Abstract
Background
Diet has long been hypothesized to play an important role in hyperuricemia, and weight gain is a factor that is strongly associated with the rise in serum urate. We aimed to clarify the mediating role of obesity in the relationship between diet and hyperuricemia and to determine whether a weight-loss diet is an effective way to prevent hyperuricemia.
Methods
This cross-sectional study analysed representative samples of United States (n = 20,081; NHANES 2007–2016) adults. Nutrient patterns were derived with two methods: principal component analysis (PCA) and reduced rank regression (RRR) with obesity. Logistic regression and multivariable linear regression were applied to analyse the association between nutrient patterns in obesity and hyperuricemia. Mediation analyses were used to determine whether four obesity indicators, including body mass index (BMI), waist circumference (WC), visceral adiposity index (VAI) and lipid accumulation product index (LAP), mediated the relationship between nutrient patterns and hyperuricemia.
Results
PCA revealed three nutrient patterns (including “Low energy diet”, “Lower vitamin A, C, K pattern” and “Vitamin B group”), and only Vitamin B group had a total effect on hyperuricemia. RRR revealed one main nutrient pattern associated with obesity, which was characterized by High fat and low vitamin levels and was significantly associated with hyperuricemia. Mediation analysis showed that obesity mostly or even completely mediated the relationship between nutrient patterns and hyperuricemia, especially traditional obesity indicators, which played a key intermediary effect. The proportions of indirect effects for BMI and WC were as high as 53.34 and 59.69, respectively.
Conclusions
Our findings suggest that the direct effect of diet on hyperuricemia is weak, and obesity plays a critical mediating role in the relationship between diet and hyperuricemia, which confirms that a weight-loss diet such as a “Low fat and high vitamin diet” may be useful in preventing hyperuricemia.
Similar content being viewed by others
Introduction
The latest Global Burden of Disease (GBD) showed that gout, the most common cause of inflammatory arthritis, affects 41 million people worldwide [1]. Hyperuricemia, as the early stage and major aetiologic factor of gout, needs to be given more attention. Hyperuricemia is caused by the elevation of plasma uric acid concentration in the human body and is defined as blood uric acid levels higher than 7.0 mg/dL (416 μmol/L) in men and 6.0 mg/dL (360 μmol/L) in women under normal dietary conditions [2, 3]. Hyperuricemia is also a potential risk factor for cardiovascular disease, type 2 diabetes, chronic kidney disease and mortality [4]. Recently, the prevalence of hyperuricemia has increased markedly worldwide, but management remains suboptimal [5].
As an important factor in many chronic diseases, diet is also hypothesized to be a contributing factor in hyperuricemia, and an increase in dietary purines leads to increased urate production [6]. According to the update on gout management, dietary modifications may be useful adjuncts to urate-lowering therapy [7]. Therefore, there has been much interest in the potential effects of dietary approaches in hyperuricemia management, and a large amount of literature has focused on evaluating the association between diet and hyperuricemia. For example, red meat, seafood, sugar-sweetened beverages, alcohol, and animal protein have been identified to be associated with a greater risk of hyperuricemia [8]. Many popular dietary patterns, such as the Med Diet Score [9], Dietary Approaches to Stop Hypertension (DASH) diet [10] and plant-based diets [11], have also been studied in relation to hyperuricemia.
On the other hand, obesity has also been hypothesized to be an important cause of elevated uric acid. For example, a longitudinal study of 2611 young adults reported that baseline BMI was positively related to a 10-year change in serum uric acid (UA) [12]. Bidirectional Mendelian randomization analyses showed that BMI was causally associated with elevated serum UA but not vice versa [13]. A randomized controlled trial found that bariatric surgery was associated with a significant urate reduction when compared with traditional therapy [14]. Another study also showed that bariatric surgery could reduce the incidence of gout, implying that obesity may be an important cause of gout [15].
Further, it is well known that dietary factors are important factors in obesity. Based on the above relationships among diet, obesity, and hyperuricemia, we naturally hypothesized that the relationship between diet and hyperuricemia may be mediated by obesity. Furthermore, we were interested in whether a weight-loss diet could have a preventive effect on hyperuricemia. In addition to body mass index (BMI) and waist circumference (WC), two other novel indicators of obesity, the visceral adiposity index (VAI) [16] and lipid accumulation product index (LAP), are also low-cost indicators and are often used to reflect obesity from different perspectives [17].
In addition, compared with a single dietary factor, dietary patterns have been widely used in nutritional research because they can reflect the overall dietary characteristics of individuals. Further, in an international research context, nutrients are universal and the nutrient patterns can be compared across varied ethnicities, so nutrient patterns may be more interpretable and much easier to translate into public health recommendations across populations [18], whereas dietary patterns may be affected by social, cultural and geographical scenarios [19]. Various approaches to dietary patterns were discussed in a review, and each method has a unique feature and serves a distinct purpose [20]. In addition to investigator-driven methods such as the Med Diet Score and Dietary Approaches to Stop Hypertension (DASH) diet, principal component analysis (PCA) and reduced rank regression (RRR) are also often used, where RRR is a hybrid method that combines a priori professional knowledge of health outcomes and the relevant relational structure of nutrients and is often used to complement data-driven methods [20].
Therefore, to further explore the relationship among nutrient patterns, obesity and hyperuricemia, the current study first identified the nutrient patterns based on two methods: principal component analysis and reduced rank regression with obesity. Furthermore, we aimed to examine the possible mediating role of multiple obesity indicators in the link between nutrient patterns and hyperuricemia by conducting mediation analyses.
Methods
Study populations
The National Health and Nutrition Survey (NHANES) is an ongoing continuous survey conducted by the Centers for Disease Control and Prevention (NCHS) to describe the health and nutritional status of the United States population [21]. Data are collected by using a complex, stratified, multistage probability cluster sampling design, and each survey cycle covers demographic data, body measurements, laboratory test results, and diet information [22]. The details of the programs, collection procedures and data files are publicly available at http://www.cdc.gov/nchs/nhanes.html. Participants in the NHANES provided written informed consent, and the study protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics and the US Army Research Institute of Environmental Medicine Human Use Review Committee [23].
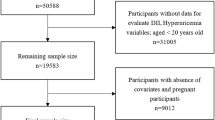
For this study, a total of 22,712 participants with reliable dietary NHANES data from 2007 to 2016 aged 20 years or older constituted the initial sample. After excluding pregnant women; individuals with missing uric acid, BMI, WC and VAI information; and those with extreme energy intake, 20,081 participants (9537 men and 10,544 women) were included in our final analyses (see Fig. 1).
Dietary information
The dietary intake data were collected via two 24-h dietary recall interviews; the first dietary recall was collected with face-to-face inquiry, and the second dietary survey was conducted by telephone 3 to 10 days after the initial recall interview [22]. The food energy and nutrient contents of each food were calculated using the USDA Food and Nutrient Database for Dietary Studies [24]. We calculated the average intake of all nutrients from the two 24-h recalls. For simplicity, we did not take into account the specific saturated, monounsaturated and polyunsaturated fatty acids because we considered the sum of them. Finally, we considered 41 major nutrients.
Assessment of mediators
Anthropometric and biochemical data were measured by NHANES researchers. WC was measured at the iliac crest by a tape measure to the nearest millimetre [22]. To assess the height and weight, participants wore their underwear, disposable paper robes and foam slippers [25]. BMI was calculated as weight in kilograms divided by the square of height in metres. A blood specimen was drawn from all study participants’ antecubital veins by a trained phlebotomist [25]. Laboratory testing details for haemoglobin A1c (HbA1c), direct HDL-cholesterol, and fasting triglycerides are provided in the NHANES Laboratory/Medical Technician Procedures Manual [22]. VAI was the integration of BMI, WC, TG and HDL: for males, \(\textrm{VAI}=\left[\frac{\textrm{WC}\left[\textrm{cm}\right]}{39.68}+\left(1.88\times \textrm{BMI}\right)\right]\times \left(\frac{\textrm{TG}\left[\textrm{mmol}/\textrm{L}\right]}{1.03}\right)\times \left(\frac{1.31}{\textrm{HDL}\left[\textrm{mmol}/\textrm{L}\right]}\right);\) for females, \(\textrm{VAI}=\left[\frac{\textrm{WC}\left[\textrm{cm}\right]}{36.58}+\left(1.89\times \textrm{BMI}\right)\right]\times \left(\frac{\textrm{TG}\left[\textrm{mmol}/\textrm{L}\right]}{0.81}\right)\times \left(\frac{1.52}{\textrm{HDL}\left[\textrm{mmol}/\textrm{L}\right]}\right)\)[26]. LAP was the indicator used to evaluate lipid accumulation, and it combined WC and triglycerides (TGs): for males, LAP = (WC[cm] − 65) × TG[mmol/L]; for females, LAP = (WC[cm] − 58) × TG[mmol/L] [27].
Serum uric acid measurement and hyperuricemia
Uric acid concentration was detected on a Beckman Synchron LX20 (Beckman Coulter, Inc., Brea, CA) using a colorimetric method [21]. Hyperuricemia was defined as uric acid ≥420 mmol/L in males and ≥ 360 mmol/L in females [28] or the use of uric acid-lowering drugs.
Confounders
Based on the associations with nutrient patterns, hyperuricemia and obesity measures, the following factors were considered confounders: age (20–39, 40–59, > 59 years), sex (male, female), race (Mexican American, non-Hispanic white, non-Hispanic black, others), income status based on poverty index (0–1.3, 1.3–3.5, > 3.5) [29], smoking status (smoking at least 100 cigarettes in lifetime or not), drinking status (had at least 12 alcohol drinks/year or not), vigorous physical activity (yes or no), creatinine level and energy intake, and history of diseases (including diabetes, hypertension, cardiovascular diseases, cancer, liver disease and dyslipidaemia). Information on all of these confounders was obtained via standardized questionnaires or instrumental measurement. Hypertension was defined as a mean systolic blood pressure (SBP) ≥140 mmHg, a mean diastolic blood pressure (DBP) ≥90 mmHg, or a self-reported hypertension diagnosis [30]. Cardiovascular diseases were defined as a positive answer to the question “Have you ever been told you had congestive heart failure/coronary heart disease/angina/heart attack/stroke?” [31]. Dyslipidaemia was defined as the use of lipid-lowering medications or a low-density lipoprotein cholesterol level of ≥140 mg/dL, a high-density lipoprotein cholesterol level of < 40 mg/dL, or a triglyceride level of ≥150 mg/dL [32].
Statistical analysis
We considered masked variance and used the weighting methodology in all analyses [33]. The survey package of R (version 4.0) was used to account for the complex sampling design [34]. The general characteristics of the participants were summarized and compared according to hyperuricemia status. All continuous variables are presented as the mean with standard deviation, and the categorical variables are presented as frequencies and percentages. Student’s t test (normally distributed data) or nonparametric test (nonnormally distributed data) was applied for continuous variables, and chi-squared tests were used for categorical variables.
Nutrient patterns were derived from 41 nutrients based on two main approaches: principal component analysis (PCA) and reduced rank regression (RRR) with obesity indicators as the response variable. PCA is a data-driven analysis, and the number of factors was decided based on eigenvalues, scree tests, and factor interpretability [35]. Nutrients with factor loadings ≥|0.2| were considered major contributors to the corresponding pattern and were retained. Orthogonal varimax rotation was applied to increase interpretability between the patterns. RRR was the second statistical approach used to derive nutrient patterns. For this method, patterns were identified based on a set of predefined response variables [36]. Four obesity indicators after log transformation were used as response variables, and we retained the main nutrient patterns in which coefficients of nutrients were below or above |0.15|. The number of nutrient patterns was determined by the number of response variables. Each participant obtained a factor score for each pattern, which indicated the degree of adherence to the specific pattern. As simple linear dose–response relationships are unlikely to be found in nutritional epidemiology, we classified participants based on the quartile of the factor scores [37]. In addition, we computed the mean of main nutrient intakes across categories of nutrient pattern scores and compared them using analysis of variance.
Both crude and adjusted weighted logistic regression models were used to investigate the association between the scores for each nutrient pattern derived by PCA and RRR with hyperuricemia: Model 1 unadjusted; Model 2 adjusted for age, sex, and race; and Model 3 additionally adjusted for smoking, drinking, vigorous physical activity, pox ratio, creatinine level, energy intake, history of diabetes, hypertension, cardiovascular diseases, cancer, liver disease and dyslipidaemia. In addition, we used multivariable-adjusted models to identify nutrient patterns associated with obesity indicators, and the lowest quartile was used as the reference group. Trend tests were also conducted. Finally, as suggested by VanderWeele [38], mediation analysis was performed to examine the potential mediating role of four obesity indicators on the relationship between nutrient patterns and hyperuricemia. Odds ratios (ORs) and 95% CIs for direct effects and indirect effects were calculated using the bootstrap method. The proportion of mediation was calculated using ORDE(ORIE − 1)/(ORDEORIE − 1), where ORDE is the OR for the direct effect and ORIE is the OR for the indirect effect.
Sensitivity analyses were applied as the same steps above for mediation analysis in a new population that further excluded individuals who were taking uric acid-lowering drugs based on the above study populations and used continuous urate level as the outcome. All statistical analyses were conducted with SAS 9.4 and R 3.6.3. All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
General characteristics of study participants
The baseline characteristics of the participants according to hyperuricemia status are shown in Table 1. Of the 20,081 study participants, 18.38% had hyperuricaemia. In general, participants with hyperuricemia were more likely to be older, male, and non-Hispanic black and have higher levels of obesity indicators (BMI, WC, VAI and LAP) and creatinine and lower levels of physical activity than those without hyperuricemia. In addition, a higher proportion of those classified as hyperuricemia smoked more, and a higher proportion of them suffered from other diseases.
In addition, in terms of demographics and health-related factors, there were no significant differences between the sample analysed in this study and the total NHANES (2007–2016) sample (Supplemental Table S1).
Nutrient patterns
Principal component analysis
We derived 3 independent nutrient patterns based on the principal component analysis of a complex survey, which explained 71.6% of the total variance.
The first pattern was negatively correlated with protein, fat, carbohydrate, cholesterol, choline, sodium and selenium, therefore it was termed “Low energy intake”. The second pattern was negatively correlated with vitamin A, vitamin C, vitamin K, carotene, and lutein, therefore it was termed “Lower vitamin A, C, K pattern”. The third pattern was positively correlated with vitamin B6, B12, and folate, therefore it was termed “Vitamin B group”. The factor loadings for each nutrient pattern are shown in Supplemental Table S2.
Reduced rank regression
Only “High fat and low vitamin diet”was kept for further analyses based on RRR, since it explained the largest variance (20.01%) of the response variables. It was positively correlated with fat and cholesterol and a positive correlation with vitamin A, C, D, K, fibre and folate, therefore it was termed “High fat and low vitamin diet”. The factor loadings of the pattern and the correlation coefficients with the response variables are shown in Supplemental Table S3.
Supplemental Table S4 additionally showed the mean of main nutrient intakes across categories of nutrient pattern scores. The average intake of major nutrients showed a significant increase or decrease trend with the increase of the corresponding nutrient pattern scores (p < 0.001).
Nutrient patterns and the risk of hyperuricemia and obesity
Multivariate logistic regression analyses of the associations between the 4 nutrient patterns and hyperuricemia are shown in Table 2. After adjusting for all confounders (Model 3), there were two patterns that were significantly related to hyperuricemia. Among them, “Vitamin B group” wasbased on principal component analysis, compared to the first quartile as a reference, and the ORs were 0.81 (0.67, 0.99), 0.75 (0.63, 0.89) and 0.63 (0.51, 0.77), respectively. In addition, “High fat and low vitamin diet”, based on RRR, was significantly related to hyperuricemia compared with the lowest quartile, and the adjusted OR indicated a dose-dependent relationship with each quartile increment (P for trend < 0.001). The OR in the highest quartile was 1.23 (1.06, 1.41).
The results of multivariable linear regression analysis are shown in Table 3. All four nutrient patterns were correlated with BMI, WC and LAP (P for trend < 0.05). Furthermore, the VAI was also significantly correlated with “High fat and low vitamin diet”.
Mediating role of obesity indicators in the association between nutrient patterns and hyperuricemia
Table 4 presents the direct and indirect effects of nutrient patterns on hyperuricemia with obesity measures as mediators. Overall, all four obesity indicators mediated the relationship between each nutrient pattern and hyperuricemia. Furthermore, the direct effects of the other three nutrient patterns in relation to hyperuricemia were almost nonsignificant except for Vitamin B group. The findings suggest that the association of each nutrient pattern with hyperuricemia was mediated by obesity. Although the indirect and direct effects were in opposite directions for the two nutrient patterns and the proportion of indirect effects in this case could not be explained, we found that obesity measures (BMI, WC, LAP) fully mediated the relationship between “High fat and low vitamin diet”, based on RRR, and hyperuricemia. In particular, two common obesity measures (BMI and WC) had significant mediating effects on the relationships between all four nutrient patterns and hyperuricemia, and the mediating proportions were as high as 53.34 and 59.69, respectively. In addition, LAP also mediated the relationship between three nutrient patterns and hyperuricemia, although the indirect effect was not as large as that of BMI and WC.
Sensitivity analyses
Sensitivity analysis showed similar results: “Vitamin B group” was negatively correlated with blood uric acid while “High fat and low vitamin pattern” were positively associated with urate level (Supplemental Table S5). Compared to the first quartile as reference, the subjects in the highest quartile of the Vitamin B group were associated with lower uric acid levels (p < 0.01), for the High fat and low vitamin pattern, the uric acid level of the highest quantile increased by 0.19 (0.13,0.25) compared with the lowest quantile. In addition, all four nutrient patterns were correlated with BMI, WC and LAP (Supplemental Table S6), which were also shown to be important mediators in the nutrient patterns and uric acid pathways (Supplemental Table S7).
Discussion
In this study, we used both PCA and RRR to derive the nutrient patterns and explored their relationship with hyperuricemia and obesity. We found that Vitamin B group and the nutrient pattern related to weight loss had a significant total effect on hyperuricemia. Furthermore, the associations between all four nutrient patterns and hyperuricemia were mediated by obesity in a large proportion. The significant mediating effect of obesity combined with the significant total effect of hyperuricemia based on a reduced-rank regression suggests that a weight-loss diet may be an effective way to prevent elevated uric acid.
With principal component analysis, we obtained three nutrient patterns, and only Vitamin B group had a significant total effect on hyperuricemia after adjusting for all covariates. The relationship between Vitamin B group and urate level is still controversial, but there are some studies about individual B vitamins that may support our conclusions. For example, another NHANES study on individual B vitamins indicated that the intakes of folate and vitamin B12 were inversely related to the risk of HU in males, and only folate was found in females [39]. The findings from an in vitro and in vivo animal study showed that Aster glehni along with vitamin B6 might be used as functional nutrients in reducing serum uric acid levels in gout [40]. Another randomized controlled trial showed that uric acid was significantly decreased after 4 and 8 weeks of supplementation with vitamin B-12 and fish oil [41]. Furthermore, according to the results of a substudy of the China Stroke Primary Prevention Trial, compared with enalapril alone, the combination of enalapril and folic acid could reduce the magnitude of the increase in UA concentrations in hypertensive adults, which implied that high folic acid intake may be an adjuvant nutritional recommendation for preventing and treating hyperuricemia [42]. However, a Norwegian randomized controlled study in patients with coronary artery disease did not find any significant effect of folic acid and vitamin B-12 treatment on the risk of hyperuricemia [43]. According to the literature, this discrepancy could be due to important differences in the population characteristics and treatment regimen between the 2 studies [44]. Our study found that overall, Vitamin B group had a certain effect on hyperuricemia; however, more clinical trials are required for further verification and need to further clarify which B vitamins should be included and at what dose to maximize uric acid reduction.
Furthermore, the indirect effect of obesity was significant in the relationship of all four nutrient patterns to hyperuricemia, while the direct effect was small to none. This finding is consistent with recent studies on the relationship of diet to hyperuricemia by using the method of population attributable fractions (PAFs). In a Mendelian randomized study, the effects of four dietary patterns on hyperuricemia were fully mediated by BMI. In addition, the article also points out BMI produced PAFs for hyperuricemia of 59–69%, while diet had a relatively minor role in lowering uric acid in the three nongout cohorts [45]. Another study of the Third National Health and Nutrition Examination Survey reported that the corresponding PAFs of hyperuricemia cases for overweight or obese and nonadherence to a DASH-style diet were 44 and 8%, respectively [46]. All these findings supported our results that the association between diet and hyperuricaemia was mediated by obesity in a large proportion [47]. It is well known that diet plays a critical role in obesity and further has a subsequent risk of hyperuricemia, so the total effect of diet is greater than the PAF estimated for diet (direct effect and independent of BMI), as shown in previous studies [46, 48]. In addition, only the direct effect of vitamin B group on hyperuricemia was significant, which implied that other potential mechanisms existed in addition to obesity. Previous studies have shown several other potential mechanisms. According to reports, folic acid can effectively reduce total homocysteine, thereby reducing intracellular adenosyl-homocysteine, which may induce marked DNA damage and release purine nucleotides that result in the generation of UA [43].
To further illustrate that the relationship between diet and hyperuricemia is largely mediated by obesity, we performed reduced rank regression and obtained one main pattern associated with obesity, “High fat and low vitamin” diet, which had a significant promoting effect on hyperuricemia, and the effect was entirely caused by weight gain. In fact, a variety of studies show that greater BMI is associated with an increased risk of hyperuricemia and that weight loss is an important measure for the prevention and management of hyperuricemia and gout [46, 49, 50]. For example, a cohort study including 1189 patients observed over 6.45 years showed that obesity (BMI > 27) was an independent risk factor for incident gout among women with and without hyperuricaemia [51]. Furthermore, Mendelian randomization studies have found that obesity is causally associated with serum urate levels in the general population [52], a systematic review of randomized controlled trials found a significant reduction in serum uric acid levels following orlistat therapy in adults [50]. In addition, a meta-analysis including 20 cohort studies assessed the relationship of bariatric surgery with gout and serum urate, and the results showed that uric acid decreased by an average of 0.73 mg/dL 3 months after bariatric surgery and 1.91 mg/dL 3 years after surgery [53]. The 2020 ACR gout treatment guideline also recommends that people with hyperuricemic gout who are obese or overweight should use a weight loss program [49]. Furthermore, our research also found that VAI and LAP have certain direct and indirect effects, suggesting that metabolism-related obesity has an important effect on hyperuricemia, and we cannot ignore them. In conclusion, the results of the reduced rank regression further suggest that obesity is an important mediator in the pathway between dietary patterns and hyperuricemia. The significant total effect suggests that nutrient patterns for weight loss may be an effective way to prevent and treat hyperuricemia. This pattern is consistent with current recommended diets (e.g., Mediterranean, Dietary Approaches to Stop Hypertension (DASH), Nordic, vegan, vegetarian) that are associated with weight loss [54], and this pattern has a lower correlation with obesity-related chronic diseases [55, 56].
The present study has several strengths. First, we identified nutrient patterns based on two methods: principal component analysis and reduced rank regression with obesity. The two methods complement each other, and both suggest that obesity largely mediates the relationship between diet and hyperuricemia. Therefore, we further determined that one nutrient pattern for weight loss had a significant effect on the prevention of hyperuricemia. Second, a variety of obesity indicators (BMI, WC, VAI and LAP) were used to examine the potential mediating role of obesity in the relationship between nutrient patterns and hyperuricemia. Different obesity indicators can reflect multiple perspectives of obesity. In particular, VAI and LAP incorporate indicators such as sex and metabolism, which makes our analysis more comprehensive. Third, our analyses were based on a large sample population and used a complex sampling design, so the results can be extrapolated to the general population. Fourth, the patterns were based on nutrients rather than food groups, which makes it easier for nutritionists to provide public nutrition recommendations. Finally, we adjusted for many key confounding variables.
However, our study has some limitations that need to be noted. First, due to the observational design, a possible causal relationship cannot be established, and this needs to be addressed in future cohort studies. Second, we adjusted for many potential confounders, but we cannot completely rule out unmeasured confounders. Third, a more detailed analysis needs to be performed in future studies. For example, future studies should consider subdividing the population into two subgroups, those suffering from gout and those not suffering from gout, and then analyse the subgroups separately. In addition, each obesity index was used as the response variable to perform reduced rank regression to obtain a specific nutrient pattern and analyse its effect on hyperuricemia.
Conclusion
In summary, this study explored the relationship among diet, various obesity indicators and hyperuricemia based on two methods. Studies have shown that obesity largely mediates the relationship between nutrient patterns and the risk of hyperuricemia. Our findings suggest that the direct effect of diet on hyperuricemia is weak, and obesity plays a critical mediating role in the pathway between diet and hyperuricemia, which confirms the findings of recent studies that dietary factors contribute far less to hyperuricemia than obesity because diet has little direct effect on uric acid. Furthermore, the significant mediating effect of obesity combined with the significant total effect of hyperuricemia, based on a reduced-rank regression, suggests that a weight-loss diet may be a useful adjunct to urate-lowering therapy.
Availability of data and materials
The datasets generated and analysed during the current study are publicly available from the National Center for Health Statistics at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Abbreviations
- BMI:
-
Body mass index
- VAI:
-
Visceral adiposity index
- WC:
-
Waist circumference
- LAP:
-
Lipid accumulation product index
- NHANES:
-
The National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- PCA:
-
Principal component analysis
- RRR:
-
Reduced rank regression
References
Collaborators, G.D.a.H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–922. https://doi.org/10.1016/s0140-6736(18)32335-3.
Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. https://doi.org/10.1038/ncomms1756.
Trivedi N. Uric acid and cardiovascular risk. N Engl J Med. 2009;360(5):539 author reply 540-1.
Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Ren Physiol. 2006;290(3):F625–31. https://doi.org/10.1152/ajprenal.00140.2005.
Robles-Cervantes JA, Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, Valencia-Sandoval C, Torres-Chávez A, et al. Relationship between serum concentration of uric acid and insulin secretion among adults with type 2 diabetes mellitus. Int J Endocrinol. 2011;2011:107904. https://doi.org/10.1155/2011/107904.
Danve A, Sehra ST, Neogi T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35(4):101723. https://doi.org/10.1016/j.berh.2021.101723.
Afinogenova Y, Danve A, Neogi T. Update on gout management: what is old and what is new. Curr Opin Rheumatol. 2022;34(2):118–24. https://doi.org/10.1097/bor.0000000000000861.
Gaffo AL, Saag KG. Management of hyperuricemia and gout in CKD. Am J Kidney Dis. 2008;52(5):994–1009. https://doi.org/10.1053/j.ajkd.2008.07.035.
Zhu Y, Pandya B, Choi H. Prevalence of gout and hyperuricemia in the US general population: the National Health and nutrition examination survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–41. https://doi.org/10.1002/art.30520.
Chrysohoou C, Georgiopoulos GA, Georgousopoulou EN. Chapter 31 - how nutrition affects kidney function in aging. In: Malavolta M, Mocchegiani E, editors. Molecular basis of nutrition and aging. San Diego: Academic Press; 2016. p. 423–31.
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–103. https://doi.org/10.1056/NEJMoa035700.
Rathmann W, Haastert B, Icks A, Giani G, Roseman JM. Ten-year change in serum uric acid and its relation to changes in other metabolic risk factors in young black and white adults: the CARDIA study. Eur J Epidemiol. 2007;22(7):439–45. https://doi.org/10.1007/s10654-007-9132-3.
Feng Y, Fu M, Guan X, Wang C, Yuan F, Bai Y, et al. Uric acid mediated the association between BMI and postmenopausal breast Cancer incidence: a bidirectional Mendelian randomization analysis and prospective cohort study. Front Endocrinol (Lausanne). 2021;12:742411. https://doi.org/10.3389/fendo.2021.742411.
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. https://doi.org/10.1056/NEJMoa035622.
Maglio C, Peltonen M, Neovius M, Jacobson P, Jacobsson L, Rudin A, et al. Effects of bariatric surgery on gout incidence in the Swedish obese subjects study: a non-randomised, prospective, controlled intervention trial. Ann Rheum Dis. 2017;76(4):688–93. https://doi.org/10.1136/annrheumdis-2016-209958.
Wu J, Gong L, Li Q, Hu J, Zhang S, Wang Y, et al. A novel visceral adiposity index for prediction of type 2 diabetes and pre-diabetes in Chinese adults: a 5-year prospective study. Sci Rep. 2017;7(1):13784. https://doi.org/10.1038/s41598-017-14251-w.
Wiltgen D, Benedetto IG, Mastella LS, Spritzer PM. Lipid accumulation product index: a reliable marker of cardiovascular risk in polycystic ovary syndrome. Hum Reprod. 2009;24(7):1726–31. https://doi.org/10.1093/humrep/dep072.
Jacobs DR, Tapsell LC. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 2010;10:439–50.
Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Feizi A, Feinle-Bisset C, et al. Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: findings from the SEPAHAN study. Eur J Nutr. 2016;55(2):505–18. https://doi.org/10.1007/s00394-015-0867-4.
Zhao J, Li Z, Gao Q, Zhao H, Chen S, Huang L, et al. A review of statistical methods for dietary pattern analysis. Nutr J. 2021;20(1):37. https://doi.org/10.1186/s12937-021-00692-7.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. 2013;1(56):1–37.
Borrud L, Chiappa MM, Burt VL, Gahche J, Dohrmann SM. National Health and nutrition examination survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat. 2014;162:1–24.
Ferguson C, Knol L, Ellis A. Visceral adiposity index and its association with dietary approaches to stop hypertension (DASH) diet scores among older adults: National Health and nutrition examination surveys 2011-2014. Clin Nutr. 2021;40(6):4085–9. https://doi.org/10.1016/j.clnu.2021.02.008.
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–34. https://doi.org/10.3945/an.115.009258.
Mazidi M, Kengne A, George E, Siervo M. The association of red meat intake with inflammation and circulating intermediate biomarkers of type 2 diabetes is mediated by central adiposity. Br J Nutr. 2021;125(9):1043–50. https://doi.org/10.1017/s0007114519002149.
Amato M, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2. https://doi.org/10.2337/dc09-1825.
Onat A, Avci GS, Barlan MM, Uyarel H, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28(8):1018–25.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk — NEJM; 2008.
Sun Y, Sun J, Zhang P, Zhong F, Cai J, Ma A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019;10(8):4932–40. https://doi.org/10.1039/c8fo01917g.
Carey R, Whelton P. Prevention, detection, evaluation, and Management of High Blood Pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association hypertension guideline. Ann Intern Med. 2018;168(5):351–8. https://doi.org/10.7326/m17-3203.
Moon S, Yu SH, Chung HS, Kim YJ, Yu JM, Kim SJ, et al. Association of nocturia and cardiovascular disease: data from the National Health and nutrition examination survey. Neurourol Urodyn. 2021;40(6):1569–75. https://doi.org/10.1002/nau.24711.
Kuwabara M, Kuwabara R, Niwa K, Hisatome I, Smits G, Roncal-Jimenez CA, et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia according to level of body mass index in Japanese and American subjects. Nutrients. 2018;10(8). https://doi.org/10.3390/nu10081011.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll M, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital and health statistics. Series 2. Data Eval Methods Res. 2013;161(161):1–24.
Meneses-León J, León-Maldonado L, Macías N, Torres-Ibarra L, Hernández-López R, Rivera-Paredez B, et al. Sugar-sweetened beverage consumption and risk of hyperuricemia: a longitudinal analysis of the health workers cohort study participants in Mexico. Am J Clin Nutr. 2020;112(3):652–60. https://doi.org/10.1093/ajcn/nqaa160.
Iwasaki Y, Arisawa K, Katsuura-Kamano S, Uemura H, Tsukamoto M, Kadomatsu Y, et al. Associations of nutrient patterns with the prevalence of metabolic syndrome: results from the baseline data of the Japan multi-institutional collaborative cohort study. Nutrients. 2019;11(5):990. https://doi.org/10.3390/nu11050990.
Hoffmann K, Schulze MB, Schienkiewitz A, Nöthlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159(10):935–44. https://doi.org/10.1093/aje/kwh134.
Willett W. Nutritional Epidemiology; 2012.
VanderWeele TJ. Mediation analysis: a Practitioner's guide. Annu Rev Public Health. 2016;37:17–32. https://doi.org/10.1146/annurev-publhealth-032315-021402.
Zhang Y, Qiu H. Folate, vitamin B6 and vitamin B12 intake in relation to Hyperuricemia. J Clin Med. 2018;7(8). https://doi.org/10.3390/jcm7080210.
Han EH, Lim MK, Lee SH, Kim HJ, Hwang D. Synergic effect in the reduction of serum uric acid level between ethanol extract of Aster glehni and vitamin B(6). Food Sci Biotechnol. 2018;27(5):1439–44. https://doi.org/10.1007/s10068-018-0394-1.
Huang T, Li K, Asimi S, Chen Q, Li D. Effect of vitamin B-12 and n-3 polyunsaturated fatty acids on plasma homocysteine, ferritin, C-reaction protein, and other cardiovascular risk factors: a randomized controlled trial. Asia Pac J Clin Nutr. 2015;24(3):403–11. https://doi.org/10.6133/apjcn.2015.24.3.19.
Qin X, Li Y, He M, Tang G, Yin D, Liang M, et al. Folic acid therapy reduces serum uric acid in hypertensive patients: a substudy of the China stroke primary prevention trial (CSPPT). Am J Clin Nutr. 2017;105(4):882–9. https://doi.org/10.3945/ajcn.116.143131.
Dierkes J, Seifert R, Gregory JF, Nygård O. Long-term supplementation with folic acid and vitamin B-12 has no effect on circulating uric acid concentrations in Norwegian patients with coronary artery disease. Am J Clin Nutr. 2018;107(1):130–2. https://doi.org/10.1093/ajcn/nqx017.
Qin X, Li Y, Hou FF. Reply to J Dierkes et al. Am J Clin Nutr. 2018;107(1):132–3. https://doi.org/10.1093/ajcn/nqx018.
Topless RKG, Major TJ, Florez JC, Hirschhorn JN, Cadzow M, Dalbeth N, et al. The comparative effect of exposure to various risk factors on the risk of hyperuricemia: diet has a weak causal effect. Arthritis Res Ther. 2021;23(1):75. https://doi.org/10.1186/s13075-021-02444-8.
Choi HK, McCormick N, Lu N, Rai SK, Yokose C, Zhang Y. Population impact attributable to modifiable risk factors for Hyperuricemia. Arthritis. Rheumatol. 2020;72(1):157–65. https://doi.org/10.1002/art.41067.
Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92(2):246–9. https://doi.org/10.2105/ajph.92.2.246.
Major TJ, Topless RK, Dalbeth N, Merriman TR. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. Bmj. 2018;363:k3951. https://doi.org/10.1136/bmj.k3951.
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60. https://doi.org/10.1002/acr.24180.
Noori S, Mirzababaei A, Amini MR, Clark CCT, Mirzaei K. Effect of orlistat on serum uric acid level in adults: a systematic review and meta-analysis of randomised controlled trials. Int J Clin Pract. 2021;75(11):e14674. https://doi.org/10.1111/ijcp.14674.
Chen JH, Pan WH, Hsu CC, Yeh WT, Chuang SY, Chen PY, et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care Res. 2013;65(1):133–40. https://doi.org/10.1002/acr.21824.
Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, et al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7(6):e39321. https://doi.org/10.1371/journal.pone.0039321.
Yeo C, Kaushal S, Lim B, Syn N, Oo AM, Rao J, et al. Impact of bariatric surgery on serum uric acid levels and the incidence of gout-a meta-analysis. Obes Rev. 2019;20(12):1759–70. https://doi.org/10.1111/obr.12940.
Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. https://doi.org/10.1038/nutd.2017.3.
Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8(16):e012865. https://doi.org/10.1161/jaha.119.012865.
Shahavandi M, Amini MR, Shahinfar H, Shab-Bidar S. Major dietary patterns and predicted cardiovascular disease risk in an Iranian adult population. Nutr Health. 2021;27(1):27–37. https://doi.org/10.1177/0260106020952591.
Acknowledgements
The data used in this study were from the NHANES. We thank all the staff of and participants in the NHANES for their contribution.
Funding
This work was supported by the funds of the National Natural Science Foundation of China (Grant numbers: 82073674). This work was also supported in part by the Shanxi Science and Technology Research of China (202103021223234) and the Innovation Foundation for Graduate Student of Shanxi Province (2020BY065).
Author information
Authors and Affiliations
Contributions
Tong Wang designed the study and Juping Wang wrote the main manuscript text. Data preprocessing and analysis were carried out by Junkang Zhao and Jie Liang; Shuting Chen and Simin He prepared all the tables; Qian Gao and Xue Gao reviewed and edited the manuscript; and supervision was carried out by Tong Wang. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the institutional review board of the National Center for Health Statistics (NCHS). Written informed consent was obtained from all participants/patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Comparison of the characteristics between the analytical sample and full NHANES (2007–2016) sample. Table S2. Factor loadings of three nutrient patterns based on principal component analysis. Table S3. Factor loadings of nutrient pattern based on RRR and Spearman’s correlation coefficient with four obesity measures. Table S4. Mean of nutrient intakes across quarters of nutrient pattern scores. Table S5. Multivariate regression analysis relating nutrient patterns to uric acid level. Table S6. The association between nutrient patterns and obesity indicators for sensitivity analysis. Table S7. Mediating effects of obesity on the association between nutrient patterns and urate level.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Chen, S., Zhao, J. et al. Association between nutrient patterns and hyperuricemia: mediation analysis involving obesity indicators in the NHANES. BMC Public Health 22, 1981 (2022). https://doi.org/10.1186/s12889-022-14357-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14357-5