Abstract
Background
The ability of interleukins (ILs) to differentiate tuberculous pleural effusion from other types of effusion is controversial. The aim of our study was to summarize the evidence for its use of ruling out or in tuberculous pleural effusion.
Methods
Two investigators independently searched PubMed, EMBASE, Web of Knowledge, CNKI, WANFANG, and WEIPU databases to identify studies assessing diagnostic role of ILs for tuberculous pleural effusion published up to January, 2017. Study quality was assessed using Quality Assessment of Diagnostic Accuracy Studies-2. The pooled diagnostic sensitivity and specificity of ILs were calculated by using Review Manager 5.3. Area under the summary receiver operating characteristic curve (AUC) was used to summarize the overall diagnostic performance of individual markers.
Results
Thirty-eight studies met our inclusion criteria. Pooled sensitivity, specificity and AUC for chosen ILs were as follows: IL-2, 0.67,0.76 and 0.86; IL-6, 0.86, 0.84 and 0.90; IL-12, 0.78, 0.83 and 0.86; IL-12p40, 0.82,0.65 and 0.76; IL-18, 0.87, 0.92 and 0.95; IL-27, 0.93, 0.95 and 0.95; and IL-33, 0.84, 0.80 and 0.88.
Conclusions
Some of these ILs may assist in diagnosing tuberculous pleural effusion, though no single IL is likely to show adequate sensitivity or specificity on its own. Further studies on a large scale with better study design should be performed to assess the diagnostic potential of ILs.
Similar content being viewed by others
Background
Tuberculosis remains a leading cause of morbidity and mortality, especially in Asia and Africa with high tuberculosis burden. In China, the prevalence of active pulmonary tuberculosis in 2010 among those older than 15 years was 459/100,000, and the prevalence of smear-positive pulmonary tuberculosis was 66/100,000. [1] Up to 30% of patients with tuberculosis have tuberculous pleural effusion (TPE), in which extrapulmonary involvement causes pleural effusions [2]. Properly treating pleural effusions requires determining whether the effusions are TPEs or another type of effusion.
The gold standard for diagnosing TPE is the isolation of Mycobacterium tuberculosis (M. tuberculosis) from samples of either pleural effusion or pleural biopsy. This culturing offers 100% diagnostic specificity, but it usually takes several weeks, delaying diagnosis and increasing the risk that patients are lost to follow-up. In addition, pleural biopsy is invasive and technically difficult to some extent, particularly in children, such that success can depend strongly on the individual performing the biopsy. [3] Detecting granulomas in pleural biopsies can diagnose TPE with approximately 95% specificity, [2,3,4] but the sensitivity of culture- or granuloma-based methods is limited. [5] Although image-guided biopsies and local anesthetic thoracoscopic (LAT) biopsies can highly evaluated the sensitivity compared to blind pleural biopsy, both those techniques are not recommended as the first procedure for patients presenting with pleural effusions. Thus, this highlights the need for alternative less invasive diagnostic strategies.
TPE is largely the result of pathological immune reactions associated with an increase in cytokines, including interleukins (ILs). [6] ILs are secreted proteins that bind to specific receptors and help mediate communication among leukocytes. For example, IL-12 is essential for initially activating inrerferon(INF)-γ-mediated T cell responses to primary M. tuberculosis infection. [7, 8] ILs can promote various types of inflammatory responses, playing a role in activation-induced death of skin keratinocytes, mucosal epithelial cells, and T cells. [9] Evidence that pleural levels of some ILs are elevated in patients with TPE has led investigators to explore their potential for differentiating TPE from other types of pleural effusion. Most studies have looked at only one or a few ILs, and some studies looking at the same ILs have arrived at different conclusions. This led us to systematically review the literature and meta-analyze available data to gain a more comprehensive understanding of the potential of ILs for diagnosing TPE.
Methods
Search strategy and study selection
The systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. [10] Two investigators independently searched PubMed, EMBASE, Web of Knowledge, CNKI, WANFANG, and WEIPU databases to identify studies assessing the role of ILs in diagnosing TPE published up to January, 2017. Before the full search, we performed a preliminary search to decide on the ILs to include in the review. The following search terms were used: “interleukins or IL” and “IL-2 or IL-6 or IL-12 or IL-12p40 or IL-18 or IL-27 or IL-33” and “tuberculosis” and “pleural effusion/pleural fluid” and “sensitivity or specificity or accuracy”. Reference lists in retrieved studies and review articles were examined manually to identify additional studies.
Two authors (NZ and CW) independently assessed each study for eligibility; disagreements were resolved by consensus. Studies were included if they fulfilled all the following criteria: (1) the work was an original research article published in English or Chinese, (2) human samples were analyzed, (3) standard methods were used to definitively diagnose the type of effusion as TPE or other type, and (4) data sufficient for calculating specificity and sensitivity were reported. Conference proceedings, letters to the editor, and studies including fewer than 10 patients with TPE were excluded.
Quality assessment and data extraction
The same two authors (NZ and CW) assessed the quality of included studies using the Quality Assessment of Diagnostic Accuracy Studies-2 tool (QUADAS-2). [11] For each criterion, a response of “yes” was assigned if it was fulfilled; “unclear”, if doubt existed whether it was fulfilled; or “no” if it was not fulfilled. The following data were retrieved from each study: authors, country, publication year, population characteristics, testing methods, cut-off value, methodological quality, and 2-by-2 tables showing rates of true positives (TPs), true negatives (TNs), false positives (FPs) and false negative (FNs).
Statistical analysis
Data were compiled in Excel, then transferred to Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and STATA Version 12.0 (Stata Corp., College Station, TX) for statistical analysis. For each study, sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated, together with 95% confidence intervals (CIs). A summary ROC (SROC) curve was generated for each IL in each study, [12] from which a single test threshold value was determined and used to calculate sensitivity and specificity. [13] Overall diagnostic performance for that IL was assessed as the area under the SROC curve (AUC).
The Q test and inconsistency index (I2) were used to detect potential heterogeneity in the natural logarithm of DOR (lnDOR) meta-analyzed across studies. [14] Presence of implicit cut-off point effects and correlation between sensitivity and specificity were assessed for each IL by calculating the Spearman rank correlation coefficient for each IL. Deeks’ funnel plot and Egger’s test were used to detect publication bias [15]. All statistical tests were two-sided, with P < 0.05 taken as the threshold of significance.
Results
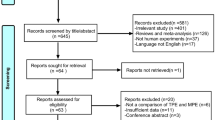
Our systematic review included 38 studies examining the ability of pleural concentrations of IL-2, IL-6, IL-12, IL-12p40, IL-18, IL-27, and IL-33 to diagnose TPE. [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] Other ILs in the Table 1 were excluded for meta-analysis because relevant data were available from fewer than 3 studies [54,55,56,57,58] (Fig. 1). Two authors (NZ and CW) assessed studies for possible overlap in the populations analyzed. Data were pooled from overlapping populations as long as the different studies reported on different ILs or IL combinations. Otherwise, if studies with overlapping populations reported on the same IL or IL combination, only the data from the largest study were used.
Study characteristics
Table 1 summarizes clinical characteristics of patients in the 38 studies that used for quantitative meta-analysis [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Average sample size was 98 (range, 43 to 431) for each IL (Table 2). 23 studies stated that the pleural effusion samples were collected before any drug treatment [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], while the rest 15 studies didn’t report such information [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Diagnosis of TPE or other type of pleural effusion was based only on clinical course in 5 studies, [22, 23, 28, 35, 41] i.e. on clinical presentation, pleural fluid analysis, radiology and responsiveness to anti-tuberculosis chemotherapy. Diagnosis was based on bacteriology, histology or both (gold standard) in 11 studies. In the remaining 21 studies, some patients were diagnosed with TPE based on clinical course and others based on the gold standard. One study [51] did not report the diagnostic standard for TPE. All but 3 studies [35, 37, 52] measured IL levels using enzyme-linked immunosorbent assays (ELISA), with the remaining 3 studies using radioimmunoassays.
Determination of statistical pooling model
Diagnostic studies are typically meta-analyzed using an SROC-based fixed-effects model, [59] a random-effects model using a bivariate normal approximation, [60] or a hierarchical SROC (HSROC)-based full Bayesian [61] or empirical Bayes method [62]. In our study, lnDOR heterogeneity was statistically significant and associated with high I2 values for most ILs (Table 3). These indications of substantial heterogeneity in lnDOR made the use of a SROC-based fixed-effects model inappropriate [63].
The possible presence of implicit cut-off point effects was examined for each included IL, using the Spearman rank correlation between sensitivity and specificity (Table 3). A negative correlation was found for most ILs, indicating no detectable implicit cut-point effect. Therefore, we used a random-effects model to estimate the mean sensitivity and specificity and associated CIs.
Diagnostic accuracy
These data were meta-analyzed using a random-effects model (Table 3). Fig. 2 summarizes the sensitivities and specificities for IL-27 and IL-18 reported by each study. (Results for the other ILs are reported in Additional file 1: Figure S1.) Sensitivity of IL-27 ranged from 0.80 to 1.00, and the pooled value was 0.93 (95%CI 0.90–0.95). Sensitivity of IL-18 ranged from 0.44 to 0.97, and the pooled value was 0.87 (95%CI 0.79–0.92). Specificity of IL-27 varied from 0.85 to 0.99, and the pooled value was 0.95 (95%CI 0.90–0.98). Specificity of IL-18 varied from 0.82 to 1.00, and the pooled value was 0.92 (95% CI 0.88–0.95). The pooled parameters for all included ILs are shown in Table 4.
Unlike a traditional ROC plot, each data point on an SROC curve represents a separate study, allowing the curve to provide an overall assessment of diagnostic performance. Plotting the rate of TP against the rate of FP gave curves showing AUCs of 0.95 for IL-18 and IL-27 (Fig. 3). Among all ILs, IL-27 showed the highest overall accuracy, with a sensitivity of 93% and specificity of 95%.
Study quality and publication bias
QUADAS-2 assessment of included studies showed that most studies had low risk of bias (Fig. 4). Both Egger’s and Deeks’ tests suggest no evidence of bias among the studies for any ILs meta-analyzed (Table 3). Funnel plots indicate low risk of publication bias (Additional file 1: Figure S2).
Summary of QUADAS-2 assessments of included studies. QUADAS-2: Quality Assessment of Diagnostic Accuracy Studies-2. Patient Selection: Describe methods of patient selection; Index Text: Describe the index test and how it was conducted and interpreted; Reference Standard: Describe the reference standard and how it was conducted and interpreted; Flow and Timing: Describe any patients who did not receive the index tests or reference standard or who were excluded from the 2 × 2 table, and describe the interval and any interventions between index tests and the reference standard
Discussion
Assaying pleural levels of ILs may be a cost-effective and minimally invasive alternative to traditional tests for differentiating TPE from other types of pleural effusion. Our meta-analysis of the available evidence suggests that IL-27 and IL-18 show relatively high diagnostic accuracy for TPE, while five other well-studied ILs do not (IL-2, IL-12, IL-27, IL-33 and IL-12p40). Even IL-27 and IL-18 do not appear to have adequate diagnostic potential on their own, so they would need to be used in conjunction with other methods or conventional markers.
Our meta-analysis showed that IL-2, despite being centrally involved in the regulation of immune tolerance and activation, [64] is associated with quite low sensitivity and specificity. This may reflect the fact that IL-2 data were available from only 5 studies, all of which were conducted in China. Future work, preferably in Caucasians and other groups of Asians, should investigate the diagnostic potential of this IL.
DOR combines sensitivity and specificity into a single indicator of test performance. [65] Higher DOR indicates better discriminatory test performance. Mean DOR was 64.12 for IL-18 and 227.9 for IL-27, indicating high overall accuracy. Potentially more clinically meaningful than DOR are likelihood ratios. [66] A likelihood ratio > 10 or <0.1 suggests a 10-fold difference between the pre- and post-test probability that a condition is present. Of the ILs meta-analyzed here, only IL-18 and IL-27 had PLRs >10, suggesting that a positive test result for these ILs indicates a relatively high probability of TPE. In addition, IL-27 was associated with an NLR of 0.08, indicating an 8% probability that a negative IL-27 test result is a false negative for TPE. This may be sufficient for ruling out TPE in the clinic.
Pleural levels of a number of biomarkers have been proposed as aids in the diagnosis of TPE, including adenosine deaminase (ADA) and interferon-γ(INF-γ), both of which are present in patients with TPE at significantly higher concentrations than in patients with other types of pleural effusion. The diagnostic performance determined here for IL-18 and IL-27 compares favorably with that of ADA and INF-γ. Meta-analyses [67, 68] indicate that these latter two assays on their own are associated with the following diagnostic indices: sensitivity, 0.89 (95%CI 0.87–0.91) and 0.92 (95%CI 0.90–0.93); specificity, 0.97 (95%CI 0.96–0.98) and 0.90 (95%CI 0.89–0.91); PLR, 23.45 (95%CI 17.31–31.78) and 9.03 (95%CI 7.19–11.35); NLR, 0.11 (95%CI 0.07–0.16) and 0.10 (95%CI 0.07–0.14); and DOR, 272.7 (147.5–504.2) and 110.08 (95%CI 69.96–173.20). Although the available evidence suggests that IL-18 and IL-27 seem to have higher accuracy than ADA, the higher-cost and more complicated determination of IL-27 and IL-18 may limit their practical applicability. [69, 70] In addition, it has been reported that the combination of positive IL-27 with positive ADA values [16, 31, 46], can reach a sensitivity of 100% for the identification of TBP, Our meta-analysis, combined with previous ones, suggests that combining IL-18 and IL-27 with INF-γ and ADA may strengthen TPE diagnosis. We also suggest further studies should be carried out to determine the diagnostic accuracy of IL-27 and IL-18 combination or their combination with ADA or INF-γ.
Our meta-analysis suggests an association between elevated levels of at least certain pleural ILs and TPE. TPE has been characterized as a hypersensitive T cell reaction to mycobacteria or antigens in the pleural space, leading to the accumulation of protein-rich fluid. [6] ILs are divided into different families based on sequence homology, receptor chains or functional properties. IL-18 and IL-33 belong to the IL-1 family, [71] which contains inflammatory mediators playing a major role in early innate immune responses. IL-6, which belongs to a cytokine family of the same name, is a multifunctional, pleiotropic regulator of immune responses, acute-phase responses, hematopoiesis, and inflammation. [72] IL-2, a member of the γ-chain cytokine family, is produced mainly by CD4+ and CD8+ T cells and is essential for Treg cell development. [73] Although both blood and pleural fluid samples can be processed for all ILs, these assays are limited by their inability to differentiate drug resistant TB, consequently, cannot replace appropriate microbiological and molecular investigations. Future work is needed to examine how ILs may affect onset and/or progression of TPE and the probable association between ILs and drug sensitivity of TB.
To ensure reliable results, we meta-analyzed only ILs for which sensitivity and specificity data were available from at least 3 studies. As a result, we did not analyze several ILs for which levels appear to be elevated in tuberculosis [74], including IL-8 [57] and IL-22 [58]. Further work should examine the diagnostic potential of these ILs. In addition, more work should also examine the diagnostic performance of these and other ILs in combination, which we could not do for lack of studies including such combinations.
Our meta-analysis has additional limitations. First, exclusion of conference abstracts, letters to journal editors and unpublished data may have given rise to publication bias, such that our results overestimate actual diagnostic performance. Second, patients were diagnosed with TPE based on both bacteriological and histological assessment in only a few studies; in most studies, patients were diagnosed on the basis of one or the other, alone or in combination with clinical course, and they were diagnosed based solely on clinical course in a few studies. This increases risk of misclassification bias. Third, description of methodology was incomplete in many studies, leading to a QUADAS-2 assessment of “unclear”. In addition, we did not perform meta-regression analysis to determine the source of heterogeneity, because of the limited numbers of the studies included. Our results highlight the need for more rigorous studies of ILs in the diagnosis of TPE. Future work should also examine the diagnostic potential of IL levels in serum, since most studies have focused on pleural levels.
Conclusion
The available evidence suggests that assaying pleural levels of certain ILs may aid in the diagnosis of TPE when used in combination with other biomarkers and approaches. By confirming such diagnosis, ILs may help avoid the need for more invasive diagnostic procedures.
Abbreviations
- ADA:
-
Adenosine deaminase
INF-γ
Interferon-γ
- AUC:
-
The area under the SROC curve
- DOR:
-
Diagnostic odds ratio
- FN:
-
False negative
- FP:
-
False positive
- IL:
-
Interleukins
- NLR:
-
Negative likelihood ratio
- PLR:
-
Positive likelihood ratio
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- SROC:
-
Summary ROC
- TN:
-
True negative
- TP:
-
True positive
- TPE:
-
Tuberculous pleural effusion
References
Technical guidance group of the fifth national TB epidemiological survey. The fifth national tuberculosis epidemiological survey in 2010 [in Chinese]. Chin J Antituberc. 2012;34(08):485–508.
Qiu L, Teeter LD, Liu Z, Ma X, Musser JM, Ea G. Diagnostic associations between pleural and pulmonary tuberculosis. J Inf Secur. 2006;53(6):377–86.
Trajman A, Pai M, Dheda K, Smit R, Zyl V, Zwerling AA, Joshi R, Kalantri S, Daley P, Menzies D. Novel tests for diagnosing tuberculous pleural effusion: what works and what does not? Eur Respir J. 2008;31(5):1098–106.
Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15(3):451–8.
Mcgrath EE, Anderson PB. Diagnostic tests for tuberculous pleural effusion. Eur J Clin Microbiol Infect Dis. 2010;29(10):1187–93.
Sharma SK, Mitra DK, Balamurugan A, Pandey RM, Mehra NK. Cytokine polarization in Miliary and pleural tuberculosis. J Clin Immunol. 2002;22(6):345–52.
Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27(1):393–422.
Reddy VM, Andersen BR. Immunology of tuberculosis. Annu Rev Immunol. 2001;19(4):70–4.
Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, Meyer N, Akdis CA, Schmidt-Weber CB. Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol. 2009;123(3):1–7.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, Group Q. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12(14):1293–316.
Lau J, Ioannidis JPA, Balk EM, Milch C, Terrin N, Chew PW, Salem D. Diagnosing acute cardiac ischemia in the emergency department: a systematic review of the accuracy and clinical effect of current technologies. Ann Emerg Med. 2001;37(5):453–60.
Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–45.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Wu YB, Ye ZJ, Qin SM, Wu C, Chen YQ, Shi HZ. Combined detections of interleukin27, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J. 2013;126(17):3215–21.
Chen L, Jiang H, Lin C. Detections of interferon-γ, IL18, IL12 and sIL2R for diagnosis of tuberculosis pleural effusion and malignant pleural effusions [in Chinese]. Chin J Clinicians (Electrionc Edition). 2011;05(12):3602–4.
Dai B, Xu X. Diagnostic value of interleukin-18 on tuberculous and malignant pleural effusion [in Chinese]. J Clin Pulm Med. 2015;20(2):228–30.
Ding YM. Combined detections of interleukin 18, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy and malignant pleurisy [in Chinese]. China: Zhejiang University; 2008.
Gu XY, Li DY, Wang SY, Zhao WG, Li RY, Li XH. Diagnostic significance of Interleukin-12 in tuberculous pleurisy [in Chinese]. Chin J Antitubercul. 2002;24(4):240.
Hu J, Wang Y, Chen J, Yang M, Jiang L. Significance of interleukin-18 (IL-18) and vascular endothelial growth factor (VEGF) in differentiating tuberculous pleural effusion from malignant pleural effusions (in Chinese). Clinical Misdiagnosis&Mistherapy. 2009;22(3):1–3.
Jiang ZD, Wang WR, Liu X. Diagnostic significance of interleukin-18 in tuberculous and malignant pleurisy [in Chinese]. Chin Prac Med. 2009;4(35):83–4.
Jiang ZD, Wang WR, Liu X. Diagnostic value of interleukin-12 in tuberculous pleurisy [in Chinese]. Chin Prac Med. 2010;5(1):50–1.
Kiropoulos TS, Kostikas K, Oikonomidi S, Tsilioni I, Nikoulis D, Germenis A, Gourgoulianis KI. Acute phase markers for the differentiation of infectious and malignant pleural effusions. Respir Med. 2007;101(5):910–8.
Ren ZQ, Xing WC, Sun Y, Liu F, Lin CZ. The differential diagnostic value of interferon-γ, interleukin-2, tumor necrosis factor α and adenosine deaminase in tuberculous and malignant pleural effusion. Prog Modern Biomed. 2014;14(23):4471–4.
Lee KS, Kim HR, Kwak S, Choi KH, Cho JH, Lee YJ, Lee MK, Lee JH, Park SD, Park DS. Association between elevated pleural interleukin-33 levels and tuberculous pleurisy. Ann Lab Med. 2013;33(1):45–51.
Luo JH, Wu N. Diagnostic value of IL-27, ADA and INF-γ in Tuberculous pleural effusion and malignant pleural effusion [in Chinese]. Hebei Med J. 2015;37(4):512–4.
Tian RX. Clinical investigation of the diagnostic value of interferon-γ, interlukin-12 and adenosine deaminase isoenzyme in tuberculous pleurisy. Chin J Tubere Respir Dis. 2004;27(7):435–8.
Sun M, Yan D, Jiang S, Gu X, Ma W. Diagnostic value of interleukin-27 in tuberculous pleural effusion [in Chinese]. Natl Med J China. 2014;94(34):2641–4.
Valdes L, San-Jose ED, Jm AD, Golpe A, Valle J, Penela P, Gonzalez-Barcala F. Diagnostic value of interleukin-12 p40 in tuberculous pleural effusions. Eur Respir J. 2009;33(33):816–20.
Valdés L, San JE, Ferreiro L, Golpe A, Gude F, Á-D JM, Pereyra MF, Toubes ME, González-Barcala FJ. Interleukin 27 could be useful in the diagnosis of tuberculous pleural effusions. Respir Care. 2014;59(3):399–405.
Wang A. Diagnostic significance of adenosine deaminase activity, interferon-γ andiInterleukin-6 in tuberculous pleural effusions [in Chinese]. J Hubei Med Univ. 2005;26(1):117–20.
Wu XM. Level of interlukin 18 and its significance in tuberculous pleural effusion and malignant pleural effusion [in Chinese]. Chin J Mod Med. 2006;16(15):2356–60.
Xiong DS, Xu GP, Xiong WN, Gao YP, Ni W, Chen SX. Combined detections of interleukin 18 and adenosine deaminase for diagnosis of tuberculous pleural effusion and malignant pleural effusion [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(10):779–80.
Zan H, Xie W, Tao S. Diagnostic significance of adenosine deaminase activity and interleukin-6 in tuberculous pleural effusions [in Chinese]. Sichuan Med J. 2014;35(5):588–91.
Xuan WX, Zhang JC, Zhou Q, Yang WB, Li-Jun MA. IL-33 levels differentiate tuberculous pleurisy from malignant pleural effusions. Oncol Lett. 2014;8(1):449–53.
Yang C. Level of IL-6, IL-8 and its significance in tuberculous pleural effusion and malignant pleural effusion [in Chinese]. China: Dalian Medical University; 2006.
Niu CM. Interlukin-27 in tuberculous and malignant pleural effusion [in Chinese]. China: Huazhong University of Science and Technology; 2012.
Klimiuk J, Krenke R, Safianowska A, Korczynski P, Chazan R. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol. 2014;852:21–30.
Li D, Shen Y, Fu X, Li M, Wang T, Wen F. Combined detections of interleukin-33 and adenosine deaminase for diagnosis of tuberculous pleural effusion. Int J Clin Exp Pathol. 2015;8(1):888–93.
Fernández dLC, Duplat A, Giampietro F, de Waard JH, Luna J, Singh M, Araujo Z. Diagnostic accuracy of immunological methods in patients with tuberculous pleural effusion from Venezuela. Investig Clin. 2011;52(1):23–34.
Liu J, Zhang L, Feng S, Zhang L, Sun H, Liu G, Xiao H, Wu M, Du Y, Liu S. Evaluating the value of detecting cytokines for diagnosis of tuberculous pleural effusion by liquid array technology [in Chinese]. Chin J Lab Med. 2015;38(8):562–6.
Liu D, Xu XL. Diagnostic value of CD1c, IL-2, IL-18 in tuberculous pleural effusion and malignant pleural effusion [in Chinese]. World Latest Medicine Information. 2015;15(54):159–60.
Okamoto M, Kawabe T, Iwasaki Y, Hara T, Hashimoto N, Imaizumi K, Hasegawa Y, Shimokata K. Evaluation of interferon-γ, interferon-γ-inducing cytokines, and interferon-γ–inducible chemokines in tuberculous pleural effusions. J Lab Clin Med. 2005;145(2):88–93.
Önür ST, Sökücü SN, Dalar L, Seyh EC, Akbaş A, Alti S. Are soluble IL-2 receptor and IL-12p40 levels useful markers for diagnosis of tuberculous pleurisy? Infect Dis (Lond). 2015;47(3):1–6.
Skouras VS, Magkouta SF, Psallidas I, Tsilioni I, Maragozidis P, Gourgoulianis KI, Kalomenidis I. Interleukin-27 improves the ability of adenosine deaminase to rule out tuberculous pleural effusion regardless of pleural tuberculosis prevalence. Infect Dis (Lond). 2015;47(7):1–7.
Wang XY, Han L, Yang ZH. Combined detections of interleukin 18 and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleural effusion and malignant pleural effusion [in Chinese]. Chin J Gerontol. 2008;28(21):2125–7.
Wong C, Yew W, Leung S, Chan C. Assay of pleural fluid interleukin-6, tumour necrosis factor-alpha and interferon-gamma in the diagnosis and outcome correlation of tuberculous effusion. Respir Med. 2003;97(12):1289–95.
Wu J, Li Y, Jiang S. Diagnostic value of combined determination of serum and chest fluid adenosine deaminase(ADA), IL-2, IL-6, IL-10 contents for differentiation of tuberculous from malignant pleural effusion. J of Radioimmunology. 2005;18(2):97–9.
Yang WB, Liang QL, Ye ZJ, Niu CM, Ma WL, Xiong XZ, Du RH, Zhou Q, Zhang JC, Shi HZ. Cell origins and diagnostic accuracy of interleukin 27 in pleural effusions. PLoS One. 2012;7(7):e40450.
Yu L, Wang M, Ma X, Zhang Y, Zuo L. Diagnostic value of IL-18 and INF-gama levels in tuberculous and malignant pleural effusions [in Chinese]. Shanghai J of Med Lab Sci. 2003;18(1):39.
Zhang XQ, Hua ZX, Lu DM, Liu HS, Lv XY, Chen LH. The differential diagnostic value of interleukin-2 in tuberculous pleural or ascitic effusion [in Chinese]. J Chengde Med Coll. 1998;15(2):108–9.
Zhou SX, Tan J, Xiao XP. Comparison of clinical values of adenosine deaminase,tumor necrosis factor-α and interleukin-12 in diagnosing tuberculous pleural effusion [in Chinese]. Lab Med Clin. 2012;9(12):1436–7.
Gao Y, Ou Q, Wu J, Zhang B, Wen X, Zhang W, Shao L. Expressions and diagnostic value of interleukin-31 in tuberculous pleural effusion [in Chinese]. Chin J Infect Dis. 2015;33(6):323–6.
Yuan YM, Wang ZY, Wang XJ, Cheng XX, Hui-Ru AN, Zhai SH, Zhang LL, Shuang-Mei L. The application of Mtb-antigen-specific Interleukin-22 in the differential diagnosis of Tuberculous pleurisy [in Chinese]. Modern Instruments & Medical Treatment. 2014;20(3):1–3.
Yang J, Liu W. Role of combination of CA50、TSA、CEA、SF and IL-8 assays in differentiation of benign from malignant pleural effusion [in Chinese]. Chin Med J Metall Industry. 2001;18(5):264–6.
Yamada Y, Nakamura A, Hosoda M, Kato T, Asano T, Tonegawa K, Itoh M. Cytokines in pleural liquid for diagnosis of tuberculous pleurisy. Respir Med. 2001;95(7):577–81.
Jin D, Chen Y, Wang Z, Wang S, Bunjhoo H, Zhu J, Cao Y, Xiong W, Xiong S, Xu Y. Diagnostic value of interleukin 22 and carcinoembryonic antigen in tuberculous and malignant pleural effusions. Exp Ther Med. 2011;2(6):1205–9.
Littenberg B, Moses LE. Estimating diagnostic accuracy from multiple conflicting reports a new meta-analytic method. Med Decis Mak. 1993;13(13):313–21.
Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84.
Macaskill P. Empirical Bayes estimates generated in a hierarchical summary ROC analysis agreed closely with those of a full Bayesian analysis. J Clin Epidemiol. 2004;57(9):925–32.
Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. 2009;28(21):2653–68.
Boyman O, Kolios AG, Raeber ME. Modulation of T cell responses by IL-2 and IL-2 complexes. Clin Exp Rheumatol. 2015;33(4):S54–7.
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7322):1188.
Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. Diagnostic value of interferon-γ in tuberculous pleurisy : a meta-analysis. Chest. 2007;131(4):1133–41.
Liang QL, Shi HZ, Ke W, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102(5):744–54.
Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, Schito M, Zumla A. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13(4):362–72.
Porcel JM. Advances in the diagnosis of tuberculous pleuritis. Ann Transl Med. 2016;4(15):282.
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37.
Hurst SM, Wilkinson TS, Mcloughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(14):705–14.
Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26(1):453–79.
Chomej P, Bauer K, Bitterlich N, Hui DSC, Chan KS, Gosse H, Schauer J, Hoheisel G, Sack U. Differential diagnosis of pleural effusions by fuzzy-logic-based analysis of cytokines. Respir Med. 2004;98(4):308–17.
Acknowledgments
Not applicable
Funding
This work was supported by grants from the National Natural Science Foundation of China (81300032) and Science Foundation for Young Scholars of Sichuan University (2015SCU11999–10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
NZ and CW: conceived the article and contributed the systematic review, meta-analysis, and manuscript writing. JQ, YQW, TY, and FQW: contributed to the systematic review and manuscript writing. YCS and LC: contributed to the conception and design and been involved in the revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
Ni Zeng and Chun Wan share joint first authorship.
Additional files
Additional file 1: Figure S1.
Forest plot of the sensitivities and specificities reported by each interleukin. The Forest plots of the sensitivities and specificities reported by A. interleukin-6; B.interleukin-33; C interleukin-12; D interleukin-2; E interleukin-12p40. Figure S2. Funnel graph for the assessment of potential publication bias in each interleukin. The Funnel graphs for the assessment of potential publication bias in each interleukin: A for IL-27; B for IL-18;C for IL-6; D for IL-33; E for IL-12; F for IL-2; G for IL-12p40. (DOC 231 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zeng, N., Wan, C., Qin, J. et al. Diagnostic value of interleukins for tuberculous pleural effusion: a systematic review and meta-analysis. BMC Pulm Med 17, 180 (2017). https://doi.org/10.1186/s12890-017-0530-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-017-0530-3