Abstract
Background
Despite the human papillomavirus (HPV) vaccine being a safe, effective cancer prevention method, its uptake is suboptimal in the United States (U.S.). Previous research has found a variety of intervention strategies (environmental and behavioral) to increase its uptake. The purpose of the study is to systematically review the literature on interventions that promote HPV vaccination from 2015 to 2020.
Methods
We updated a systematic review of interventions to promote HPV vaccine uptake globally. We ran keyword searches in six bibliographic databases. Target audience, design, level of intervention, components and outcomes were abstracted from the full-text articles in Excel databases.
Results
Of the 79 articles, most were conducted in the U.S. (72.2%) and in clinical (40.5%) or school settings (32.9%), and were directed at a single level (76.3%) of the socio-ecological model. Related to the intervention type, most were informational (n = 25, 31.6%) or patient-targeted decision support (n = 23, 29.1%). About 24% were multi-level interventions, with 16 (88.9%) combining two levels. Twenty-seven (33.8%) reported using theory in intervention development. Of those reporting HPV vaccine outcomes, post-intervention vaccine initiation ranged from 5% to 99.2%, while series completion ranged from 6.8% to 93.0%. Facilitators to implementation were the use of patient navigators and user-friendly resources, while barriers included costs, time to implement and difficulties of integrating interventions into the organizational workflow.
Conclusions
There is a strong need to expand the implementation of HPV-vaccine promotion interventions beyond education alone and at a single level of intervention. Development and evaluation of effective strategies and multi-level interventions may increase the uptake of the HPV vaccine among adolescents and young adults.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
The human papillomavirus (HPV) is the most common infection that can lead to cancer later in life. There are 570,000 incident cancer cases per year in females and 60,000 incident cancer cases in males attributable to HPV globally [1].HPV can lead to cancers of the cervix, vagina, and vulva for females, penis cancer for males, and anus and oropharyngeal cancers for both [1]. The World Health Organization has a vision to eliminate HPV-related cancers, particularly cervical cancer, worldwide by 2030 [2]. Similarly, in the U.S., Healthy People 2030 has an objective to increase the proportion of adolescents who receive recommended doses of the HPV vaccine from a baseline of 48.0% to 80.0% [3].
HPV vaccination can prevent more than 90% of cancers due to HPV infections [4, 5]. Vaccination starts at age 9 and the catch up is recommended through age 26. If not adequately vaccinated, persons up to the age of 45 can be considered for vaccination but with shared decision-making between the patient and provider [6]. Primary prevention is from ages 9–14 globally [7]. The HPV vaccine is commonly recommended during routine vaccinations to children ages 11–12 and there is a push from public health professionals and providers to start as early as 9 in the U.S [8]. Globally, an estimated 15% of girls are fully vaccinated against HPV [9]. In the U.S., about 58.5% of adolescents were up-to-date on HPV vaccination in 2020, with 61% of females being fully vaccinated versus 56% of males [10]. Public health efforts are needed to increase the global rates of HPV vaccination.
Worldwide, there have been a few reviews of interventions focused on improving HPV vaccination rates [11,12,13,14,15]. Interventions to promote HPV vaccination have typically targeted parents, adolescents, young adults, and providers.. HPV vaccination interventions have targeted various socio-ecological levels that influence HPV vaccination to ultimately effect change. Some focus only on the individual level (e.g., via education such as informational text included with reminders), whereas others may include changes to policy (e.g., via formalized requirements, such as school mandates). Multi-level and multi-component interventions are increasingly used [12, 13, 15] and address health disparities [16, 17]. Multi-level interventions target two or more levels of influence at or around the same time; the approaches implemented at each level typically may vary in type (e.g., behavioral, health systems, or policy) [16, 18]. It is important to understand the wide range of levels that can be utilized in interventions from single-level to multi-level and how those levels can impact the desired outcome of vaccination.
This study aimed to conduct a systematic review of HPV interventions by synthesizing literature published from May 2015 to March 2020, related to promoting HPV vaccine uptake and/or completion in the U.S and internationally. A previous systematic review and meta-analysis in the United States found a combination of provider- and community-level interventions were effective [11]. Our review was intended to update this review of interventions for HPV vaccine promotion with more rigorous methodology, including exploration of sources of heterogeneity and quality assessment. Another purpose of the study was to improve the understanding of multi-level interventions for HPV vaccine promotion. The review questions included: 1) What are the targeted audiences and levels of intervention for HPV vaccination interventions?, 2) What are common components of the interventions?, 3) What were facilitators and barriers to implementation of the vaccination interventions?, and 4) What are the study outcomes measured including the rates of HPV vaccination initiation and completion and their effectiveness? Our resulting study provides a strong contribution to the literature that can be used to inform future promotion efforts that aim to increase HPV vaccine uptake.
Methods
We conducted a systematic review of the peer-reviewed published literature, using methods following the PRISMA guidelines [19]. The team included cancer control researchers and master’s and doctoral students in public health and nursing fields.
Search strategy
The lead author, in collaboration with a health sciences librarian, created a search strategy using text and MeSH terms (Supplemental Table 1). We searched for relevant articles in six bibliographic databases, including Medline, CINAHL, Embase, Web of Science, Cochrane Reviews, and SCOPUS. Some of the keywords searched alone or in combination were children, pediatric, young adult, parent, behavioral therapy, prevention, and human papilloma virus. An additional manual search was performed of the bibliographies of relevant studies identified from the database search. The team reviewed the articles found in the search and removed duplicates.
Inclusion criteria
To be included in the review, an article had to: a) aim to increase HPV vaccination through at least one intervention; b) report an outcome based on the intervention (e.g., increase knowledge of HPV, report on HPV vaccine outcomes determined either by self-report or medical records; c) be published between May 2015 through March 2020; and d) be published in English. Studies that tested single or multi-level interventions were included. Screening was conducted in two stages with the initial stage evaluating titles and abstracts reviewed by 3 authors (CE, CA, and MD), and a second stage screening full text articles independently reviewed by the same 3 authors. Discrepancies were resolved through discussion at team meetings. Studies were excluded if they did not describe a primary intervention aimed at increasing HPV vaccination, were systematic reviews or articles with just a program description, or had no study outcomes. Those that met eligibility through abstract review were included in the full-text review. After the full article review, the articles were examined further to see if they met the eligibility criteria, and 33 were excluded.
Data extraction
We retrieved the full text of eligible studies for review and abstraction. We then created a detailed codebook for data collection. Data extraction tables for the article and quality assessment were developed and maintained in an Excel database. They were modified following discussions between three reviewers before data extraction. Data extracted included study location, target population, sample description, and setting; intervention details consisted of study design, description of the intervention (e.g., control group components, if applicable), level(s) of intervention, delivery and barriers to implementation and vaccination, and outcomes of the study. We piloted the forms with five studies and made refinements to the codebook and Excel database. We invited cancer and implementation science researchers from the Cancer Prevention and Control Research Network [20] and doctoral and MPH students from the participating institutions to be trained as data abstractors and abstract data from the final included articles. There were a total of 15 reviewers (CA, CP, CE, MD, SS, CB, MF, AE, LS, ED, GR, KY, SL, TV, and PM). For quality control, we had 2 abstractors for each study, and we merged the data when consensus was reached for each article. The abstractors also performed study quality assessment for the articles they abstracted. The pair of abstractors came to an agreement if there were discrepancies. If there was a disagreement or question about a study quality answer, then the core team (CA, CP, and CE) had a discussion and came to an agreement on the study quality question.
Quality assessment
For this assessment, we employed the NCI Quality Rating assessment for Pre and Posttest Designs to conduct quality assessment of the included articles [21]. This assessment included 12 items which included: whether the objectives, intervention, and eligibility requirements were clearly stated, had a sample adequate for confidence in the data, had a loss to follow-up of 20% or less, and measured changes in outcomes of interest before and after the intervention.
Synthesis of the results
We compiled all article abstractions into one database. We ran descriptive statistics and created summary scores for study setting and program component descriptions. The Community Guide categories (education, technology, vaccine access, incentive, provider education, health system change, community wide campaign, and policy) were used to organize the interventions into informational; behavioral change for participants, providers or both; or environmental (small-no government involvement such as organizational policy change or large policy-formal laws, rules or regulations, national or local government involvement). These categories also were applied in the Walling et al. systematic review [12]. We also created summary tables for study characteristics, outcomes, and quality ratings. The primary outcome was HPV vaccine initiation and/or completion, although we reported on other outcomes related to HPV vaccination determinants, or factors to increase vaccination (i.e., parental knowledge, awareness, self-efficacy, acceptability, attitudes and beliefs, and vaccine intention). We examined the range of HPV vaccine initiation and completion for adolescents and/or young adults.
Results
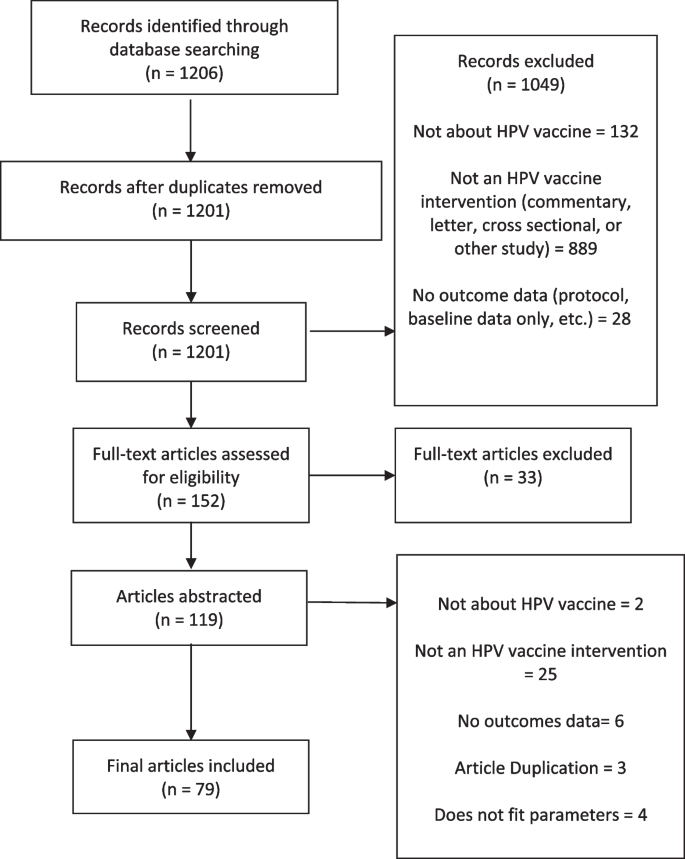
The search identified 1,201 studies after removing duplicates. As a result of the title and abstract screen, 1,045 studies were excluded due to not being an intervention study or not reporting outcomes. The full-text of the remaining 152 articles were reviewed, leading to the exclusion of an additional 72 articles that did not have descriptions of the intervention or outcome data. This resulted in 79 articles included in the review for data extraction (Fig. 1). Table 1 shows the main characteristics of the included studies published between 2015 and 2020.
Study setting and design
Of the 79 intervention articles, 57 (72.2%) were conducted in the U.S. Other studies were conducted in Europe (n = 10, 12.7%), Africa (n = 4, 5.1%), Asia (3, 3.80%), Australia (3, 3.80%), Central/South America (1, 1.27%), and Canada (1, 1.27%). Forty-five studies (57.0%) employed an experimental design, 18 (22.8%) used a quasi-experimental design, and 16 (20.3%) employed a non-experimental design.
Setting and population focus
Intervention settings included clinics (32, 40.5%), schools (26, 32.9%), communities (10, 12.7%), an organization (1, 1.3%), a health insurance system, and online (10, 11.4%). Study samples ranged from 36 to 8,062.
Of the 79 studies, most interventions targeted adolescents only (39 studies, 49%) [22, 25, 27, 29, 31, 32, 34,35,36, 40, 43, 44, 46, 48, 50,51,52,53,54,55, 60,61,62,63,64,65, 68, 69, 72,73,74,75, 85, 90,91,92, 94, 98, 100], of which 15 (38%) included girls only, 17 (44%) included both boys and girls, 3 (8%) included boys only, and 4 (10%) did not report. Other interventions focused on young adults ages 18–34 years (20 studies, 25%) [22,23,24,25,26, 28, 34, 38, 47, 49, 57, 58, 69, 73, 78, 83, 89, 93, 97, 99], parents (27 studies, 34%) [25, 33, 41, 43, 45, 50,51,52, 56, 61, 63, 66, 70, 75, 76, 78, 79, 81, 82, 84, 86, 90,91,92,93, 96, 100], healthcare providers (13 studies, 17%) [30, 37, 39, 47, 59, 66, 67, 69, 71, 80, 87, 88, 95], or did not report (1 study, 1%) [77].
Twenty-one interventions included multiple target populations as participants. Common combinations of participants included parents and adolescents (11 studies) [43, 50,51,52, 61, 63, 75, 90,91,92, 100], adolescents and young adults (4 studies) [22, 26, 34, 73], clinicians and young adults (1 study) [47], parents and young adults (3 studies) [25, 78, 93], parents and clinicians (1 study) [66], and clinicians, adolescents, and young adults (1 study) [69]. Only three studies included only male adolescents or young adult study populations (2 were adolescents only, and the last one was both adolescents and young adults).
Eight of the 79 studies (10.1%) included a large proportion of parents from diverse racial and ethnic identities (defined as ≥ 50% other races than White) [33, 45, 56, 70, 76, 79, 81, 100], 6 (7.6%) included adolescents from diverse groups [27, 40, 64, 65, 74, 98], 8 (10.1%) included both parents and children from diverse groups [38, 51, 52, 61, 63, 75, 90, 91], 6 (7.6%) included young adults from diverse groups [26, 35, 57, 60, 80, 97], and 1 included both young adults and children from diverse groups (1.3%) [34].
Socio-ecological levels
Based on a review of the reported intervention components, the audiences they targeted, and the socio-ecological model, most studies were conducted at the individual level (44, 55.7%), followed by interpersonal level (10, 12.7%), community level (3, 3.8%), and clinic level (4, 5.0%).
Multi-level interventions
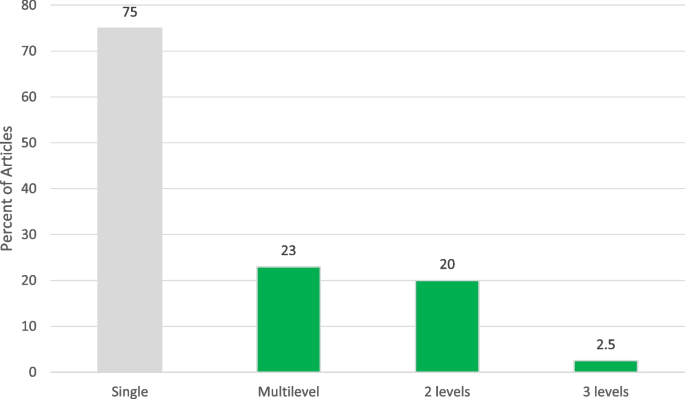
Although most interventions were directed at a single level of the socio-ecologicl level (n = 61, 76.3%), 23.7% (n= 18) were multi-level. Sixteen (88.9%) combined two levels [27, 32, 39, 42, 43, 55, 63, 66, 69, 71, 73, 75, 77, 82, 98, 100], and 2 (9.1%) combined three levels (Fig. 2) [47, 67]. Common combinations of the levels included provider and clinical (5 studies) [66, 69, 71, 73, 82], interpersonal and clinical (4 studies) [27, 39, 43, 77], individual and interpersonal (2 studies) [32, 100], individual and clinical (2 studies) [42, 98], and individual and community (2 studies) [55, 75]. Meyer et. al aimed to use an electronic point-of-care prompt and 2-h lecture for providers to increase HPV vaccine uptake in retail clinics (provider and clinical interventions) [73]. Staras et al. sought to increase HPV vaccine initiation among publicly insured Florida adolescents ages 11–17 using a quasi-experimental factorial design with four study arms: 1) postcard campaign, 2) in-clinic Health Information Technology (HIT) system, 3) postcard campaign and in-clinic HIT system, and 4) usual care (individual and clinical interventions) [98]. Paskett et al. developed a program focused on HPV vaccine uptake among parents who have adolescent girls ages 9–17 who have not received the HPV vaccine, which would include vaccinations (individual and provider interventions) [82]. The 3-level combinations included: 1 study with individual, interpersonal, and clinical interventions [67], and 1 study with individual, clinical, and community interventions [47]. For example, Malo et al. created a 3-level intervention for parents to analyze which messages were most motivating to persuade them to administer the HPV vaccine to their child, for educating and training physicians, physician assistants, nurse practitioners and nurses who serve at primary clinics specialized in pediatrics or family medicine about the most persuasive messages in speaking to parents about the HPV vaccine for their children (individual, interpersonal, and clinical interventions) [67].
Intervention components
The duration of interventions ranged from 10 min to 18 months among the studies reporting intervention time frames. Twenty-seven interventions (33.8%) reported using theory in intervention development [23, 24, 31, 35, 36, 40, 45, 47, 48, 50, 53, 54, 58, 61, 63, 67, 70, 72, 74,75,76, 81,82,83, 86, 100]. Theories or frameworks referenced included the Elaboration Likelihood Model, Culture-centric narrative theory, Health Belief Model, Theory of Reasoned Action/Planned Behavior, Moral Norm and Social Cognitive Theory.
Intervention components varied from education to offering vaccination (vaccine access). The most common intervention components were individual education of parents and/or adolescents (60, 76.0%); use of technology such as websites, PowerPoints, and text messages (21, 26.6%); and provider education (16, 20.3%). Examples of educational messaging were: expressing the benefit of the HPV vaccine, providing cervical and breast cancer prevention education, supplying educational handouts at an eighth-grade reading level, and displaying facts on posters about HPV and the HPV vaccine (i.e. both genders can receive the vaccine). The websites provided factual information on HPV and the HPV vaccine including statistics on the incidence of HPV infection and cervical cancer, risks associated with HPV infection, costs of vaccination, safety and efficacy of the HPV vaccine, and suggestions for how to talk to a doctor about the vaccine. Other components included patient reminders (13, 16.5%) [27, 50, 51, 62, 63, 70, 71, 74, 77, 83, 89, 90, 99], improving access to the HPV vaccine (6, 7.6%) [29, 55, 64, 75, 85, 89], health systems change (6, 7.6%) [43, 69, 75, 77, 81, 98], incentives (4, 5.1%) [46, 62, 68, 92], and community-wide campaigns or outreach (3, 3.8%) [32, 45, 75]. Patient reminders included phone calls, text messages, mailing reminders, and reminder-recall letters prompting adolescents to sign up for an appointment via a website. Several ways to improve access to the HPV vaccine consisted of utilizing school-based programs and expanding HPV vaccination programs in countries where there were no existing HPV vaccine programs. For incentives, gift cards (e.g., general merchandise and department stores, fashion and footwear retailers, bookstores, jewelry shops, motoring stores, and home improvement stores) and vaccine vouchers were used. Some studies combined two components (29, 36.7%) [24, 27,28,29, 31,32,33,34,35, 39, 40, 44, 45, 47, 50, 55, 65, 66, 71, 72, 81, 83, 85,86,87,88, 90, 97, 98], three components (6, 7.6%) [51, 62, 63, 69, 74, 82] or four components (3, 3.8%) [75, 77, 89]. Common intervention combinations included education and technology (18 studies, 23%) [24, 28, 31, 33,34,35, 40, 44, 51, 63, 65, 72, 74, 82, 86, 88, 89, 97], education and reminders (9 studies, 11%) [50, 51, 62, 63, 74, 77, 83, 89, 90], education and vaccine access (5 studies) [29, 55, 75, 85, 89], and provider education and technology (4 studies, 5%) [39, 69, 82, 87].
Community guide intervention categorization
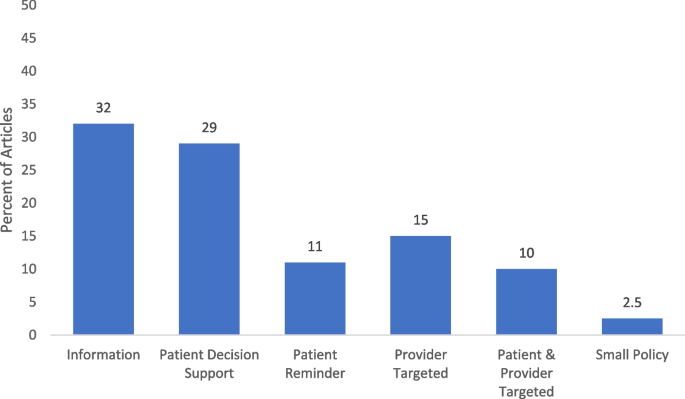
We reported on the categorization of the interventions based on the Community Guide’s categorization framework to assess the design and execution of health-related evidence-based interventions [12]. The most common type of HPV vaccination interventions were informational interventions (25, 31.7%). Of the behavioral interventions, 23 (29.1%) [24, 26, 32, 34,35,36, 38, 40, 44, 46, 48, 51, 52, 55, 61, 68, 76, 79, 81, 91, 92, 97, 100] were patient-targeted decision support, 9 (11.4%) [50, 62, 63, 70, 74, 83, 89, 90, 99] were patient-targeted reminders, 12 (15.2%) [22, 30, 37, 39, 43, 59, 67, 69, 71, 73, 87, 95] were provider-targeted, 8 (10.1%) [27, 47, 66, 75, 77, 82, 85, 98] were both patient and provider targeted interventions. Only 2 (2.5%) [29, 64] were related to environmental interventions related to small policies (i.e., organizational guidelines, no government involvement) (Fig. 3).
Facilitators and barriers to intervention implementation
Several studies reported facilitators (13 studies, 16.5%) [26, 27, 29, 30, 32, 36, 37, 40, 45, 47, 48, 63, 65] and barriers (22 studies, 27.58%) [24, 26, 27, 30, 36, 37, 40, 43, 44, 50, 61, 67, 68, 73, 74, 77, 79, 87, 89, 93, 98, 99] to intervention implementation. Facilitators included use of patient navigators and user-friendly resources [26, 27, 36], interactive information sessions [29, 30, 37], low-cost interventions [30, 40, 47], and quality improvement initiatives [30, 37]. Barriers to implementation were related to cost [24, 27, 93, 99], time constraints with the given intervention [30, 36, 43, 67, 87] and integrating the intervention into clinical workflow [37, 73, 87, 98]. Other barriers included mobility of parents and technology challenges.
HPV Intervention outcomes
Forty-two studies (53.2%) [22, 24, 26, 27, 29, 32, 34, 37,38,39,40, 44, 47, 48, 50,51,52,53, 55, 61,62,63, 68, 69, 71, 73,74,75,76,77, 79, 81,82,83, 85, 89,90,91, 97,98,99,100] reported on HPV vaccination outcomes, with 38 (48.1%) [22, 24, 26, 27, 29, 32, 34, 37,38,39,40, 44, 47, 50,51,52,53, 55, 61, 62, 68, 69, 71, 73,74,75,76,77, 79, 81,82,83, 85, 91, 97,98,99,100] reporting HPV vaccine initiation and 26 (32.9%) [22, 26, 27, 29, 34, 37,38,39,40, 48, 50, 52, 55, 63, 68, 71, 74,75,76,77, 81, 89,90,91, 97, 99, 100] reporting vaccine series completion. Post-intervention vaccine initiation ranged from 5% to 99.2%, while series completion ranged from 6.8% to 93%. For the experimental studies (n= 47), 11 (23.4%) measured vaccine initiation [24, 34, 38, 48, 51, 54, 61, 82, 83, 85, 86], and 3 (6.4%) measured completion [89, 90, 99]. Eleven (23.4%) assessed initiation and completion as outcomes (Table 2) [39, 40, 44, 50, 52, 53, 68, 71, 81, 91, 100]. Of the interventions that only measured vaccine initiation, 3 out of 11 (27%) found a significant increase in vaccine initiation [48, 82, 85]. For the interventions that measured both as an outcome, 3 out of the 11 (27%) found a significant increase in vaccine initiation [50, 71, 100]. Therefore, a total of 6 (12.8%) interventions demonstrated a significant increase in vaccine initiation [48, 50, 71, 82, 85, 100]. For the interventions that measured both vaccine initiation and completion, 1 (9.1%) reported a significant increase in completion only [81] and 2 (18.2%) in both vaccine initiation and completion [39, 68]. Of the interventions with quasi-experimental studies (n = 16), 5 (31.3%) were studies with comparison groups [30, 55, 62, 69, 98] and 11 (68.8%) were studies with pre and post intervention data collection (Table 3) [22, 25, 47, 59, 63, 73, 76, 79, 93, 95, 97]. Out of the quasi-experimental interventions with comparison groups (n= 5), 3 (60%) measured vaccine initiation [62, 69, 98], and 1 (20%) assessed both initiation and completion [55]. Of those, 2 (40%) demonstrated significant increase in vaccine initiation [62, 98], 0 in completion, and 1 (20%) in both as an outcome [55]. Out of the quasi-experimental interventions with pre and post-intervention designs (n= 11), 2 (18.2%) measured initiation [47, 79], 1 (9.1%) measured completion [63], and 4 (36.4%) assessed both as outcomes [22, 73, 76, 97]. One (9.1%) reported a significant increase in vaccine initiation [47] 1 (9.1%) in completion [63]; and 2 (18.2%) in both [73, 76].
Other common intervention outcomes included measures of parental knowledge (18, 32.1%), self-efficacy (7, 12.5%), acceptability (7, 12.5%), and attitudes and beliefs (6, 10.7%). For adolescents, other outcome measures were knowledge (8, 34.5%), awareness (3, 13.0%), and attitudes and beliefs (3, 13.0%). For young adults, these measures included knowledge (14, 35.9%), attitudes and beliefs (7, 17.9%), and self-efficacy (4, 10.3%). Out of 79 studies, 15 (19%) measured vaccine intention.
Quality assessment
The study quality (SQ) assessment included 12 criteria items with response options as 0 = no or 1 = yes. The results showed that SQ1 (the study had a clear objective) was the most common criterion met, with 79 (98.8%) studies meeting this criterion. This was followed by SQ3 (participants in the study are representative of those who would be eligible), which was met by 68 (85%) studies. SQ2 (eligibility criteria clearly described) and SQ6 (delivered consistently across the study population) were tied for third place, with 67 (83.75%) studies meeting these criteria. On the other hand, SQ8 (people assessing the outcomes blinded to participants' exposures/interventions) was the least met criterion, with only 9 (11.25%) studies meeting this criterion. SQ12 (the study took into account the use of individual-level data to determine effects at the group level) was met by 15 (18.75%) studies. SQ11 (outcome measured multiple times) was met by 19 (23.75%) studies, while SQ9 (loss to follow-up after baseline 20% or less) was met by 30 (37.50%) studies. Overall, 60% (n = 48) and 32.5% (n = 26)were rated as Good or Fair in quality, respectively. Six (0.75%) studies were rated as Poor. For a detailed presentation of the quality elements and overall quality scores, please refer to Supplementary Table 2.
Discussion
We conducted a systematic review to assess interventions for HPV vaccine promotion. Our goal was to better describe common target populations of HPV vaccine interventions, common intervention levels and components, barriers and facilitators to intervention implementation, and the relationship between types of interventions and HPV-vaccine related outcomes. Previous systematic reviews have identified the breadth of intervention designs and contributed to our understanding of relative effectiveness of different intervention types [12, 14, 101]; however, given the advances in HPV vaccination research over the last several years, an update to these reviews was warranted. Moreover, previous systematic reviews have had a limited scope in terms of study settings, study designs, or topics and our goal here was to conduct a global and comprehensive review of interventions [14, 15, 102, 103]. We reported on the level of socio-ecological that each intervention targets, barriers and facilitators to the implementation of these interventions, and intervention with outcomes such as initiation and completion rates from the U.S. and other countries. In our update to these reviews, we found that while intervention components were described thoroughly to contribute to our knowledge of types of interventions being implemented, fewer details about barriers and facilitators and HPV vaccine-related outcomes (particularly vaccination rates) were included. There were few patterns to be discerned in which types of interventions were found to be most effective, and in fact, among those that did report, only 20.3% reported significant increases in either initiation or completion or both. Despite this, our findings offer six key insights into the types of interventions being implemented that make effective interventions.
From intervention research, we know that there are certain “components” that can help to promote successful intervention implementation and outcomes. For HPV vaccination specifically, we know that working with healthcare providers is an effective strategy [11]. More broadly, literature suggests that interventions are more effective when they focus on implementation at multiple levels [82] and use theory in intervention development [104]. However, in our review, we found that overall, many of the interventions identified did not adhere to these best practices; only 23% of the interventions were multi-level (18 total) and 34% employed theory (27 total).
We used the Community Guide and the Walling et al. systematic review classification of interventions such as informational, behavioral, and environmental to categorize and rank interventions [11]. Firstly, our review revealed the most commonly implemented interventions were not the types of interventions that had previously been shown to have the greatest impact. For example, while the success of behavioral provider and clinic-focused interventions (particularly ones that promote changes to systems like utilizing reminder-recall and encouraging strong recommendations) is well-documented [11], in our study we found other types of interventions were more often used. For example, information-providing interventions (used to increase knowledge of HPV, HPV-associated cancers and the HPV vaccine [11]) were most common (31.7%) followed by patient decision support interventions (29.1%). Among these intervention categories, the intensity of the activities ranged widely. For example, in our study among information-providing interventions some studies employed a passive approach by offering pamphlets and educational materials [60] whereas others were more active and included live presentations [57, 65]. Yet, educational, or information-giving interventions have been found to be less effective in increasing uptake or completion [103]. The interventions being implemented are not the types that have been shown to be most effective, which is consistent with other research that has identified a discrepancy between the implementation of interventions or strategies that are most effective compared to interventions that may be deemed “easiest” to implement [105, 106].
Secondly, despite extensive research showing the increased effectiveness of multi-level interventions [82], there were limited interventions included in this review that were multi-level (23%). For example, The Community Guide has found insufficient evidence for provider or patient education alone to increase vaccination, but it has found that using education in combination with provider-focused interventions (i.e. provider reminders; assessment and feedback) has been successful [107]. In this review, 75% of the interventions reported intervening on only a single level, most commonly in clinical or school-based settings focused on individuals or providers. Future interventions to promote HPV vaccination should prioritize intervening at multiple levels to more effectively improve vaccine outcomes and discern which combination of levels results in higher vaccination.
Thirdly, using theory is well-documented as a best practice in intervention development and implementation [104, 108]; however only one-third of the interventions in this review used theory in the design of their program strategies. It is highly possible that some of these interventions did in fact use theory or theoretical constructs to guide their research, but did not report it explicitly. The Health Belief Model, Theory of Planned Behavior, Social Cognitive Theory and the Elaboration Likelihood Model were the most commonly utilized; this is consistent with a recent systematic review exploring the use of theory in HPV vaccine interventions [102]. Using theory allows for understanding why specific interventions may be effective (or not effective) and for comparison across multiple studies. Thus, future HPV vaccine interventions should report more broadly on the use of theory in their intervention development and how constructs are employed in their design of intervention components or assessed in evaluation.
Fourthly, the effectiveness of these interventions was difficult to discern due to heterogeneity in measurement, outcomes, and study designs. Unfortunately, it is difficult to speak to what types of interventions were most effective as only about half reported on vaccine initiation (48%) and less than a third (32%) reported on vaccine series completion. Other commonly assessed outcomes included parental knowledge [33, 90, 91, 100], self-efficacy [35, 48, 54, 70, 72, 75, 76, 82, 86, 96], attitudes/beliefs [23, 31, 48, 49, 51, 54, 58, 65, 72, 75, 80, 82, 86, 97, 100], and acceptability [28, 34, 41, 43, 50, 72, 78, 79, 92]. There is mixed evidence on whether these outcomes are associated with uptake. For example, one meta-analysis found that parents’ beliefs, attitudes and intentions were positively associated with HPV vaccine uptake [109], while other studies have found intention to be unrelated to uptake, particularly in multivariable models, other factors seem to attenuate the effect of intention [110]. Moreover, many of the studies included in this review were quasi- or non-experimental, making it difficult to draw inferences about the effectiveness of any of the outcomes reported. Only about half focused on vaccine series initiation and completion. There are promising findings that a proportion of the interventions that reported significant changes in vaccination uptake or completion are multi-level and multi-component. Future intervention studies should focus on using rigorous methods to assess the effectiveness of different types of interventions, including investigating vaccination outcomes of series initiation or completion, and having longer-term follow-up to be able to assess longer-term outcomes. In addition, evaluation of multi-level interventions for the promotion of HPV vaccination should be conducted to contribute to their evidence of effectiveness.
Fifthly, related to the lack of reporting on intervention outcomes was a lack of reporting on implementation barriers and facilitators. Less than 20% of studies reported on facilitators and less than 30% reported on barriers. This is a similar finding to the review conducted by Smulian et al. (2016), who also reported a lack of reporting on barriers and facilitators [11]. This kind of information is critical in understanding program implementation, adaptation, and tailoring for different settings [24, 68, 93]. Recently, the use of hybrid trials, which can be used to assess both effectiveness and implementation outcomes, is emerging among implementation research [111, 112]. In the future, researchers could prioritize conducting these hybrid trials so that we can not only identify those interventions that are most effective, but also important implementation determinants that can inform sustainability and scalability in multiple types of healthcare settings.
Finally, it is important to note that it is a critical time, in the era of the COVID-19 pandemic to disseminate effective cancer prevention interventions. HPV vaccination rates have fallen during the pandemic [113, 114] and competing priorities have led to less time for clinics to devote to vaccine promotion [115]. Coupled with recent data suggesting that concerns about HPV vaccine safety are rising [116], this is indicative of a need to identify what works and how to implement it to prevent future generations from being susceptible to HPV-associated cancers. Overall, increased reporting of both vaccine outcomes as well as barriers and facilitators to vaccination will move the field forward and provide data to help researchers determine which types of interventions to prioritize.
Strengths and limitations
Our study was strengthened by the inclusion of interventions globally and our focus on understanding multi-level intervention strategies. By categorizing interventions at different levels (e.g., individual, interpersonal, clinical) we have added to the growing literature on multi-level interventions. Additionally, almost 30% of the studies included were conducted outside of the United States. This finding helps to add to the growing global literature on HPV vaccine interventions and allows for comparability between the U.S. and other countries that continue to struggle with low HPV vaccination rates [2]. However, this should simultaneously be recognized as a potential limitation, as results may not be generalizable across all global geographies. While studies from North and South America, Europe, Africa, Asia, and Australia were included, there were only several from each continent (other than North America) which limits the generalizability of results. Similarly, less than 15% of studies included parents or children from diverse racial and ethnic identities (defined as ≥ 50% other races than White). This makes it hard to assess the impact of interventions for HPV vaccination on racially and ethnically diverse populations. Future HPV vaccination research should focus on these populations to test intervention effectiveness. We also were limited by only reporting on articles written in English and may be missing HPV vaccination interventions written in other languages.
Another key limitation is the lack of reporting vaccine-related outcomes in studies. Just over 50% reported either initiation and/or completion outcomes. This fact with varying study designs makes it difficult to collectively assess intervention effectiveness through data synthesis. Moreover, 40% of the studies were rated as “fair” or “poor” quality in our quality assessment, primarily due to studies not including multiple time points for outcome measures, not blinding participants in intervention studies, and for group-level studies not reporting on individual-level data to determine group-level effects. These limitations identify key gaps in the literature and that future research should focus on including more diverse populations in interventions, employing more rigorous study designs, and including vaccine initiation and completion rates.
Conclusions
In 2020, the World Health Organization adopted a Global Strategy to eliminate cervical cancer, aiming for 90% of girls to be fully vaccinated by age 15 [2]. Given that males can suffer from HPV-associated cancers as well, many countries have expanded their vaccination programs to include males. However, worldwide, most countries fall far short of this 90% goal. Therefore, there is a strong need to expand implementation of HPV-vaccine promotion interventions beyond education alone and at a single level and use rigorous intervention designs. Inclusion of longer-term interventions and more evaluations focusing on vaccine initiation and/or completion would be helpful to truly understand what is most effective in improving HPV vaccination rates. Many of the interventions included in this review did not report vaccine uptake data; relied on strategies found to be less effective (e.g., education alone); did not use or not report on use of theory; did not report on barriers and facilitators to implementation; or addressed a single level for intervention. Improving on the design and evaluation of HPV vaccination interventions is particularly critical at this moment as many adolescents missed vaccinations during the COVID-19 pandemic and vaccine hesitancy is growing. Improving our understanding of which interventions to prioritize for implementation will be important to ensure future generations of adolescents are protected against HPV-associated cancers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID- 19:
-
Coronavirus disease of 2019
- HIT:
-
Health Information Technology
- HPV:
-
Human papillomavirus
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. https://doi.org/10.1002/ijc.30716.
WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization. (https://www.who.int/publications/i/item/9789240014107).
USDHHS. Healthy People 2030. U.S. Department of health and human services. (https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08).
CDC. Cancers caused by HPV are preventable. Centers for disease control and prevention. (https://www.cdc.gov/hpv/hcp/protecting-patients.html).
Rosenblum HG, Lewis RM, Gargano JW, Querec TD, Unger ER, Markowitz LE. Human Papillomavirus Vaccine Impact and Effectiveness Through 12 Years After Vaccine Introduction in the United States, 2003 to 2018. Ann Intern Med 2022. https://doi.org/10.7326/M21-3798
Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. https://doi.org/10.15585/mmwr.mm6832a3.
World Health Organization. Cervical cancer. www.who.int. Published February 22, 2022. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
Petrosky E, Bocchini JA, Jr., Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64(11):300–4. (https://www.ncbi.nlm.nih.gov/pubmed/25811679).
Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144.
Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183–90. https://doi.org/10.15585/mmwr.mm7035a1.
Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: A systematic review. Hum Vaccin Immunother. 2016;12(6):1566–88. https://doi.org/10.1080/21645515.2015.1125055.
Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1):e20153863. https://doi.org/10.1542/peds.2015-3863.
Mavundza EJ, Iwu-Jaja CJ, Wiyeh AB, et al. A systematic review of interventions to improve HPV vaccination coverage. Vaccines (Basel). 2021;9(7):687. https://doi.org/10.3390/vaccines9070687.
Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr. 2015;169(7):686–92. https://doi.org/10.1001/jamapediatrics.2015.0310.
Acampora A, Grossi A, Barbara A, et al. Increasing HPV vaccination uptake among adolescents: a systematic review. Int J Environ Res Public Health. 2020;17(21):7997. https://doi.org/10.3390/ijerph17217997.
Agurs-Collins T, Persky S, Paskett ED, et al. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am J Public Health. 2019;109(S1):S86–93. https://doi.org/10.2105/AJPH.2018.304730.
Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908. https://doi.org/10.1093/ije/dyt049.
Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the national institutes of health centers for population health and health disparities. Am J Public Health. 2008;98(9):1608–15. https://doi.org/10.2105/AJPH.2006.102525.
PRISMA. Preferred reporting items for systematic reviews and meta-analyses (PRISMA). (http://www.prisma-statement.org/).
Ribisl KM, Fernandez ME, Friedman DB, et al. Impact of the cancer prevention and control research network: accelerating the translation of research into practice. Am J Prev Med. 2017;52(3 Suppl 3):S233–40. https://doi.org/10.1016/j.amepre.2016.08.026.
National Heart, Lung, and Blood Institute (NHLBI). Study quality assessment tools. (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
Austin B, Morgan H. Improving human papillomavirus vaccine uptake in the family practice setting. J Nurse Pract. 2019;15(6):e123–5. https://doi.org/10.1016/j.nurpra.2019.01.022.
Baxter CE, Barata PC. The paradox of HPV vaccines: how to reach sexually inexperienced women for protection against a sexually transmitted infection. Women’s health issues. 2011;21(3):239–45. https://doi.org/10.1016/j.whi.2010.11.007. (In eng).
Bennett AT, Patel DA, Carlos RC, et al. Human papillomavirus vaccine uptake after a tailored, online educational intervention for female university students: a randomized controlled trial. J Womens Health (Larchmt). 2015;24(11):950–7. https://doi.org/10.1089/jwh.2015.5251. (In eng).
Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A brief educational intervention increases providers’ human papillomavirus vaccine knowledge. Hum Vaccin Immunother. 2015;11(6):1331–6. https://doi.org/10.1080/21645515.2015.1022691. In eng.
Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A human papillomavirus vaccination program for low-income postpartum women. Am J Obstet Gynecol. 2016;215(3):318.e1-9. https://doi.org/10.1016/j.ajog.2016.02.032. (In eng).
Berenson AB, Rupp R, Dinehart EE, Cofie LE, Kuo YF, Hirth JM. Achieving high HPV vaccine completion rates in a pediatric clinic population. Hum Vaccines Immunother. 2019;15(7–8):1562–9. https://doi.org/10.1080/21645515.2018.1533778. Article In English.
Bonafide KE, Vanable PA. Male human papillomavirus vaccine acceptance is enhanced by a brief intervention that emphasizes both male-specific vaccine benefits and altruistic motives. Sex Transm Dis. 2015;42(2):76–80. https://doi.org/10.1097/olq.0000000000000226. (In eng).
Botha MH, van der Merwe FH, Snyman LC, Dreyer G. The vaccine and cervical cancer screen (VACCS) project: acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa. S Afr Med J. 2015;105(1):40–3. https://doi.org/10.7196/samj.8419. In eng.
Calo WA, Gilkey MB, Leeman J, et al. Coaching primary care clinics for HPV vaccination quality improvement: comparing in-person and webinar implementation. Transl Behav Med. 2019;9(1):23–31. https://doi.org/10.1093/tbm/iby008. (Journal: Article).
Carolan K, Verran J, Crossley M, Redfern J, Whitton N, Amos M. Impact of educational interventions on adolescent attitudes and knowledge regarding vaccination: a pilot study. Plos One. 2018;13(1):14. https://doi.org/10.1371/journal.pone.0190984. Article In English.
Chigbu CO, Onyebuchi AK, Onyeka TC, Odugu BU, Dim CC. The impact of community health educators on uptake of cervical and breast cancer prevention services in Nigeria. Int J Gynecol Obstetr. 2017;137(3):319–24. https://doi.org/10.1002/ijgo.12150. (Article) (In English).
Cipriano JJ, Scoloveno R, Kelly A. Increasing parental knowledge related to the Human Papillomavirus (HPV) Vaccine. J Pediatr Health Care. 2018;32(1):29–35. https://doi.org/10.1016/j.pedhc.2017.06.006. (Article) (In English).
Cory L, Cha B, Ellenberg S, et al. Effects of educational interventions on human papillomavirus vaccine acceptability: a randomized controlled trial. Obstet Gynecol. 2019;134(2):376–84. https://doi.org/10.1097/aog.0000000000003379. (In eng).
Darville G, Anderson-Lewis C, Stellefson M, et al. Customization of avatars in a HPV digital gaming intervention for college-age males: an experimental study. Simul Gaming. 2018;49(5):515–37. https://doi.org/10.1177/1046878118799472. (Article).
Davies C, Skinner SR, Stoney T, et al. “Is it like one of those infectious kind of things?” The importance of educating young people about HPV and HPV vaccination at school. Sex Educ-Sex Soc Learn. 2017;17(3):256–75. https://doi.org/10.1080/14681811.2017.1300770 (Article) (In English).
Dawson R, Lemmon K, Trivedi NJ, Hansen S. Improving human papilloma virus vaccination rates throughout military treatment facilities. Vaccine. 2018;36(11):1361–7. https://doi.org/10.1016/j.vaccine.2018.02.007. (In eng).
Dempsey AF, Maertens J, Sevick C, Jimenez-Zambrano A, Juarez-Colunga E. A randomized, controlled, pragmatic trial of an iPad-based, tailored messaging intervention to increase human papillomavirus vaccination among Latinos. Human Vaccines Immunother. 2019;15(7–8):1577–84. https://doi.org/10.1080/21645515.2018.1559685. (Article) (In English).
Dempsey AF, Pyrznawoski J, Lockhart S, et al. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: a cluster randomized clinical trial. JAMA Pediatr. 2018;172(5):e180016. https://doi.org/10.1001/jamapediatrics.2018.0016. (In eng).
DiClemente RJ, Murray CC, Graham T, Still J. Overcoming barriers to HPV vaccination: A randomized clinical trial of a culturally-tailored, media intervention among African American girls. Hum Vaccin Immunother. 2015;11(12):2883–94. https://doi.org/10.1080/21645515.2015.1070996 (In eng).
Donahue K, Hendrix K, Sturm L, Zimet G. Provider communication and mothers’ willingness to vaccinate against human papillomavirus and influenza: a randomized health messaging trial. Acad Pediatr. 2018;18(2):145–53. https://doi.org/10.1016/j.acap.2017.07.007. (In eng).
Dreyer G, van der Merwe FH, Botha MH, et al. School-based human papillomavirus vaccination: An opportunity to increase knowledge about cervical cancer and improve uptake of screening. S Afr Med J. 2015;105(11):912–6. https://doi.org/10.7196/SAMJ.2015.v105i11.9814.
Edwards T, Hooper GL. A school-based intervention to increase HPV vaccination rates. J Doct Nurs Pract. 2019;12(2):196–201. https://doi.org/10.1891/2380-9418.12.2.196
Esposito S, Bianchini S, Tagliabue C, et al. Impact of a website based educational program for increasing vaccination coverage among adolescents. Human Vaccines Immunother. 2018;14(4):961–8. https://doi.org/10.1080/21645515.2017.1359453 (Article) (In English).
Ford ME, Cannady K, Nahhas GJ, et al. Assessing an intervention to increase knowledge related to cervical cancer and the HPV vaccine. Adv Cancer Res. 2020;146:115–37.
Forster AS, Cornelius V, Rockliffe L, Marlow LA, Bedford H, Waller J. A cluster randomised feasibility study of an adolescent incentive intervention to increase uptake of HPV vaccination. Br J Cancer. 2017;117(8):1121–7. https://doi.org/10.1038/bjc.2017.284 (In eng).
Gerend MA, Murdock C, Grove K. An intervention for increasing HPV vaccination on a university campus. Vaccine. 2020;38(4):725–9. https://doi.org/10.1016/j.vaccine.2019.11.028. (Article).
Grandahl M, Rosenblad A, Stenhammar C, et al. School-based intervention for the prevention of HPV among adolescents: a cluster randomised controlled study. BMJ open. 2016;6(1):e009875 (Journal Article; Randomized Controlled Trial; Research Support, Non‐U.S. Gov't).
Gualano MR, Thomas R, Stillo M, et al. What is the most useful tool in HPV vaccine promotion? Results from an experimental study. Hum Vaccin Immunother. 2019;15(7–8):1607–14. https://doi.org/10.1080/21645515.2018.1526552. (In eng).
Henrikson NB, Zhu W, Baba L, et al. Outreach and reminders to improve human papillomavirus vaccination in an Integrated primary care system. Clin Pediatr (Phila). 2018;57(13):1523–31. https://doi.org/10.1177/0009922818787868 (In eng).
Hofstetter AM, Barrett A, Camargo S, Rosenthal SL, Stockwell MS. Text message reminders for vaccination of adolescents with chronic medical conditions: A randomized clinical trial. Vaccine. 2017;35(35):4554–60. https://doi.org/10.1016/j.vaccine.2017.07.022 (Article).
Joseph NP, Bernstein J, Pelton S, et al. Brief client-centered motivational and behavioral intervention to promote HPV vaccination in a hard-to-reach population: a pilot randomized controlled trial. Clin Pediatr (Phila). 2016;55(9):851–9. https://doi.org/10.1177/0009922815616244 (In eng).
Juraskova I, Bari RA, O’Brien MT, McCaffery KJ. HPV vaccine promotion: does referring to both cervical cancer and genital warts affect Intended and Actual Vaccination Behavior? Women’s Health Issues. 2011;21(1):71–9. https://doi.org/10.1016/j.whi.2010.08.004 (Journal: Article).
Juraskova I, O’Brien M, Mullan B, Bari R, Laidsaar-Powell R, McCaffery K. HPV vaccination and the effect of information framing on intentions and behaviour: an application of the theory of planned behaviour and moral norm. International journal of behavioral medicine. 2012;19(4):518–25. https://doi.org/10.1007/s12529-011-9182-5 (Journal Article; Randomized Controlled Trial).
Kaul S, Do TQN, Hsu E, Schmeler KM, Montealegre JR, Rodriguez AM. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 2019;8:8. https://doi.org/10.1016/j.pvr.2019.100189 (Article).
Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a radionovela to promote HPV vaccine awareness and knowledge among Hispanic parents. J Commun Health. 2011;36(6):957–65. https://doi.org/10.1007/s10900-011-9395-1. (Journal Article; Randomized Controlled Trial; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.).
Kester LM, Shedd-Steele RB, Dotson-Roberts CA, Smith J, Zimet GD. The effects of a brief educational intervention on human papillomavirus knowledge and intention to initiate HPV vaccination in 18–26 year old young adults. Gynecol Oncol. 2014;132(SUPPL1):S9–12. https://doi.org/10.1016/j.ygyno.2013.12.033. (Journal: Article).
Kim J, Nan X. Effects of consideration of future consequences and temporal framing on acceptance of the HPV vaccine among young adults. Health Commun. 2016;31(9):1089–96. https://doi.org/10.1080/10410236.2015.1038774.
Kumar MM, Boies EG, Sawyer MH, Kennedy M, Williams C, Rhee KE. A brief provider training video improves comfort with recommending the human papillomavirus vaccine. Clin Pediatr (Phila). 2019;58(1):17–23. https://doi.org/10.1177/0009922818805217. (In eng).
Kwang NB, Mahayudin T, Yien HL, Abdul Karim AK, Teik CK, Shan LP. Effect of an Educational intervention on knowledge of human papillomavirus vaccination among Pre-University Students in Malaysia. Asian pacific journal of cancer prevention. 2016;17(1):267–74. https://doi.org/10.1002/central/CN-01474250/full. (Article).
Lee H, Kim M, Cooley ME, et al. Using narrative intervention for HPV vaccine behavior change among Khmer mothers and daughters: A pilot RCT to examine feasibility, acceptability, and preliminary effectiveness. Appl Nurs Res. 2018;40:51–60. https://doi.org/10.1016/j.apnr.2017.12.008 (In eng).
Lefevere E, Hens N, De Smet F, Beutels P. The impact of non-financial and financial encouragements on participation in non school-based human papillomavirus vaccination: a retrospective cohort study. Eur J Health Econ. 2016;17(3):305–15. https://doi.org/10.1007/s10198-015-0680-2 (In eng).
Lennon T, Gundacker C, Nugent M, et al. Ancillary benefit of increased HPV immunization rates following a CBPR approach to address immunization disparities in younger siblings. Journal of Community Health. 2019;44(3):544–51. https://doi.org/10.1007/s10900-018-00610-9 Article) (In English).
Lin L, Macias Parra M, Sierra VY, et al. Long-term immunogenicity and safety of the AS04-adjuvanted human papillomavirus-16/18 vaccine in four- to six-year-old girls: three-year follow-up of a randomized phase III trial. Pediatr Infect Dis J. 2019;38(10):1061–7. https://doi.org/10.1097/INF.0000000000002437. (Journal: Article).
Liu CR, Liang H, Zhang X, et al. Effect of an educational intervention on HPV knowledge and attitudes towards HPV and its vaccines among junior middle school students in Chengdu, China. BMC Public Health. 2019;19(1):488. https://doi.org/10.1002/central/CN-01953799/full. (Journal Article).
Malo TL, Gilkey MB, Hall ME, Shah PD, Brewer NT. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25(10):1383–91. https://doi.org/10.1158/1055-9965.Epi-16-0224. (In eng).
Malo TL, Hall ME, Brewer NT, Lathren CR, Gilkey MB. Why is announcement training more effective than conversation training for introducing HPV vaccination? A theory-based investigation. Implement Sci. 2018;13(1):57. https://doi.org/10.1186/s13012-018-0743-8. (In eng).
Mantzari E, Vogt F, Marteau TM. Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial. Health Psychol. 2015;34(2):160–71. https://doi.org/10.1037/hea0000088. (In eng).
Marchand-Ciriello L, Foustoukos A, Fantasia HC. Intervention to Increase human papillomavirus vaccine initiation rates in adolescent males. JNP-J Nurse Pract. 2020;16(1):79–82. https://doi.org/10.1016/j.nurpra.2019.06.018. (Editorial Material) (In English).
McGlone MS, Stephens KK, Rodriguez SA, Fernandez ME. Persuasive texts for prompting action: agency assignment in HPV vaccination reminders. Vaccine. 2017;35(34):4295–7. https://doi.org/10.1016/j.vaccine.2017.06.080. (Article).
McLean HQ, VanWormer JJ, Chow BDW, et al. Improving human papillomavirus vaccine use in an integrated health system: impact of a provider and staff intervention. J Adolesc Health. 2017;61(2):252–8. https://doi.org/10.1016/j.jadohealth.2017.02.019. (In eng).
McRee AL, Shoben A, Bauermeister JA, Katz ML, Paskett ED, Reiter PL. Outsmart HPV: Acceptability and short-term effects of a web-based HPV vaccination intervention for young adult gay and bisexual men. Vaccine. 2018;36(52):8158–64. https://doi.org/10.1016/j.vaccine.2018.01.009. (In eng).
Meyer AF, Borkovskiy NL, Brickley JL, et al. Impact of electronic point-of-care prompts on human papillomavirus vaccine uptake in retail clinics. Am J Prev Med. 2018;55(6):822–9. https://doi.org/10.1016/j.amepre.2018.06.027 (In eng).
Mohanty S, Leader AE, Gibeau E, Johnson C. Using facebook to reach adolescents for human papillomavirus (HPV) vaccination. Vaccine. 2018;36(40):5955–61. https://doi.org/10.1016/j.vaccine.2018.08.060 (In eng).
Molokwu J, Dwivedi A, Mallawaarachchi I, Hernandez A, Shokar N. Tiempo de Vacunarte (time to get vaccinated): Outcomes of an intervention to improve HPV vaccination rates in a predominantly Hispanic community. Prev Med. 2019;121:115–20. https://doi.org/10.1016/j.ypmed.2019.02.004. (In eng).
Morales-Campos DY, Parra-Medina DA. Predictors of HPV vaccine initiation and completion among hispanic mothers of 11–17 year old daughters living along the Texas-Mexico border. Cancer Epidemiology Biomarkers and Prevention 2016;25(3):B90. (Conference Abstract) (In English). https://doi.org/10.1158/1538-7755.DISP15-B90.
Nissen M, Kerkvliet JL, Polkinghorn A, Pugsley L. Increasing rates of human pipillomavirus vaccination in family practice: a quality improvement project. S D Med. 2019;72(8):354–60 (In eng).
Nwanodi O, Salisbury H, Bay C. Multimodal counseling interventions: effect on human papilloma virus vaccination acceptance. Healthcare (Basel). 2017;5(4):86. https://doi.org/10.3390/healthcare5040086. (In eng).
Obulaney PA, Gilliland I, Cassells H. Increasing cervical cancer and human papillomavirus prevention knowledge and HPV vaccine uptake through mother/daughter education. J Community Health Nurs. 2016;33(1):54 66-quiz 66-7. https://doi.org/10.1080/07370016.2016.1120595. (In eng).
Padmanabha N, Kini JR, Alwani AA, Sardesai A. Acceptability of human papillomavirus vaccination among medical students in Mangalore. India Vaccine. 2019;37(9):1174–81. https://doi.org/10.1016/j.vaccine.2019.01.032. (Article) (In English).
Parra-Medina D, Morales-Campos DY, Mojica C, Ramirez AG. Promotora outreach, education and navigation support for HPV vaccination to Hispanic women with unvaccinated daughters. J Cancer Educ. 2015;30(2):353–9. https://doi.org/10.1007/s13187-014-0680-4. (In eng).
Paskett ED, Krok-Schoen JL, Pennell ML, et al. Results of a multilevel intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiol Biomarkers Prev. 2016;25(4):593–602. https://doi.org/10.1158/1055-9965.Epi-15-1243. (In eng).
Patel DA, Zochowski M, Peterman S, Dempsey AF, Ernst S, Dalton VK. Human papillomavirus vaccine intent and uptake among female college students. J Am College Health. 2012;60(2):151–61. https://doi.org/10.1080/07448481.2011.580028. (Journal Article; Randomized Controlled Trial; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.).
Porter RM, Amin AB, Bednarczyk RA, Omer SB. Cancer-salient messaging for human papillomavirus vaccine uptake: a randomized controlled trial. Vaccine. 2018;36(18):2494–500. https://doi.org/10.1016/j.vaccine.2018.01.040. (In eng).
Poscia A, Pastorino R, Boccia S, Ricciardi W, Spadea A. The impact of a school-based multicomponent intervention for promoting vaccine uptake in Italian adolescents: a retrospective cohort study. Ann Ist Super Sanita. 2019;55(2):124–30. https://doi.org/10.4415/ann_19_02_04 (In eng).
Pot M, Paulussen TG, Ruiter RA, et al. Effectiveness of a web-based tailored intervention with virtual assistants promoting the acceptability of HPV vaccination among mothers of invited girls: randomized controlled trial. J Med Internet Res. 2017;19(9):e312. https://doi.org/10.2196/jmir.7449. (In eng).
Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313–20. https://doi.org/10.1080/10810730.2018.1442530. (In eng).
Rhodes D, Visker JD, Cox C, Sas A, Banez JC. Effects of an online educational module on school nurses’ knowledge of HPV vaccination. J Cont Educ Nurs. 2017;48(9):431–6. https://doi.org/10.3928/00220124-20170816-10. (Article) (In English).
Richman AR, Maddy L, Torres E, Goldberg EJ. A randomized intervention study to evaluate whether electronic messaging can increase human papillomavirus vaccine completion and knowledge among college students. J Am Coll Health. 2016;64(4):269–78. https://doi.org/10.1080/07448481.2015.1117466. (In eng).
Richman AR, Torres E, Wu Q, et al. Text and Email Messaging for Increasing Human Papillomavirus Vaccine Completion among Uninsured or Medicaid-insured Adolescents in Rural Eastern North Carolina. J Health Care Poor Underserved. 2019;30(4):1499–517. https://doi.org/10.1353/hpu.2019.0090.
Rickert VI, Auslander BA, Cox DS, Rosenthal SL, Rupp RE, Zimet GD. School-based HPV immunization of young adolescents: effects of two brief health interventions. Hum Vaccin Immunother. 2015;11(2):315–21. https://doi.org/10.1080/21645515.2014.1004022. (In eng).
Rockliffe L, Chorley AJ, McBride E, Waller J, Forster AS. Assessing the acceptability of incentivising HPV vaccination consent form return as a means of increasing uptake. BMC Public Health. 2018;18(1):382. https://doi.org/10.1186/s12889-018-5278-z. (In eng).
Roussos-Ross K, Foster L, Peterson HV, Decesare J. Do educational seminars for the human papillomavirus vaccine improve attitudes toward the value of vaccination? J Pediatr Adolesc Gynecol. 2017;30(4):456–9. https://doi.org/10.1016/j.jpag.2016.12.003. (Article) (In English).
Sadoh AE, Okonkwobo C, Nwaneri DU, et al. Effect of peer education on knowledge of human papilloma virus and cervical cancer among female adolescent students in Benin City. Nigeria Ann Glob Health. 2018;84(1):121–8. https://doi.org/10.29024/aogh.24. (In eng).
Schnaith AM, Evans EM, Vogt C, et al. An innovative medical school curriculum to address human papillomavirus vaccine hesitancy. Vaccine. 2018;36(26):3830–5. https://doi.org/10.1016/j.vaccine.2018.05.014. (In eng).
Shah PD, Calo WA, Gilkey MB, et al. Questions and concerns about HPV vaccine: a communication experiment. Pediatrics. 2019;143(2):10. https://doi.org/10.1542/peds.2018-1872. (Article) (In English).
Staples JN, Wong MS, Rimel BJ. An educational intervention to improve human papilloma virus (HPV) and cervical cancer knowledge among African American college students. Gynecol Oncol. 2018;149(1):101–5. https://doi.org/10.1016/j.ygyno.2017.10.015. (In eng).
Staras SAS, Vadaparampil ST, Livingston MD, Thompson LA, Sanders AH, Shenkman EA. Increasing human papillomavirus vaccine initiation among publicly insured florida adolescents. J Adolesc Health. 2015;56(5):S40–6. https://doi.org/10.1016/j.jadohealth.2014.11.024. (Article) (In English).
Stern L, Unger Z, Debevec E, Ginde S, Morfesis J, Patel A. Staying on track: a cluster randomized controlled trial of automated reminders for HPV vaccine series completion. Contraception. 2013;88(3):438–9. https://doi.org/10.1016/j.contraception.2013.05.036. (Journal: Conference Abstract).
Underwood NL, Weiss P, Gargano LM, et al. Human papillomavirus vaccination among adolescents in Georgia. Hum Vaccin Immunother. 2015;11(7):1703–8. https://doi.org/10.1080/21645515.2015.1035848. (In eng).
Rodriguez AM, Do TQN, Goodman M, Schmeler KM, Kaul S, Kuo YF. Human papillomavirus vaccine interventions in the U.S.: a systematic review and meta-analysis. Am J Prev Med. 2019;56(4):591–602. https://doi.org/10.1016/j.amepre.2018.10.033.
Xiao X, Lee DKL, Wong RM, Borah P. The impact of theory in HPV vaccination promotion research: a systematic review and meta-analysis. Am J Health Promot. 2021;35(7):1002–14. https://doi.org/10.1177/08901171211012524.
Fu LY, Bonhomme LA, Cooper SC, Joseph JG, Zimet GD. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901–20. https://doi.org/10.1016/j.vaccine.2014.01.091.
Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. https://doi.org/10.1146/annurev.publhealth.012809.103604.
Askelson N, Ryan G, McRee AL, et al. Using concept mapping to identify opportunities for HPV vaccination efforts: Perspectives from the Midwest and West Coast. Eval Program Plann. 2021;89:102010. https://doi.org/10.1016/j.evalprogplan.2021.102010.
Askelson NM, Ryan G, Seegmiller L, Pieper F, Kintigh B, Callaghan D. Implementation challenges and opportunities related to HPV vaccination quality improvement in primary care clinics in a rural state. J Community Health. 2019;44(4):790–5. https://doi.org/10.1007/s10900-019-00676-z.
Vaccination Programs: Health Care System-Based Interventions Implemented in Combination. The Community Guide. 2014 (https://www.thecommunityguide.org/findings/vaccination-programs-health-care-system-based-interventions-implemented-combination).
Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107–12. https://doi.org/10.1016/j.jclinepi.2004.09.002.
Newman PA, Logie CH, Lacombe-Duncan A, et al. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018;8(4):e019206. https://doi.org/10.1136/bmjopen-2017-019206.
Bowyer HL, Forster AS, Marlow LA, Waller J. Predicting human papillomavirus vaccination behaviour among adolescent girls in England: results from a prospective survey. J Fam Plann Reprod Health Care. 2014;40(1):14–22. https://doi.org/10.1136/jfprhc-2013-100583.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. https://doi.org/10.1097/MLR.0b013e3182408812.
Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2019;280:112513. https://doi.org/10.1016/j.psychres.2019.112513.
Daniels V, Saxena K, Roberts C, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–5. https://doi.org/10.1016/j.vaccine.2021.04.003.
Patel Murthy B, Zell E, Kirtland K, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations -10 U.S.Jurisdictions, March-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(23):840–5. https://doi.org/10.15585/mmwr.mm7023a2.
Ryan G, Gilbert PA, Ashida S, Charlton ME, Scherer A, Askelson NM. Challenges to adolescent HPV vaccination and implementation of evidence-based interventions to promote vaccine uptake during the COVID-19 pandemic: “HPV is probably not at the top of our list.” Prev Chronic Dis. 2022;19:E15. https://doi.org/10.5888/pcd19.210378.
Sonawane K, Lin YY, Damgacioglu H, et al. Trends in human papillomavirus vaccine safety concerns and adverse event reporting in the United States. JAMA Netw Open. 2021;4(9):2124502. https://doi.org/10.1001/jamanetworkopen.2021.24502.
Acknowledgements
Not applicable.
Funding
This study was supported by Centers for Disease Control and Prevention, SIP 19–005 Cancer Prevention and Control Research Network, U48DP006377, U48DP006389, U48DP006400, U48DP006413, U48DP006408 and U48DP006398. CBB was supported by a NIH Cancer Care Quality Training Program grant, UNC-CH, Grant No. T32-CA-116339. GR was supported by the National Cancer Institute Grant No. T32-CA-172009. PM was supported by P30CA023074-41. The funders had no role in the study design, data collection, analysis, and interpretation of data and in writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC or NIH.
Author information
Authors and Affiliations
Contributions
CE is responsible for the study design and oversight of the study. CE, CP, CA, MD, GR, SS, KY, CB, PM, SL, AE, LS, ED, TH and MF contributed to data abstraction and review of the manuscript. CE, CP, LS and SP contributed the analysis plan of the manuscript and they and MD, GR wrote the manuscript. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional files 1: Supplemental Table 1.
Systematic Review of HPV Vaccination Intervention Search Terms. Supplemental Table 2. Quality Assessment of Included Articles*.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Escoffery, C., Petagna, C., Agnone, C. et al. A systematic review of interventions to promote HPV vaccination globally. BMC Public Health 23, 1262 (2023). https://doi.org/10.1186/s12889-023-15876-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15876-5