Abstract
Background
Novel therapies need to be evaluated in normal clinical practice to allow a true representation of the treatment effectiveness in real-world settings.
Methods/design
The Salford Lung Study is a pragmatic randomised controlled trial in adult asthma, evaluating the clinical effectiveness and safety of once-daily fluticasone furoate (100 μg or 200 μg)/vilanterol 25 μg in a novel dry-powder inhaler, versus existing asthma maintenance therapy. The study was initiated before this investigational treatment was licensed and conducted in real-world clinical practice to consider adherence, co-morbidities, polypharmacy, and real-world factors. Primary endpoint: Asthma Control Test at week 24; safety endpoints include the incidence of serious pneumonias. The study utilises the Salford electronic medical record, which allows near to real-time collection and monitoring of safety data.
Discussion
The Salford Lung Study is the world’s first pragmatic randomised controlled trial of a pre-licensed medication in asthma. Use of patients’ linked electronic health records to collect clinical endpoints offers minimal disruption to patients and investigators, and also ensures patient safety. This highly innovative study will complement standard double-blind randomised controlled trials in order to improve our understanding of the risk/benefit profile of fluticasone furoate/vilanterol in patients with asthma in real-world settings.
Trial registration
Clinicaltrials.gov, NCT01706198; 04 October 2012.
Similar content being viewed by others
Background
Combination of a long-acting inhaled corticosteroid (ICS) fluticasone furoate (FF) with the novel long-acting β2-agonist (LABA) vilanterol (VI) in a novel dry-powder inhaler (DPI; Ellipta®) has been investigated as a once-daily medication for the management of asthma [1]. Following a phase III programme of randomised controlled trials (RCTs), marketing authorisation was given by the European Commission on 18 November 2013 and FF/VI (Relvar®) is now licensed across Europe for asthma and chronic obstructive pulmonary disease.
We have previously described the principles behind the Salford Lung Study (SLS) and how this pragmatic RCT (pRCT) differs from standard RCTs [2]. The study was originally designed and approved before the investigational treatment received regulatory approval, and hence is a phase III study. It compares the FF/VI combination with existing maintenance therapy, in a large population of patients with asthma, studied in real-world routine clinical practice, and monitored using an electronic medical record (EMR). Here we provide details of the SLS asthma protocol.
Methods
Study design
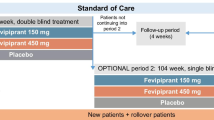
SLS is a 12-month randomised, open-label, phase III pRCT evaluating the effectiveness and safety of FF/VI (Relvar®; 100 μg/25 μg or 200 μg/25 μg once daily, delivered by a novel DPI [Ellipta®] in patients with asthma) (clinicaltrials.gov identifier NCT01706198) (Fig. 1). The study is conducted in accordance with the International Conference on Harmonisation, Good Clinical Practice (GCP), all applicable data protection requirements and the ethical principles outlined in the Declaration of Helsinki 2013 (National Research Ethics Service Committee North West, Greater Manchester South; Research Ethics Committee reference 12/NW/0455). The study protocol conforms to the SPIRIT 2013 statement (Standard Protocol Items: Recommendations for Interventional Trials [3, 4]).
Study design. *Cardiovascular risk factors collected. †Comprises: Asthma Control Test; Asthma Quality of Life Questionnaire(s); EuroQol questionnaire; Medication Adherence Report Scale for Asthma; Work Productivity and Activity Impairment Questionnaire: Asthma. FF fluticasone furoate; GP general practitioner; ICS inhaled corticosteroid; LABA long-acting β2-agonist; VI vilanterol
The Salford Lung Study team sought guidance on the study design under the joint scientific advice process from National Institute for Health and Care Excellence and the Medicines and Healthcare Products Regulatory Agency; a joint consultation process took place to seek guidance on the study design. Informal discussions and advice on the study design took place prior to formal ethics application from the National Research Ethics Service Committee North West, Greater Manchester South.
Patients
All patients with asthma at 66 primary care sites (at the time of manuscript preparation) in and around Salford and South Manchester are identified from practice databases, and invited to participate in the study by their own general practitioner (GP) (Fig. 1).
Eligibility criteria include:
-
aged ≥18 years
-
symptomatic asthma diagnosed by a GP
-
regular maintenance inhaler therapy with ICS or ICS/LABA
-
symptoms within the week prior to visit 2.
There are minimal exclusion criteria such as a recent history of life-threatening asthma, chronic obstructive pulmonary disease or other clinically significant disease that would jeopardise patient safety. Eligible patients are recruited for the study in GPs’ offices. At visit 1, patients are offered study participation through written consent. At visit 2 (0–30 days after visit 1), patients are randomised 1:1 to receive FF/VI or continue on usual asthma maintenance therapy (ICS or ICS/LABA). The randomisation at visit 2 is stratified by Asthma Control Test (ACT) score (≥20, 16 to 19, or ≤15) and by previous asthma maintenance therapy (ICS or ICS/LABA). Patients randomised to continue their existing asthma maintenance therapy arm do not receive FF/VI. During the study, the doses of all medication may be adjusted at the GP’s discretion in the usual way. Both study groups receive free prescriptions for study medication, which is collected by the patients from local community pharmacies, and prescription data are captured on the electronic case report forms (eCRFs).
Participating sites
Primary care
To preserve the real-world nature of the study, the patient experience is as close to routine care as possible. The study’s principal investigators are the patients’ own GPs who may make treatment adjustments according to their clinical opinion. GPs make repeat prescriptions of study medication as usual, which are collected by patients from their usual pharmacy. GPs are ideally placed to facilitate recruitment, identify and report serious adverse events (SAEs) or serious adverse drug reactions (ADRs), and report study endpoints. As very few participating GPs had experience of clinical trial participation, all GPs have received training and support in GCP, patient recruitment, the study protocol, coding of healthcare issues and general research procedures.
Pharmacy
Every pharmacy in Salford and others in South Manchester have agreed to participate in the study, even though very few of the pharmacists had experience of clinical trial participation. Standard operating procedures were established, and more than 500 staff at participating pharmacies have received training in GCP and safety reporting. Initially pharmacies faxed copies of all prescriptions for collected study treatments to the study coordination centre, but as the trial progressed this has been collected electronically.
Hospitals
The large majority of admissions are to the local Salford Royal Hospital and the University Hospital of South Manchester where admissions are tracked electronically and in near-real time. Occasionally patients are admitted to other hospitals. These admissions are tracked via the primary care records. Information relating to all hospitalisations is reviewed by a dedicated study safety team.
Data monitoring
All hospital admissions, outpatient and emergency department visits are identified from the EMR database (whenever and wherever they occur). From primary care, all healthcare contacts, out-of-hours activity and prescriptions of antibiotics or oral steroids can be identified. These events are reviewed by the study research team and classified as asthma or non-asthma related. Furthermore, the EMR captures suspected unexpected serious adverse reactions (e.g. reduced kidney function or elevated liver function tests) and, for the purposes of SLS, includes data from external sources to identify, for example, deaths or National Health Service (NHS) hospital admissions outside Salford. Northwest EHealth [5] manages the EMRs, enabling data on study endpoints and patient safety to be collected continuously and remotely in near-real time.
Endpoints
Efficacy
The primary endpoint is the percentage of patients in each treatment arm, who have either an ACT total score of ≥20 or an increase from baseline of ≥3 in ACT total score at week 24 assessment. Secondary efficacy endpoints include: the percentage of patients who have either an ACT total score of ≥20 or an increase from baseline of ≥3 in ACT total score at weeks 12, 40 and 52; the mean change from baseline in ACT total score at weeks 12, 24, 40 and 52; and the percentage of patients with an ACT total score ≥20 at weeks 12, 24, 40 and 52. Further details of trial endpoints can be found in Table 1. Randomisation is stratified by baseline asthma therapy (ICS or ICS/LABA) and ACT score (≤15, 16–19, ≥20) to ensure treatment groups are balanced on disease severity and level of asthma control. These randomisation stratification variables will be included in the primary analysis model as covariates. Subgroup summaries and/or analysis will also be provided by the randomisation stratification variables, when appropriate.
Safety
Safety endpoints include the frequency and type of SAEs and ADRs, and the incidence of SAEs of pneumonia during the study. SAEs are monitored continually through the patient’s EMR. GPs/investigators or site staff are responsible for detecting, documenting and reporting SAEs and non-serious ADRs, identified by hospitalisation alerts through EMR and reported on eCRFs, with additional monitoring by telephone every 3 months.
Statistical analysis
A sample size of 2906 patients (1453 patients per treatment group) will detect a treatment difference of 6 % between usual asthma maintenance therapies and FF/VI on the primary endpoint, at the significance level 0.05 and 90 % power (assuming 50 % response rate in the usual asthma maintenance group at 6 months). A total of 4036 patients are required in the intent-to-treat (ITT) population (randomisation of 2018 patients per treatment arm) in order to have at least 2906 patients in the primary efficacy analysis population, assuming 80 % of patients in the ITT population have an ACT score of <20 at baseline and a 10 % dropout rate over the first 6-month period.
Primary efficacy analysis population is all ITT patients, who have an ACT total score <20 at baseline (randomisation visit). Treatment difference between the two treatment arms will be analysed using logistic regression adjusting for baseline ACT total score, baseline asthma therapy per randomisation stratification (ICS or ICS/LABA), age and gender. Subgroup analyses, when appropriate, will be provided for efficacy and safety endpoints based on baseline disease characteristics per randomisation stratification.
Discussion
Guidelines on treatment options are primarily based on double-blind RCTs (DBRCTs) [6, 7]. However, RCTs for registration purposes do not represent real life. Multiple inclusion and exclusion criteria mean that only a small proportion of patients with asthma (~5 %) are represented in DBRCTs, and patients with co-morbidities are excluded. In addition, only restricted outcomes are assessed (e.g. forced expiratory volume in 1 second), and studies are of short duration (<6 months). Finally, patients are closely monitored and inhaler technique repeatedly checked, so that adherence levels exceed 90 %, compared with less than 40 % in observational studies.
Although observational studies provide opportunities to assess real-world outcomes, the lack of suitable comparison between treatment groups and the small patient numbers in prospective trials can be limiting [8]. Consequently, the true impact and value of treatments for asthma may not be fully reflected by observational studies and DBRCTs.
The SLS is the first phase III pRCT study in asthma initiated while the investigational treatment was an un-licensed medicine, where supervision and monitoring of patients are reduced to a minimum. Clinical endpoints are collected and patient safety is guaranteed with remote clinical surveillance in near to real-time through the EMR. The study should provide evidence for prescribers, payers and healthcare providers to assess effectiveness, adherence levels and the true value of FF/VI treatment in the real world.
The primary endpoint, ACT, was chosen to reflect impact of treatments on patients’ overall asthma control. The rate of severe asthma exacerbations as a primary endpoint would not have been feasible due to the infrequent occurrence of such events in a general asthma population [9]; consequently, the number of eligible patients with asthma in the study population is insufficient to have adequate statistical power for an exacerbation study. However, although ACT may reflect a dimension of asthma that is different from exacerbations, it is not merely a proxy for exacerbations.
This pRCT has been a major challenge in study design, operational planning and study support. It has been possible because the SLS is focused on a single geographical area in the UK, with a stable population with high respiratory morbidity, and where healthcare is managed by the NHS. Salford and the surrounding area are unique in having a longstanding EMR connecting primary and secondary care together with local pharmacies. The extent to which these data will be transferable will depend on local health service provision, but SLS should provide a model for future assessments of the clinical effectiveness of new treatments.
In summary, data from the SLS will complement results from conventional registration trials, allowing a better understanding of the risk/benefit profile of the FF/VI combination in the wider community of patients with asthma.
Abbreviations
- ACT:
-
Asthma Control Test
- ADR:
-
Adverse drug reaction
- AQLQ:
-
Asthma Quality of Life Questionnaire
- DBRCT:
-
Double-blind randomised controlled trial
- DPI:
-
Dry-powder inhaler
- eCRF:
-
Electronic case report form
- EMR:
-
Electronic medical record
- EQ-5D:
-
EuroQol questionnaire
- FF:
-
Fluticasone furoate
- GCP:
-
Good Clinical Practice
- GP:
-
General practitioner
- ICS:
-
Long-acting inhaled corticosteroid
- ITT:
-
Intent-to-treat
- LABA:
-
Long-acting β2-agonist
- MARS-A:
-
Medication Adherence Report Scale for Asthma
- NHS:
-
National Health Service
- pRCT:
-
pragmatic randomised controlled trial
- RCT:
-
Randomised controlled trial
- SAE:
-
Serious adverse event
- SLS:
-
Salford Lung Study
- VI:
-
Vilanterol
- WPAI:
-
Work Productivity and Activity Impairment Questionnaire
References
Busse WW, O'Byrne PM, Bleecker ER, Lotvall J, Woodcock A, Andersen L, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients > =12 years old with asthma: a randomised trial. Thorax. 2013;68:513–20.
New JP, Bakerly ND, Leather D, Woodcock A: Obtaining real-world evidence: the Salford Lung Study. Thorax 2014;69:1152–4.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;2013(158):200–7.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346, e7586.
Northwest EHealth: Website. [www.nweh.org.uk].
Herland K, Akselsen JP, Skjonsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger "real life" population of patients with obstructive lung disease? Respir Med. 2005;99:11–9.
Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219–23.
Price D, Chisholm A, van der Molen T, Roche N, Hillyer EV, Bousquet J. Reassessing the evidence hierarchy in asthma: evaluating comparative effectiveness. Curr Allergy Asthma Rep. 2011;11:526–38.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99.
Acknowledgements
This study is sponsored by GlaxoSmithKline who reviewed the manuscript for factual accuracy and funded medical writing services. The authors thank Sandra Brave of iMed Comms, Macclesfield, UK, who provided editorial writing support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
NDB’s employing organisation provides IT support to GlaxoSmithKline. He has received educational grants and speaker’s fees from GlaxoSmithKline and Novartis, and support for attending educational conferences from Boehringer Ingelheim, GlaxoSmithKline and Novartis. AW has acted on advisory boards and provided consultancy for Almirall, Chiesi, Cytos and GlaxoSmithKline. He has received travel support to speak at an international meeting from Boehringer Ingelheim and GlaxoSmithKline. He is an investigator on cough and asthma studies for Afferent and GlaxoSmithKline. JPN has received consulting and speaker’s fees, and an educational grant from GlaxoSmithKline. MG’s institution has received funding from GlaxoSmithKline as the SLS study sponsor. WW is an employee of, and holds shares/stock options in, GlaxoSmithKline. DL is an employee of, and holds shares/stock options in, GlaxoSmithKline. JV has received travel support and consultancy fees from GlaxoSmithKline (related to the SLS study); in addition, he has received consultancy fees from Almirall, AstraZeneca, Bioxydyn, Chiesi, GlaxoSmithKline (outside the SLS study), Novartis, Syntaxin and Takeda (Nycomed), and speaker’s fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis and Takeda (Nycomed). His wife has previously worked for AstraZeneca, Ferring and GlaxoSmithKline (until 2009).
Authors’ contributions
AW: led with DL on the initial design, implementation, discussions with regulatory authorities, and ethics application, co-chairs the trial governance committee, member of trial science committee. NDB: involved in the setting up of the Salford Lung Study and in developing clinical safety alerting system. JPN: involved with setting up the study and protocol, and led the development of the information technology platform used to deliver the Salford Lung Study. JMG: involved in the design, set up and technical infrastructure that supports the Salford Lung Study. WW: was the author of section 8 of the study protocol, Data Analysis and Statistical Considerations, and a contributing author for the other sections of the study protocol. JV: involved with the development of the Salford Lung Study real-world trial. DL: conceived concept for the real-world trial, and involved in setting up the Salford Lung Study, co-wrote the protocol, set up the operational model, co-led on ethics, regulatory submissions and co-chairs the study governance board. All authors contributed equally to the preparation of this paper, including development of the outline, review of all drafts, final approval and decision to submit the manuscript to BMC Pulmonary Medicine. Information for this paper was based on the authors’ personal knowledge and relevant published journal articles.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Woodcock, A., Bakerly, N.D., New, J.P. et al. The Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in asthma. BMC Pulm Med 15, 160 (2015). https://doi.org/10.1186/s12890-015-0150-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-015-0150-8