, and V, and at 60.5 °C for 45 s for SCCmec subtypes IVa, IVb, IVc, and IVd, and extension at 72 °C for 40 s. For agr typing, the program was similar to nucA except for annealing at 57 °C for 1 min. The final extensions were continued at 72 °C for 5 min and performed in a thermocycler (Bio-Rad, T100, and the USA). The following MRSA strains were used as positive controls: COL (SCCmec type I); XU642 (EMRSA-16, SCCmec type II); WBG525 (EMRSA-1, SCCmec type III3); and WBG9465 (EMRSA-15, SCCmec type IV2), kindly provided by Dr. Mohammad Emaneini. The sequences of oligonucleotide primers for the detection of SCCmec types and agr typing are shown in Table 1 [26, 27].
Repetitive sequence-based polymerase chain reaction
The primers REP1 (5′-GCGCCGICATCAGGC-3′) and REP2 (5′- ACGTCTTATCAGGCCTAC-3′) were used for repetitive sequence-based polymerase chain reaction (rep-PCR). Amplification reactions were performed in a final volume of 25 μL, containing 12.5 µl Master Mix (Amplicon, Denmark), 25 ρmol of each primer, and 5 µl bacterial DNA. The PCR protocol included an initial denaturation at 94 °C for 10 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 45 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 16 min. The PCR products were electrophoresed on a 2% agarose gel at 90 V for 45 min (for rep-PCR 60 V for 150 min), stained with DNA Safe Stain, and visualized under UV light using a gel documentation system (Major Sciences, Taiwan).
Results
Demographic and clinical characteristics of patients
A total of 58 patients participated in the study. At least one bacterial isolate was identified in 52 (89.7%) patients using the culture method. The average age of culture-positive patients was 57.92 ± 18.59 years (ranging between 18 and 94), 52% of whom were female. Besides, 36 (69%) patients were VAP, and 16 (31%) were NV-HAP. The most common clinical symptoms and laboratory results were leukocytosis (100%), fever (82.7%), and sputum (82.7%). Table 2 presents the demographic and clinical characteristics of patients with VAP and NV-HAP.
Detection of bacteria using the culture and PCR methods
Using the culture method, 54 bacterial isolates were collected from 52 patients. Among them, Gram-negative bacilli were obtained in 39 (72.2%) and Gram-positive cocci (S. aureus) in 15 (27.8%). Among Gram-negative bacteria, A. baumannii was obtained in 20 (37%), P. aeruginosa in 13 (24.1%), and E. coli in 6 (11.1%) patients. Most bacterial isolates from patients with VAP were Gram-negative (84.6%), while 56.3% were prominent in NV-HAP Gram-positive bacteria (S. aureus). A. baumannii was the most predominant isolate identified in 37% of patients. A. baumannii was identified more in patients with VAP than those with NV-HAP, and the difference was statistically significant (P = 0.016). Moreover, S. aureus was detected more in patients with NV-HAP than those with VAP, and the difference was statistically significant (P = 0.003). (More details are shown in Table 3).
Using the PCR method, 56 bacteria were detected, 69.6% from patients with VAP and 30.4% from those with NV-HAP. The detection rate of microorganisms was as follow: 35.7% (N = 20) for A. baumannii, 26.8% (N = 15) for S. aureus, 23.2% (N = 13) for P. aeruginosa, 12.5% (N = 7) for E. coli, and 1.8% (N = 1) for K. pneumoniae. There were no significant differences between bacteria isolated by either the culture or PCR methods.
Biofilm production
Totally, 94.4% of the isolates were biofilm-producing. Among them, the highest rate of biofilm formation was observed in A. baumannii and P. aeruginosa (100%), followed by S. aureus (86.7%) and E. coli (83.4%) (Table 4).
Antibiogram
Among Gram-negative bacteria, the highest rate of resistance was observed in levofloxacin, cefotaxime, and ceftriaxone. S. aureus isolates have a high rate of resistance to levofloxacin and penicillin. Table 5 presents the antibiotic resistance patterns of the Gram-positive and Gram-negative bacterial isolates. All of the Gram-negative isolates were susceptible to colistin.
SCCmec typing
The mecA gene was identified in 12 (80%) S. aureus isolates and defined as MRSA, among which 11 cases (91.6%) harbored SCCmec type III (the most frequent SCCmec type) and 1 case (8.4%) harbored SCCmec type I. Types II, IVa, IVb, IVc, IVd, and V were not recognized in any isolates.
Agr typing
Among 15 S. aureus isolates, 73% (N = 11) were successfully typed using the agr typing method. agr type III was the most prevalent agr type identified in 54% (N = 6), followed by agr type I (36%, N = 4) and agr type II (9%, N = 1). No agr types IV were observed in the isolates.
Rep-PCR typing
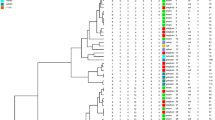
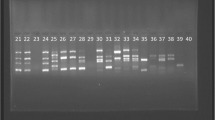
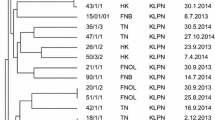
In Gram-negative bacteria, the rep-PCR typing pattern of isolates (based on band pattern similarity) generally exhibited great genetic diversity among isolates. The rep-PCR analysis showed seven different patterns in 20 A. baumannii isolates, six patterns in 13 P. aeruginosa isolates, and four patterns in 6 E. coli isolates (Table 6 and Fig. 1).
Discussion
HAP is a common, serious, and costly problem in hospitals with dangerous complications and an important cause of death in patients admitted to the ICUs [28,29,30]. In the United States, HAP occurs at a rate of 5–20 cases per 1000 hospital admissions [31]. In various studies, Gram-negative bacteria are more prevalent than Gram-positive bacteria in HAP [32,33,34]. In the present study, Gram-negative bacteria (A. baumannii, P. aeruginosa, E. coli, and K. pneumoniae) were isolated at a rate of 72.2%. The frequency of HAP-causing bacteria may vary by hospital, geographic area, patient status, immune system, and health status. A. baumannii is an opportunistic pathogen associated with nosocomial infections, especially in ICUs. In the present study, like previous studies [35,36,37,38], A. baumannii was the most common microorganism isolated from HAP, implying the important role of A. baumannii in HAP. The high percentage of A. baumannii isolates in this study indicated its colonization capacity (in hospital environments, staff hands, and patient equipment), biofilm formation, the transmission of organisms to lower airways during suctioning in ventilated patients. S. aureus plays an important role in nosocomial infections, especially in HAP. Herein, it was the second organism(27.8%), similar to Ronald et al. [39] study, whose reported values were higher than in other studies [32, 40, 41]. The presence of S. aureus in hospital environments and its long-term colonization capacity may be attributed to microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), biofilm formation, and teichoic acid (TA). In accordance with our study, Feng et al. detected A. baumannii in patients with VAP rather than those with NV-HAP, while S. aureus was detected in patients with NV-HAP more than those with VAP [8]. This can be due to the fact that A. baumannii tends to aquatic environments. P. aeruginosa, one of the most common opportunistic pathogens leading to severe nosocomial infections and a stable pathogen that easily forms biofilm and colonizes the body, was the third (24%) isolated pathogen in the present study. A. baumannii and P. aeruginosa were the most common isolated organisms in patients with VAP, which can be due to their tendency to aquatic environments. Herein, E. coli was isolated from11.2% of patients with HAP, higher than reported earlier [37, 39, 41]. In contrast, K. pneumoniae was not isolated in any case using the culture method, unlike many earlier studies [37, 40, 42].
The development of nosocomial infections in hospitalized patients (especially in the ICUs) is usually associated with ventilators, venous catheters, and urinary catheters. Many infections caused by these devices are related to contamination caused by biofilm-producing microorganisms. In general, colonization and biofilm formation are two important factors in HAP-causing bacteria [10]. Herein, 94% of the isolates were able to form biofilm. Additionally, all of the A. baumannii and P. aeruginosa isolates were biofilm-producing, similar to the studies by Asadian et al. [43] and Alonso et al. [44], indicating the significant role of biofilm in HAP-causing bacteria. Totally,86.7% of the S. aureus isolates were biofilm-positive, higher than what was reported by El-Nagdy [45]. Endotracheal tube, a potential source of intubated patients’ pulmonary contamination. It can cause damage to and transfer bacteria from the pharyngeal cavity to the lower airways, on which biofilm can form by bacteria, which is then removed during suctioning and delivered to the lower device [11]. In addition, microorganisms can be established due to biofilm and colonization in hospital environments for longer time periods. Biofilm formation in bacteria can lead to increased antibiotic resistance, development of chronic and persistent infection, and increased mortality rates in patients with HAP. Therefore, preventing biofilm formation requires the surveillance of hygiene standards, appropriate replacement of endotracheal tube, and proper sterilization of medical instruments.
The rep-PCR analysis showed seven different patterns in 20 A. baumannii isolates, six patterns in 13 P. aeruginosa isolates, and four patterns in 6 E. coli isolates, suggesting high pattern diversity among bacterial isolates, which could be related to the transmission of bacteria from patients or healthcare workers among different wards. Analyzing the rep-PCR typing method in Gram-negative bacteria demonstrated that bacterial isolates with similar patterns are isolated from patients in consecutive intervals caused by a common source of infection.
Herein, 80% of S. aureus harbored the mecA gene and was identified as MRSA, which is in accordance with the studies by Abbasi-Montazeri et al. [46] and Khoshnood et al. [47], which was lower than what was reported by Mohammadi et al. [48]. Like earlier studies, SCCmec type III was the most prevalent [49, 50]. However, in a study by Abbasi Montazeri et al., SCCmec type I was identified as predominant in their methicillin-resistant coagulase-negative staphylococci (MR-CoNS)) isolates [51]. The predominance of SCCmec type III suggested that the S. aureus isolates in the present study are of hospital origin. Hence, the Infection Control and Prevention Program (ICPP) is necessary to limit or eradicate the bacteria circulating in hospitals.
The majority of the S. aureus isolates belonged to agr type III. In accordance with our study, two recent studies [52, 53] reported that agr group III was the most predominant in their isolates, while in other studies, agr type I and II [54] and agr type I and III [55] were the most common. Our findings revealed that the S. aureus isolates with agr group III were more prevalent in nosocomial infections.
Antibiotic resistance is turning into a public health crisis, especially in developing countries. The highest rate of resistance was observed among Gram-negative bacteria to levofloxacin, cefotaxime, and ceftriaxone. Most of the A. baumannii isolates in this study were MDR. The highest rate of antibiotic resistance was observed to levofloxacin, ceftriaxone, meropenem, cefotaxime, and piperacillin-tazobactam, which is consistent with other studies indicating that most of the A. baumannii isolates were MDR [42, 56, 57].
More than 60% of P. aeruginosa isolates were resistant to levofloxacin, ceftriaxone, meropenem, cefotaxime, piperacillin-tazobactam. Unlike this study, Delle Rose et al. [58] from Italy reported that only 33.3% of P. aeruginosa isolates were resistant to meropenem. They also concluded that 31.2%, 25%, and 45% of the isolates were resistant to levofloxacin, ceftriaxone, and tazobactam, respectively, which is lower than that reported in our study. In comparison, in another study from India by Tiwari et al. [42], 80% of their isolates showed resistance to meropenem. In general, the high antibiotic resistance rate of P. aeruginosa and A. baumannii makes treatment difficult. According to the studies, the proper antibiotic selection is challenging because these bacteria are resistant to different antibiotics by using various antibiotic resistance mechanisms. All Gram-negative bacteria herein were colistin-sensitive, similar to studies by Moosavian et al. [59] and Sharifi et al. [60]. It can be used as a treatment against MDR Gram-negative isolates. Varying antibiotic resistances in different studies may be related to healthcare policies in different countries, length of hospital stay, and the geographical distribution of bacterial isolates. The present study results indicated a dramatic increase in the rate of antibiotic resistance, which could be due to antibiotic overuse, empiric therapy without antibiogram results, lack of proper sterilization of ventilators after each use, and use of non-standard ventilation systems.
Conclusions
The most common isolated bacteria in HAP were A. baumannii, S. aureus, P. aeruginosa, and E. coli. Among them, A. baumannii was more prevalent in patients with VAP. Meanwhile, S. aureus is a major pathogen in patients with NV-HAP, which could be related to the tendency of A. baumannii to aquatic environments. Most bacterial isolates were biofilm-producing, leading to increased antibiotic resistance and chronic and persistent infection in HAP. All Gram-negative bacteria isolated herein were sensitive to colistin, which can be used to treat MDR Gram-negative isolates. Using the rep-PCR typing method, high diversity of patterns were observed among bacterial isolates, which may be due to the transmission of bacteria from patients or healthcare workers among different wards.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999–2006.
Kumar A, Raghavendran M. Ventilator associated pneumonia. RJNS. 2021;11(2):38–41.
James DB, Finley E, Authority PPS. The breadth of hospital-acquired pneumonia: nonventilated versus ventilated patients in pennsylvania. NLM. 2012;9(3):99–105.
Andre C, Mark L, Michael K, John M, Daniel A, Lucy B, Lena M, Naomi P, John G, Jordi C, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111.
Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–7.
Chou CC, Shen CF, Chen SJ, Chen HM, Wang YC, Chang WS, Chang YT, Chen WY, Huang CY, Kuo CC, et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect. 2019;52(1):172–99.
Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S, Hraiech S, Jung B, Kipnis E, Launey Y, et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med. 2018;37(1):83–98.
Feng DY, Zhou YQ, Zou XL, Zhou M, Zhu JX, Wang YH, Zhang TT. Differences in microbial etiology between hospital-acquired pneumonia and ventilator-associated pneumonia: a single-center retrospective study in Guang Zhou. Infect Drug Resist. 2019;12:993–1000.
Bonadonna L, Briancesco R, Coccia AM. Analysis of microorganisms in hospital environments and potential risks. Indoor Air Quality in Healthcare Facilities. 2017: 53–62.
Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care. 2012;16(3):R93.
Diaconu O, Siriopol I, Poloșanu LI, Grigoraș I. Endotracheal tube biofilm and its impact on the pathogenesis of ventilator-associated pneumonia. J Crit Care Med (Targu Mures). 2018;4(2):50–5.
Meshkat Z, Salimizand H, Amini Y, Khakshoor M, Mansouri D, Farsiani H, et al. Molecular characterization and genetic relatedness of clinically Acinetobacter baumanii isolates conferring increased resistance to the first and second generations of tetracyclines in Iran. Ann Clin Microbiol Antimicrob. 2017;16(1):51.
Nowak J, Zander E, Stefanik D, Higgins PG, Roca I, Vila J, et al. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2017;72(12):3277–82.
Khare N, Kaushik M, Martin JP, Mohanty A, Gulati P. Genotypic diversity in multi-drug-resistant E. coli isolated from animal feces and Yamuna River water, India, using rep-PCR fingerprinting. Environ Monit Assess. 2020;192(11):681.
Han LZ, Ho PL, Ni YX, Zhang H, Jiang YQ, Chu HQ, et al. Panton-valentine leukocidin–positive MRSA, Shanghai. Emerg Infect Dis. 2010;16(4):731–3.
Yang X, Dong F, Qian S, Wang L, Liu Y, Yao K, Song W, Zhen J, Zhou W, Xu H, et al. Accessory gene regulator (AGR) dysfunction was unusual in Staphylococcus aureus isolated from Chinese children. BMC Microbiol. 2019;19(1):95.
Bibalan MH, Shakeri F, Javid N, Ghaemi A, Ghaemi EA. Accessory gene regulator types of Staphylococcus aureus isolated in Gorgan, North of Iran. J Clin Diagnostic Res. 2014;8(4):DC07–9.
Singh-Moodley A, Lowe M, Mogokotleng R, Perovic O. Diversity of SCC mec elements and spa types in South African Staphylococcus aureus mec A-positive blood culture isolates. BMC Infect Dis. 2020;20(1):1–12.
Lim KT, Yeo CC, Suhaili Z, Thong KL. Comparison of methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains isolated from a tertiary hospital in Terengganu. Malaysia Jpn J Infect Dis. 2012;65:502–9.
Wang JL, Wang JT, Chen SY, Chen YC, Chang SC. Distribution of Staphylococcal cassette chromosome mec types and correlation with comorbidity and infection type in patients with MRSA bacteremia. PLoS ONE. 2010;5(3):e9489.
Jabalameli F, Mirsalehian A, Khoramian B, Aligholi M, Khoramrooz SS, Asadollahi P, et al. Evaluation of biofilm production and characterization of genes encoding type III secretion system among Pseudomonas aeruginosa isolated from burn patients. Burns. 2012;38(8):1192–7.
Avila-Novoa MG, Iñíguez-Moreno M, Solís-Velázquez OA, González-Gómez JP, Guerrero-Medina PJ, Gutiérrez-Lomelí M. Biofilm formation by Staphylococcus aureus isolated from food contact surfaces in the dairy industry of Jalisco, Mexico. J Food Qual. 2018. https://doi.org/10.1155/2018/1746139.
Askarinia M, Ghaedi M, Manzouri L, Khoramrooz SS, Sharifi A, Ghalamfarsa G, et al. The effect of Cu-BPDCA-Ty on antibacterial activity and the expression of meca gene in clinical and standard strains of methicillin-resistant Staphylococcus aureus. Jundishapur J Microbiol. 2018;11(3):e60680.
Noller AC, McEllistrem MC, Stine OC, Morris JG Jr, Boxrud DJ, et al. Multilocus sequence typing reveals a lack of diversity among Escherichia coli O157:H7 isolates that are distinct by pulsed-field gel electrophoresis. J Clin Microbiol. 2003;41(2):675.
Zamani A, Mashouf RY, Namvar AME, Alikhani MY. Detection of magA gene in Klebsiella spp. isolated from clinical SamplesDetection of magA. Iran J Basic Med Sci. 2013;16(2):173–6.
Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–33.
Shopsin B, Mathema B, Alcabes P, Said-Salim B, Lina G, Matsuka A, et al. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J Clin Microbiol. 2003;41(1):456–9.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Bassi GL, Luna CM, Martin-Loeches I, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50(3):1700582.
Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(3):250–6.
Iwata K, Igarashi W, Oba Y, Ohji G, Honjo M, Oka H, et al. Hospital-acquired pneumonia in Japan may have a better mortality profile than HAP in the United States: a retrospective study. J Infect Chemother. 2012;18(5):734–40.
Jiao J, Li Z, Wu X, Cao J, Liu G, Liu Y, Li F, Zhu C, Song B, Jin J, et al. Risk factors for 3-month mortality in bedridden patients with hospital-acquired pneumonia: a multicentre prospective study. PLoS ONE. 2021;16(3):e0249198.
Farid GA, Moghaddam AB, Bojdy A. Nosocomial pneumonia in patients admitted to the intensive care unit of a tertiary care center in Mashhad, northeast of Iran; an etiologic survey. Arch Clin Infect Dis. 2018;13(4):e64239.
Djordjevic ZM, Folic MM, Jankovic SM. Distribution and antibiotic susceptibility of pathogens isolated from adults with hospital-acquired and ventilator-associated pneumonia in intensive care unit. J Infect Public Health. 2017;10(6):740–4.
Boostani V, Dehghan F, Karmostaji A, Zolghadri N, Shafii A. Incidence of hospital-acquired bacterial pneumonia and its resistance profiles in patients admitted to intensive care unit. Glob J Health Sci. 2017;9(3):73–9.
Japoni A, Vazin A, Davarpanah MA, Ardakani MA, Alborzi A, Japoni S, et al. Ventilator-associated pneumonia in Iranian intensive care units. J Infect Dev Ctries. 2011;5(04):286–93.
Salehifar E, Abedi S, Mirzaei E. Profile of microorganisms involved in nosocomial pneumonia and their antimicrobial resistance pattern in intensive care units of Imam Khomeini Hospital, Sari, 2011–2012. J Mazandaran Univ Med Sci. 2013;23(1):151–62.
Bozorgmehr R, Bahrani V, Fatemi A. Ventilator-associated pneumonia and its responsible germs; an epidemiological study. Emerging (Tehran). 2017;5(1):e26.
Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: etiology, clinical outcomes, and impact of antimicrobial resistance. J Med Assoc Thai. 2010;93(Suppl 1):S126–38.
Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Supplement_1):S81–7.
Yin Y, Zhao C, Li H, Jin L, Wang Q, Wang R, et al. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: a 10-year prospective observational study in China. Eur J Clin Microbiol Infect Dis. 2020;7:1–8.
Naidus EL, Lasalvia MT, Marcantonio ER, Herzig SJ. The diagnostic yield of non-invasive microbiologic sputum sampling in a cohort of patients with clinically diagnosed hospital-acquired pneumonia. J Hosp Med. 2018;13(1):34–7.
Tiwari U, Loomba P, Dogra V, Mishra B. Bacteriological profile of nosocomial pneumonia patients in a superspeciality hospital. Natl J Integr Res Med. 2016;7(3):60–3.
Asadian M, Azimi L, Alinejad F, Ostadi Y, Lari AR. Molecular characterization of Acinetobacter baumannii isolated from ventilator-associated pneumonia and burn wound colonization by random amplified polymorphic DNA polymerase chain reaction and the relationship between antibiotic susceptibility and biofilm production. Adv Biomed Res. 2019;8:58.
Alonso B, Fernández-Barat L, Domenico EGD, Marín M, Cercenado E, Merino I, et al. Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect Dis. 2020;20(1):1–8.
El-Nagdy AH, Abdel-Fattah GM, Emarah Z. Detection and control of biofilm formation by Staphylococcus aureus from febrile neutropenic patient. Infect Drug Resist. 2020;13:3091–101.
Abbasi-Montazeri E, Khosravi AD, Feizabadi MM, Goodarzi H, Khoramrooz SS, Mirzaii M, et al. The prevalence of methicillin resistant Staphylococcus aureus (MRSA) isolates with high-level mupirocin resistance from patients and personnel in a burn center. Burns. 2013;39(4):650–4.
Khoshnood S, Shahi F, Jomehzadeh N, Montazeri EA, Saki M, Mortazavi SM, et al. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among methicillin-resistant Staphylococcus aureus strains isolated from burn patients. Acta Microbiol Immunol Hung. 2019;66(3):387–98.
Mohammadi A, Goudarzi M, Dadashi M, Soltani M, Goudarzi H, Hajikhani B. Molecular detection of genes involved in biofilm formation in Staphylococcus aureus strains isolates: evidence from shahid motahari hospital in Tehran. Jundishapur J Microbiol. 2020;13(7):e102058.
Nejad ZS, Darabzadeh Z, Mazloomirad F, Khoramrooz SS, Ghatee MA, Sisakht SN, et al. Antimicrobial susceptibility pattern in the bacteria isolated from surgical site infection: emphasis on Staphylococcus Aureus; Yasuj City, Southwest Iran. Clin Lab. 2021. https://doi.org/10.7754/Clin.Lab.2020.200530.
Hesari Y, Sohrabi N, Abiri R, Babaei S, Amiri Z. staphylococcal cassette chromosome mec typing of meticillin-resistant Staphylococcus aureus in Kermanshah Province, West of Iran. Jundishapur J Microbiol. 2020;13(2):e98852.
Abbasi Montazeri E, Seyed-Mohammadi S, Asarehzadegan Dezfuli A, Khosravi AD, Dastoorpoor M, Roointan M, et al. Investigation of SCCmec types I-IV in clinical isolates of methicillin-resistant coagulase-negative staphylococci in Ahvaz, Southwest Iran. Biosci Rep. 2020;40(5):BSR20200847.
Maleki DT, Ghalavand Z, Laabei M, Nikmanesh B, Houri H, Kodor M, et al. Molecular analysis of accessory gene regulator functionality and virulence genes in Staphylococcus aureus derived from pediatric wound infections. Infect Genet Evol. 2019;73:255–60.
Derakhshan S, Navidinia M, Haghi F. Antibiotic susceptibility of human-associated Staphylococcus aureus and its relation to agr typing, virulence genes, and biofilm formation. BMC Infect Dis. 2021;21(1):627.
Azmi K, Qrei W, Abdeen Z. Screening of genes encoding adhesion factors and biofilm production in methicillin resistant strains of Staphylococcus aureus isolated from Palestinian patients. BMC Genomics. 2019;20(1):578.
Cheraghi S, Pourgholi L, Shafaati M, Fesharaki SH, Jalali A, Nosrati R, et al. Analysis of virulence genes and accessory gene regulator (agr) types among methicillin-resistant Staphylococcus aureus strains in Iran. J Glob Antimicrob Resist. 2017;10:315–20.
Alikiaii B, Aghadavoudi O, Emami N. Evaluating antibiotic resistance pattern of ventilator-associated pneumonia in intensive care units of Alzahra Hospital, Isfahan University of Medical Sciences, Iran. J Isfahan Med Sch. 2016;34(399):1083–9.
Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4(04):218–25.
Rose DD, Pezzotti P, Fortunato E, Sordillo P, Gini S, Boros S, Meledandri M, Gallo MT, Prignano G, Caccese R, et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur J Clin Microbiol Infect Dis. 2016;35(9):1531–9.
Moosavian M, Ahmadi K, Shoja S, Mardaneh J, Shahi F, Afzali M. Antimicrobial resistance patterns and their encoding genes among clinical isolates of Acinetobacter baumannii in Ahvaz, Southwest Iran. MethodsX. 2020;7:101031.
Sharifi H, Pouladfar G, Shakibaie MR, Pourabbas B, Mardaneh J, Mansouri S. Prevalence of β-lactamase genes, class 1 integrons, major virulence factors and clonal relationships of multidrug-resistant Pseudomonas aeruginosa isolated from hospitalized patients in southeast of Iran. Iran J Basic Med Sci. 2019;22(7):806–12.
Acknowledgements
This article is a part of Mrs. Farzad Mazloomirad MSc thesis. This study has been suppored by Yasuj University of Medical Sciences, Deputy of research and technology.
Funding
This study has been supported by Deputy of Research and Technology, Yasuj University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
SSK, FM and SH assisted with data analysis, interpretation, and drafting of the manuscript. SSK, FM, NR and SH assisted with study design, statistical analysis and drafting of the manuscript. NR assisted with statistical analysis of the manuscript. GN, FM and SH contributed to measuring samples in this study. SSK, SH, AS and FM contributed to data interpretation and critically reviewed the manuscript. All authors planned the study design, contributed to the interpretation of the data, drafted and approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This protocol was approved by Yasuj University of Medical Sciences, Ethics Committee with Ethical code number: IR.YUMS.REC. 1396. 194. Prior to sample collection, written informed consents were obtained from of each individual.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mazloomirad, F., Hasanzadeh, S., Sharifi, A. et al. Identification and detection of pathogenic bacteria from patients with hospital-acquired pneumonia in southwestern Iran; evaluation of biofilm production and molecular typing of bacterial isolates. BMC Pulm Med 21, 408 (2021). https://doi.org/10.1186/s12890-021-01773-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01773-3