Abstract
Background
While antifibrotic drugs significantly decrease lung function decline in idiopathic pulmonary fibrosis (IPF), there is still an unmet need to halt disease progression. Antioxidative therapy with N-acetylcysteine (NAC) is considered a potential additional therapy that can be combined with antifibrotics in some patients in clinical practice. However, data on the efficacy, tolerability, and safety of this combination are scarce. We performed a systematic review and meta-analysis to appraise the safety, tolerability, and efficacy of the combination compared to treatment with pirfenidone alone.
Methods
We systematically reviewed all the published studies with combined pirfenidone (PFD) and NAC (PFD + NAC) treatment in IPF patients. The primary outcomes referred to decline in pulmonary function tests (PFTs) and the rates of IPF patients with side effects.
Results
In the meta-analysis, 6 studies with 319 total IPF patients were included. The PFD + NAC group was comparable to the PFD alone group in terms of the predicted forced vital capacity (FVC%) and predicted diffusion capacity for carbon monoxide (DLco%) from treatment start to week 24. Side effects and treatment discontinuation rates were also comparable in both groups.
Conclusion
This systematic review and meta-analysis suggests that combination with NAC does not alter the efficacy, safety, or tolerability of PFD in comparison to PFD alone in IPF patients.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF), the most common fibrotic interstitial lung disease (ILD), is a chronic, progressive, and irreversible disease characterized by progressive extracellular matrix accumulation leading to respiratory insufficiency. The management strategies for IPF include relieving symptoms, maintaining patient quality of life and slowing disease progression. Apart from non-pharmacological treatments such as long-term oxygen therapy or rehabilitation, antifibrotics are the gold standard and should be started as soon as possible after the diagnosis of IPF [1].
Pirfenidone (PFD), an oral pyridine with antifibrotic, anti-inflammatory and antioxidant functions, is currently approved for the treatment of IPF in most countries and recommended by the latest guidelines [1, 2]. Evidence from the CAPACITY and ASCEND randomized controlled trials (RCTs) showed a significant reduction in the relative decline in forced vital capacity (FVC) over 72 weeks compared to the placebo group [3, 4]. Furthermore, the pooled analysis and meta-analysis suggested a lower relative risk of death in PFD-treated patients than in placebo-treated patients [5]. N-acetylcysteine (NAC), a tripeptide (g-glutamyl-cysteinyl glycine), can replenish glutathione storage levels, increase the antioxidant capacity and correct the imbalance of oxidants and antioxidants associated with fibroproliferation [6]. On the basis of the negative results of the PANTHER trial [7], NAC did not receive a positive recommendation as a treatment for IPF in the latest international guidelines [1, 7].
A substantial number of IPF patients receive combined PFD and NAC therapy [8,9,10]; however, data on the efficacy, safety, and tolerability of this combination are scarce. A recent placebo-controlled trial (PANORAMA) found that the rate of skin side effects was higher in the PFD + NAC group than in the PFD alone group [11]. However, other studies, including a study with inhaled NAC, suggest a slower lung function decline and a similar side effect profile in patients undergoing PFD + NAC treatment compared with patients undergoing PFD alone [8, 12,13,14].
Here, we systematically reviewed all studies with combined PFD and NAC treatment in IPF patients and performed a meta-analysis to compare the efficacy, safety, and tolerability of treatment with combined PFD and NAC vs treatment with PFD alone.
Method and materials
Literature search
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the PRISMA 2009-checklist. In addition, the meta-analysis was registered in PROSPERO (registration number: CRD42019134890).
A structured literature search was performed for studies on the safety and efficacy of combined PFD and NAC treatment in IPF patients. The following databases were searched from the earliest available dates to May 2019: PubMed, EMBASE, the Cochrane Library, Ovid, ProQuest, Web of Science and Chinese databases (including the China National Knowledge Infrastructure (CNKI), Chinese VIP Information (VIP), and the Wan Fang database). In addition, “clinicaltrials.gov” and the bibliographies of previous meta-analyses on PFD or NAC were checked for relevant studies. The search terms included “idiopathic pulmonary fibrosis”, “IPF”, and “pulmonary fibrosis” for the disease and “pirfenidone”, “Esbriet”, and “acetylcysteine” for the intervention. No language or research type restriction was adopted.
Study selection
The inclusion criteria for the meta-analysis were as follows: (1) IPF patients diagnosed according to the 2011 American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [15]; (2) interventions referring to combined PFD and NAC treatment, regardless of whether administration was oral or inhaled; and (3) the control group consisted of patients who received PFD alone. All appropriate studies were included in the meta-analysis.
Two reviewers (HYS and DWY) inspected all studies after removing duplicate studies by reviewing titles and abstracts. Relevant studies were assessed by viewing the full-text articles to select studies that met the inclusion criteria mentioned above. Disagreements were resolved by a consensus-based discussion.
To collect data on combined PFD + NAC therapy published in observational or retrospective studies involving patients with combined PFD, NAC, and corticosteroid/proton pump inhibitor treatment, we contacted the corresponding authors and obtained the original data regarding PFD + NAC therapy from some studies and excluded those patients receiving glucocorticoids other than PFD + NAC. Other studies with a questionable combined therapy group, incomplete data or an inappropriate control group were excluded [16, 17]. All patients included in the meta-analysis had not received glucocorticoids since the pirfenidone treatment began.
Data extraction and quality scoring
Two reviewers (HYS and XRL) extracted data from the included studies, including the following baseline characteristics: (1) first author, published year, study type, numbers of patients in the PFD + NAC group and PFD group; (2) changes in pulmonary function test (PFT) parameters such as changes in the predicted forced vital capacity (ΔFVC%) and changes in the predicted diffusion capacity for carbon monoxide (ΔDLco%); and (3) the number of side effects including skin reactions (photosensitivity and skin rash) and gastrointestinal reactions (anorexia, diarrhoea, and reduced appetite); the number of intolerable side effects leading to treatment discontinuation was also recorded.
The quality of the included observational studies was estimated using the Newcastle-Ottawa Quality Assessment Scale (NOS). Two reviewers (HYS and XRL) independently assessed the quality of the included studies in the following three domains: selection, comparability, and outcome. Each study score ranges from 0 to 9 stars in the NOS scoring system [18]. The randomized controlled studies were assessed with the Cochrane Collaboration risk of bias assessment tool [19].
Data analysis
The data extracted from the selected trials were used to generate forest plots in Stata SE 13.0 software (Stata Corp, College Station, TX, USA). The risk of patients experiencing side effects and other binary parameters are expressed as odds ratios (ORs) for both the included cohort and case-control studies. The changes in the PFT parameters and other continuous parameters are presented as standardized mean differences (SMDs) for different studies that adopted various PFT inclusion standards. We examined the level of heterogeneity to determine which type of analysis to use. If there was low heterogeneity (I2 less than 40%), then we used a fixed effects model. If the I2 statistic was greater than 40%, we applied a random effects model to summarize the data. Patients with the combination of PFD and inhaled NAC were only included in one case-control study [11], and the sensitivity analysis excluding the case-control study and the secondary analysis with only oral administration studies were completed in one step. Two-tailed p values less than 0.05 were considered significant.
Results
Study characteristics and quality scores
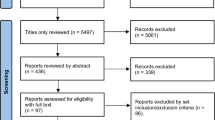
After the removal of duplicates and selection by viewing the abstracts and titles, a full-text review of 35 articles was performed. Six [8, 11,12,13,14, 20] and five [8, 12,13,14, 20] studies were included in the qualitative and quantitative analyses, respectively (Fig. 1). The systematic review comprised a total of 319 patients (PFD + NAC group n = 144, PFD alone group n = 175). The studies were conducted in Europe (n = 4), Japan (n = 1) and China (n = 2). One study was a controlled clinical trial (PANORAMA trial [11] by Behr et al), four were cohort studies [8, 12, 13, 20], and one was a case-control study [14]. Of note, one RCT was excluded because only the conference abstract was available [21]. A meta-analysis of observational real-world studies including 207 patients was performed. The general characteristics of the studies are shown in Table 1.
The average quality score for the included observational studies was 7.25 for cohort studies and 6 for the case-control study based on the NOS. The only RCT, which was conducted by Behr et al. [11], had high quality after being assessed according to the Cochrane Collaboration risk of bias assessment tool. The detailed quality characteristics are shown in the Table S1.
Effect of combined pirfenidone and acetylcysteine therapy on lung function parameters
The ΔFVC% predicted from baseline to week 24 was available in four studies with a total of 108 patients (PFD + NAC: n = 48, PFD alone: n = 60). Due to the lack of standard deviation values provided, the study by Sakamoto et al. [14] was excluded. Therefore, only three studies [8, 12, 20] were included in the meta-analysis. Given the premise of moderate heterogeneity (I2 = 62.5%, p = 0.069), the random effects model was applied for the analysis. The results showed that PFD + NAC therapy had no additional benefit in reducing the decrease in lung function (SMD = -0.09, 95% CI − 0.86-0.69, p = 0.295, Fig. 2a) compared to PFD alone.
Similarly, the ΔDLco% predicted from baseline to week 24 was available in 3 studies [8, 12, 20] with a total of 76 patients (PFD + NAC: n = 28, PFD alone: n = 48). These studies had low heterogeneity (I2 = 30.1%, p = 0.239). There was no difference in the ΔDLco% between the PFD + NAC group and the PFD group (SMD = 0.13, 95% CI -0.34-0.61, p = 0.580, Fig. 2b).
Safety and treatment tolerability of combined pirfenidone and acetylcysteine therapy
The number of patients who experienced at least one side effect was mentioned in five studies, including a total of 207 patients [8, 12,13,14, 20] (PFD + NAC: n = 84, PFD alone: n = 113). Moderate significant heterogeneity (I2 = 53.9%, p = 0.070) was detected, and a random effects model was applied. The results suggested that the rate of at least one side effect in the PFD + NAC therapy group was similar (PFD + NAC vs PFD alone: 41 vs 57, OR = 1.83, 95% CI 0.56–5.94, p = 0.314, Fig. 3a) to that in the PFD alone group. No significant differences were observed in the rates of specific side effects (PFD + NAC vs PFD alone: gastrointestinal (GI): 26 vs 47, I2 = 30.9%, p = 0.215, OR = 1.08, 95% CI 0.56–2.08, p = 0.811, Fig. 4a; skin side effects: 12 vs 17, I2 = 0%, p = 0.769, OR = 1.91, 95% CI 0.77–4.71, p = 0.162, Fig. 4b) between the treatment groups in the subgroup analysis.
Intolerable side effects leading to treatment discontinuation were reported in three studies with a total of 100 patients [8, 14, 20] (PFD + NAC n = 34, PFD alone n = 66). There was no significant heterogeneity (I2 = 0%, p = 0.762) observed among these studies. The results showed that combined PFD + NAC therapy did not increase the risk of intolerable side effects (OR = 2.85, 95% CI 0.84–9.59, p = 0.092, Fig. 3b) in comparison with PFD therapy. Patients receiving PFD + NAC therapy experienced intolerable side effects at a similar frequency as in those receiving PFD alone.
Qualitative analysis and sensitivity analysis
Funnel plots and Egger’s test could not be used to check for the existence of publication bias because our meta-analysis included fewer than 10 studies [19]. In addition, the secondary meta-analysis with only oral NAC studies and sensitivity analysis excluding the case-control study resulted in p values of 0.249, 0.611 and 0.955 for gastrointestinal, skin and intolerable side effects, respectively, and the forest plots composed of only oral NAC studies can be found in the Supplement Material (Figures S1, S2 and S3). In the ensuing quantitative analysis comparing the results of the meta-analysis and Behrs’ RCT (Table 2), the safety, tolerability, and efficacy outcomes [11] were similar in the PFD + NAC treatment group and the PFD monotherapy group except for a significantly higher rate of skin adverse effects in the RCT (p values in meta vs RCT: 0.097/0.038). Other parameters, such as the six-minute walk distance (6MWD) and progression-free survival (PFS), were available in only one study. The 6MWD results were comparable between the observational study (− 13.25 ± 6.77 vs − 16.59 ± 4.65, p = 0.159) [12] and Behr’s RCT (− 4.3 vs − 11.7, p = 0.54). In addition, PFD + NAC treatment showed favourable results regarding the PFS (median survival days 304 d vs 168 d; p = 0.016) in the case-control study [14].
Discussion
The present meta-analysis did not show superior efficacy of the combination PFD plus NAC therapy in slowing lung functional decline in IPF and showed comparable safety and tolerability compared to PFD alone.
The antifibrotic drug pirfenidone can significantly reduce lung functional decline in IPF patients; therefore, it is recommended in international guidelines as the treatment of choice [1]. However, patients still present with gradually worsening symptoms and a constant loss of quality of life [22], and the outcome is comparable to those of many malignant diseases [23]. There is still an unmet need to halt disease progression. Antioxidative therapy with NAC is discussed as a potential additional therapy in some patients in clinical practice.
The randomized placebo-controlled trial IFIGENIA investigated NAC treatment vs the standard treatment with prednisone plus azathioprine in 182 mild to moderate IPF patients over 48 weeks [24]. Combined therapy with high-dose NAC (1800 mg, d), prednisone and azathioprine significantly preserved the absolute vital capacity (VC) and DLco compared to the combination of prednisone and azathioprine [24]. However, the results of the PANTHER-IPF trial [25], which also enrolled patients with mild to moderate IPF, showed that there was no significant difference in the decline in FVC and showed a higher rate of serious adverse effects [25] and especially a higher mortality rate in patients receiving triple therapy than in patients receiving the placebo. While another report of the PANTHER also demonstrated no benefit of NAC over the placebo [7], a post hoc analysis of the PANTHER study [26] suggested that the genotypic background of IPF patients may have an impact on the effects of NAC treatment. MUC5B and TOLLIP SNPs were retrospectively investigated in a subgroup of patients in the PANTHER trial. Patients with a rs3750920 (TOLLIP) TT genotype (25% of all patients) showed favourable outcomes regarding a reduction in the risk of the composite endpoint, defined as death, transplant, hospitalization or ≥ 10% FVC decline, while patients with a CC genotype had a non-significant increase in the composite physiological index (CPI) [26].
Regarding lung function decline (especially FVC), our meta-analysis demonstrated comparable outcomes between the PFD + NAC group and PFD monotherapy group. Considering that the majority of studies included in this meta-analysis enrolled Caucasian patients with mild to moderate IPF (predicted FVC from 50 to 90%), the heterogeneity among these studies may be related to ethnicity because the studies by Ma and Sakamoto [12, 14], which showed favourable efficacy results for the combination treatment, enrolled Asian patients. In addition, a speculative explanation for this phenomenon could be that the proportion of patients with the TOLLIP TT genotype in the treatment groups differed among the studies, but the data were not available [7, 26]. Furthermore, direct antioxidant and anti-inflammatory effects on the alveoli by inhaled instead of oral NAC treatment may also contribute to the favourable outcomes in Sakamoto’s study [14].
There are some considerations regarding the safety and tolerability of PFD and NAC treatment in IPF patients. Gastrointestinal (diarrhoea, anorexia, etc.) and skin side effects (photosensitivity and skin rash) are the most common adverse effects experienced by IPF patients receiving PFD treatment [27, 28]. Compared to the findings from the PANORAMA trial, our meta-analysis showed a similar rate of side effects except for skin side effects (lower rate). The exact reason for this difference is unclear but may be related to differences in the patients’ habits, such as the time spent outdoors or the use of skin protection creams [11].
Our meta-analysis has several limitations. First is the small number of included studies. Second, the meta-analysis included only one RCT, and the rest of the studies were observational studies and real-world experiences. Third, the lung function decline assessment was partial because scarce data were available for the 6MWD and blood gas analysis; therefore, we cannot exclude improvements in other outcome measures due to treatment with combined PFD + NAC. Fourth, the random effects model, which is generally used to analyse the overall effect when moderate heterogeneity exists (I2 > 40%), was applied for the analysis of patients experiencing at least one side effect and to assess differences in the FVC% decline between groups, leading to a wider confidence interval and a more conservative conclusion.
Conclusions
In conclusion, this systematic review and meta-analysis suggests that the combination of PFD and NAC does not alter the efficacy, safety, or tolerability of PFD in comparison to PFD alone in the IPF study population. High-quality, multi-centre RCTs and large-sample real-world observational studies evaluating the safety, tolerability, and efficacy of PFD + NAC therapy vs PFD monotherapy and investigating the genetic background of patients are needed to validate these results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- ILD:
-
Interstitial lung disease
- NAC:
-
N-acetylcysteine
- PFD:
-
Pirfenidone
- PFT:
-
Pulmonary function test
- FVC:
-
Forced vital capacity
- ΔFVC%:
-
Changes in predicted forced vital capacity
- DLco:
-
Diffusion capacity for carbon monoxide
- ΔDLco%:
-
Changes in the predicted diffusion capacity for carbon monoxide
- RCT:
-
Randomized controlled trials
- SNP:
-
Single nucleotide polymorphism
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International prospective register of systematic reviews
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- ATS/ERS:
-
American Thoracic Society/European Respiratory Society
- ORs:
-
Odds ratios
- SMDs:
-
Standardized mean differences
- 6MWD:
-
Six min walk distance
- PFS:
-
Progression-free survival
References
Raghu G, Rochwerg B, Zhang Y, Garcia CAC, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19.
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20(120):85–97.
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Lancaster L, Sahn SA, Szwarcberg J, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet (London, England). 2011;377:1760–9.
King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92.
Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, Glassberg MK, Kardatzke DR, Daigl M, Kirchgaessler KU, Lancaster LH, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41.
Muramatsu Y, Sugino K, Ishida F, Tatebe J, Morita T, Homma S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir Investig. 2016;54(3):170–8.
Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–101.
Bonella F, Wessendorf T, Costabel U. Clinical experience with pirfenidone for the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2013;42:P3808.
Jo HE, Glaspole I, Grainge C, Goh N, Hopkins PM, Moodley Y, Reynolds PN, Chapman S, Walters EH, Zappala C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J. 2017;49(2):1601592.
Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, Wirtz H, Koschel D, Andreas S, Claussen M, et al. Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Respir Res. 2017;18(1):139.
Behr J, Bendstrup E, Crestani B, Günther A, Olschewski H, Sköld CM, Wells A, Wuyts W, Koschel D, Kreuter M, et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2016;4(6):445–53.
Ma L. Effect of pirfenidone combined with acetylcysteine on idiopathic pulmonary fibrosis [M]: Hebei Medical University; 2018. [In Chinese].
Mao ZX. Clinical observation of pirfenidone combined with acetylcysteine in the treatment of idiopathic pulmonary interstitial fibrosis Drugs & Clinic. 2018;8(33). [In Chinese].
Sakamoto S, Muramatsu Y, Satoh K, Ishida F, Kikuchi N, Sano G, Sugino K, Isobe K, Takai Y, Homma S. Effectiveness of combined therapy with pirfenidone and inhaled N-acetylcysteine for advanced idiopathic pulmonary fibrosis: a case-control study. Respirology (Carlton, Vic). 2015;20(3):445–52.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Huang H, Dai HP, Kang J, Chen BY, Sun TY, Xu ZJ. Double-Blind Randomized Trial of Pirfenidone in Chinese Idiopathic Pulmonary Fibrosis Patients. Medicine (Baltimore). 2015;94(42):e1600.
Okuda R, Matsushima H, Oba T, Kawabe R, Matsubayashi M, Amano M, Nishizawa T, Honda K. Efficacy and safety of inhaled N-acetylcysteine in idiopathic pulmonary fibrosis: a prospective, single-arm study. Respir Investig. 2016;54(3):156–61.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Shuster JJ: Review: Cochrane handbook for systematic reviews for interventions, version 5.1.0, published 3/2011. Julian P.T. Higgins and Sally Green, Editors. 2011, 2(2):126–130.
Oltmanns U, Kahn N, Palmowski K, Trager A, Wenz H, Heussel CP, Schnabel PA, Puderbach M, Wiebel M, Ehlers-Tenenbaum S, et al. Pirfenidone in idiopathic pulmonary fibrosis: real-life experience from a German tertiary referral center for interstitial lung diseases. Respiration. 2014;88(3):199–207.
Homma S, Kondoh Y, Kato M, Hoshino T, Mukae H, Bando M, Suda T, Kido T, Tanino Y, Kishaba T, et al. Efficacy of combined therapy with pirfenidone and inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomized controlled phase 3 trial. Eur Respir J. 2018;52:PA4795.
Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, Wirtz H, Koschel D, Andreas S, Claussen M, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry. Respir Res. 2019;20(1):59.
Vancheri C. Idiopathic pulmonary fibrosis and cancer: do they really look similar? BMC Med. 2015;13:220.
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–42.
Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al. TOLLIP, MUC5B, and the response to N-Acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(12):1475–82.
Cottin V, Koschel D, Gunther A, Albera C, Azuma A, Skold CM, Tomassetti S, Hormel P, Stauffer JL, Strombom I, et al. Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study. ERJ open research. 2018;4(4):00084–2018.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074.
Acknowledgements
All authors would like to thank to Prof. Susumu Sakamoto, Department of Respiratory Medicine, Toho University Omori Medical Center, Japan.
Funding
This study was supported by Natural Science Foundation of Tianjin Province of China (No. 15JCZDJC35000) in the process of the research design, analysis and interpretation of data, and in the decision to submit the manuscript to the journal.
Author information
Authors and Affiliations
Contributions
HYS and LQW devised and wrote the protocol and conduct the study. DWY and XRL inspected the database, review titles and abstracts. HYS and DWY contributed to data extraction, quality assessment and writing of manuscript. HYS and UO contributed to the collection of the original data. FB, MK and SCP provided some unpublished data and guidance for the analysis and interpretation of the results. In addition, LQW, FB and MK gave some critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Quality scores of observational studies in the meta-analysis based on NOS scoring system. Figure S1. Forest plot of efficacy profile (outcomes: the predicted decline in FVC% (Figure S1-a) and DLco% (Figure S1-b)) between the combined pirfenidone and acetylcysteine group and the pirfenidone alone group with only oral NAC studies. Abbreviations: FVC: forced vital capacity, PFD: pirfenidone, NAC: N-acetylcysteine. Figure S2. Forest plot of the safety profile (outcome measure: at least one side effect, Figure S2-a) and tolerability profile (outcome measure: intolerable side effects leading to treatment discontinuation, Figure S2-b) between the combined pirfenidone and acetylcysteine group and the pirfenidone alone group with only oral NAC studies. Abbreviations: PFD: pirfenidone, NAC: N-acetylcysteine. Figure S3. Forest plot of the specific safety profile (outcome measure: gastrointestinal side effects (Figure S3-a) and skin side effects (Figure S3-b)) between the combined pirfenidone and acetylcysteine group and the pirfenidone alone group with only oral NAC studies. Abbreviations: PFD: pirfenidone, NAC: N-acetylcysteine.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, H., Yin, D., Bonella, F. et al. Efficacy, safety, and tolerability of combined pirfenidone and N-acetylcysteine therapy: a systematic review and meta-analysis. BMC Pulm Med 20, 128 (2020). https://doi.org/10.1186/s12890-020-1121-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-020-1121-2