Abstract
Background
Providing universal access to safe water, sanitation and hygiene (WASH) in remote Nepal remains challenging. We investigated WASH conditions and their association with children’s nutritional status, intestinal parasitic infections and diarrhoea.
Methods
Data was collected through a cross-sectional survey of 1427 households, including questionnaires, observations, stool analysis, anthropometry, water quality measurements, and assessment of clinical signs of nutritional deficiencies.
Results
We found 55.5% of children were undernourished, 63.9% had clinical signs of nutritional deficiencies, 51.1% had intestinal parasitic infections and 52.2% had diarrhoea. Multivariate mixed logistic regression analysis revealed a statistically significant negative association between undernutrition and socio-economic level, with adjusted odds ratios (AOR) of 0.70 (95%-CI = 0.43–1.11) and 0.43 (95%-CI = 0.25–0.75) for high and intermediate levels compared to the lowest level. Undernutrition was negatively associated with regular deworming of children (AOR = 0.44, 95% CI = 0.20–0.94), food supplements (AOR = 0.57, 95% CI = 0.38–0.84), household’s own food production (AOR = 0.67, 95% CI = 0.46–0.97) and personal hygiene (AOR = 0.83, 95% CI = 0.51–1.35). Nutritional deficiency was negatively associated with handwashing after cleaning a baby’s bottom (AOR = 0.60, 95% CI = 0.40–0.92) and cleanliness of caregiver’s hands (AOR = 0.61, 95% CI = 0.41–0.89) and positively associated with keeping animals inside the house overnight (AOR = 1.71, 95% CI = 1.17–2.51) and the presence of total coliforms in the drinking water source (AOR = 10.44, 95% CI = 1.61–67.4). Diarrhoea was positively associated with intermittent water supply (AOR = 2.72, 95% CI = 1.18–6.31) and the presence of a mud floor (AOR = 2.29, 95% CI = 1.20–4.37) and negatively associated with cleanliness of the toilet (AOR = 0.68, 95% CI = 0.47–0.98), and the cleanliness of children’s hands (AOR = 0.62, 95% CI = 0.40–0.96).
Conclusions
Our study found, more than half of the survey children were in a critical health condition. Results suggest that child health improvements are dependent on multiple public health improvements, including providing better nutrition, promoting adequate hygiene behaviour, such as handwashing, keeping the latrines clean, keeping the household environment free from animal faeces and assuring a reliable supply of safe water.
Similar content being viewed by others
Background
Children in low-income countries face a range of interrelated problems, such as poor nutrition, inadequate water, sanitation, and hygiene (WASH), consequent infections, and growth and development impairments [1]. Globally, a total of 297,000 WASH-attributable diarrhoea deaths occur per year among children under 5 years, every fifth child’s growth is stunted, one in thirteen is wasted, and every seventh child is underweight. Nearly 90% of these cases occur in South Asia and Sub-Saharan Africa [2,3,4]. Furthermore, two billion people worldwide are infected with intestinal parasites, with the highest burden of this disease among children in resource-poor settings [5,6,7]. Studies have shown that infections with intestinal parasites among children are associated with stunting, physical weakness, and low educational performance [6, 8, 9].
Nutrition is closely interlinked with multiple determinants [10, 11]. While malnutrition is directly associated with insufficient dietary intake, underlying contributing factors, such as lack of access to safe water and sanitation, result in such recurrent infectious diseases as diarrhoea and intestinal worms. These parasites interfere with the digestive process by competing with the host for nutrients and inhibiting the absorption of nutrients, leading to compromised immunity [10, 11]. It is estimated that up to 45% of global malnutrition-related child deaths could be prevented by improving WASH conditions and practices [4, 12, 13].
Even though 89% of the population in Nepal currently has access to at-least basic water supply services and 62% to basic sanitation facilities, providing safe water quality at the point of consumption and ensuring adequate hygiene practices remain challenges [14, 15]. In a recent study, 31.5% of children in the Eastern region of Nepal were found to be infected with intestinal parasites. Parasitic infections were significantly associated with not using soap after defecation, the habit of thumb sucking, and not wearing sandals [16]. However, the health and nutritional status of children and their associations with nutrition and WASH have not been extensively investigated in remote hilly areas of Nepal. The Demographic and Health Survey (DHS) 2016 showed that 1 in 25 children in Nepal dies before reaching the age of 5 years, and almost 3500 die yearly from preventable causes [17, 18]. Diarrhoea is one of the most common illnesses among children and continues to be a major cause of childhood morbidity and mortality [17]. However, efforts to combat health and nutritional problems among children in these settings do not effectively incorporate WASH interventions. Hence, the aim of this study was to assess the influence of nutrition practices and WASH infrastructure on the nutritional and health status of children aged 6 months to 10 years in three rural hilly areas of Nepal. The findings from this study provide a crucial benchmark for delivering subsequent public health interventions.
Methods
Study area
The survey area was located in the districts of Surkhet (A and B), Achham, and Dailekh in the Karnali province of Nepal. The sites were selected according to the following criteria: (a) mountainous region with extremely remote location, (b) availability of a piped water supply scheme in communities WARM-P training (i.e. hygiene education) has not taken place, and (c) the population not having access to products for household water treatment.
Study design, sample population, sample size and sampling methods
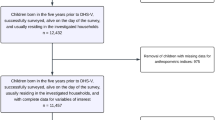
This cross-sectional study was conducted from March to May 2018 and involved 1427 households with children aged 6 months to 10 years. Sample size and statistical power were calculated using G*Power 3.1. A sample size of 300 households was required at each of the four sites to detect an effect in Cohen’s f2 at one-tailed alpha of 0.05 and a statistical power of 90% with mixed logistic regression and 15 predictor variables adjusting for clustering effect of the study site [19, 20]. We therefore randomly sampled a minimum of 345 households at each of the four sites.
Questionnaire survey
A quantitative, structured questionnaire was administered to the children’s caregivers (mostly mothers; Supplementary file 1). The questionnaire was developed following international guidelines and there were both closed and open-ended questions developed from standardized questions following international guidelines [21, 22]. The questionnaire was coded in Open Data Kit software on tablets (Samsung Galaxy note 10.1 N8010) [23] and contained questions on the use of water sources, psychological factors concerning water handling and hygiene practices, observations of WASH infrastructure, information on WASH promotion activities received, nutrition provided to children, and children’s history of waterborne illness in the past 7 days. The interviews were complemented by structured observations and the questionnaires were pretested and adapted to meet the conditions of the study site [24].
Child diet, household food security and signs of nutritional deficiency
Child dietary information was assessed following the guidelines of the Food and Agricultural Organisation (FAO) [22]. The caregivers were requested to recall whether nine different food groups were consumed within the past 7 days and, if consumed, the frequency of consumption. The supervisors randomly re-interviewed a subset of 10% of the surveyed households to assess reproducibility. Household food security was assessed with questions relating to the availability of food during the entire year.
Certified medical assistants screened children for the clinical signs of nutritional deficiencies using a standard checklist. They were examined for; (a) wasted appearance, (b) loss of hair pigment and easy pluckability, (c) bitot’s spots, (d) dry and infected cornea, (e) oedema, (f) several types of dermatitis, (g) spongy bleeding gums, (h) pale conjunctiva, (i) red inflamed tongue, (j) sub-dermal haemorrhage, (k) bowed legs, and (l) goitre [25].
Anthropometric measurements
The children were examined for anthropometric measurements; height or length and weight, adhering to standard procedures [26]. Supine lengths were obtained from children younger than 2 years using Seca BabyMat 210 and for children aged between 24 months and 10 years, using a height-measuring board and a digital scale (Seca 877; Hamburg, Germany) [24]. Anthropometric indices were calculated using AnthroPlus (WHO; Geneva, Switzerland) in accordance with the World Health Organisation (WHO) guidelines [26, 27]. Three anthropometric indices were expressed as z-scores (i.e. differences from the median in standard deviations): (a) weight for age (WAZ, underweight), (b) height for age (HAZ, stunting), and (c) body mass index for age (BMIZ, thinness) [4]. Z-scores of ≥ − 2 were regarded as normal, those between < − 2 and ≥ − 3 as moderate undernutrition and those below <− 3 as severe undernutrition. Children were considered to be undernourished if at least one of the anthropometric indices indicated undernutrition [26, 27].
Parasitological survey
Caregivers were asked to provide a fresh morning stool sample without urine contamination from the participating child on the day following the household survey. The samples were processed on the same day by experienced laboratory technicians. Each stool sample was analysed using direct wet-mount and formalin-ether concentration techniques following standard guidelines [28,29,30]. In addition, a duplicate Kato-Katz thick smear was prepared for the diagnosis of helminths [31]. The presence of infection by any worm species was defined by the detection of one or more eggs on either slide [7]. The infection intensity of helminths was calculated according to criteria defined by the WHO and multiplied by 24 to reach the total number of eggs per gram (EPG) of stool [5, 32]. Stool samples were obtained from 962 children.
Drinking water quality examination
Water samples were collected from the household’s main drinking water source and from the container used for drinking water transport and storage. The sample at the source was taken after letting the water run for 60 s from the tap. Caregivers were requested to bring fresh drinking water from the source to the household in the same container they usually use for this [33]. Water samples for analysis were poured into sterile Nasco Whirl Pak bags and immediately analysed using the membrane filtration technique: 100 mL water samples were passed through sterile 0.45 μm millipore cellulose membrane filters with sterilized filtration equipment. The filter pads were plated on Nissui Compact Dry Coli-scan plates and incubated for 24 h at 35 +/− 2 °C. Colony-forming units of total coliforms and Escherichia coli (E. coli) were counted after 24 h of incubation [15].
Data management and statistical analysis
Data cleaning was performed daily, and if any values were missing or inconsistent, the respective household was consulted the following day. Readings of intestinal parasite and nutritional deficiency screenings were double entered into an Excel 2010 spread sheet (Microsoft; Redmond, USA) and cross-checked. Numerical variables were described by means and standard deviations if normally distributed and by medians and interquartile range otherwise. Categorical variables were described by absolute and relative frequencies. We employed χ2 statistics to assess the differences in distribution of categorical variables between the study areas. Household socioeconomic status was characterized based on factor analysis of reported household assets. Three factors reflecting three socioeconomic domains were retained and divided using the k-means procedure into three categories; (a) low, (b) medium, and (c) high [34]. The same procedure was applied to create one variable for the cleanliness of containers used for transport and storage of drinking water, latrine hygiene, cleanliness of the household environment and kitchen, and personal hygiene. For each of these variables, three factors were retained and categorized, indicating (a) low, (b) intermediate, and (c) high categories.
We assessed four health-related outcome variables: (a) undernutrition (i.e. stunting, underweight and thinness) (b) nutritional deficiencies (c) intestinal parasitic infection and (d) diarrhoea. Since only a few undernutrition cases were severe, the cases were pooled into a binary variable of stunted/non-stunted, and underweight/non-underweight for the subsequent analysis. Similarly, there was a low prevalence of parasites, such as T. trichiura, E. vermicularis and Ancylostoma duodenale. Therefore, all reported intestinal parasitic infections were pooled into a binary variable of parasite infection/no infection to maximize statistical power. Nutritional deficiencies and diarrhoea outcomes were coded into binary variables for the subsequent comparative analysis.
We assessed associations between the binary outcome variables and hypothesized risk factors using mixed logistic regression models with random intercepts of study sites, controlling for potential confounding by age, sex, and socioeconomic status. First, the associations between outcome variables and risk factors were assessed using univariate models. Variables with P-values < 0.2 were retained for the final model [35]. Odds ratios were reported and the associations were considered as statistically significant if P-values were < 0.05. The statistical analysis was performed with STATA version 14 (STATA Corporation, College Station, TX, USA).
Results
Socio-demographic characteristics of the study participants
The socio-demographic and socioeconomic characteristics of the interviewed households are provided in Table A in the supplementary materials. Caregivers aged 25–39 years constituted the largest group (57.9%) of interviewees. More than 80% of the caregivers could both read and write. Agriculture was the main (60.6%) occupation of the household heads. The majority of children (99.1%) included in the study were between 6 months and 5 years of age, while 0.9% were between 6 to 10 years of age. 59.7% of the households kept animals inside the home overnight and the majority (84.1%) of the households had mud floors. Around 52.7% of the households across the study sites had access to electricity.
Child-feeding practices and household food security
Almost all caregivers (99.6%) reported having breastfed the participating child until the age of 6 months. The dietary diversity scores were low with 11.2% of the households having consumed all nine listed food groups in the previous 7 days of the survey (Table B and Table C, supplementary materials). The consumption of milk/milk products and eggs at least once per week was 9.2 and 5.3%, respectively (Table C, supplementary materials). About 40% of the households did produce their own food, among which 20.8% reported self-sufficient yearly food production.
Prevalence of nutritional deficiencies and associated risk factors
A total of 63.9% of the children in the study suffered from at least one sign of a nutritional deficiency. About one third (35.9%) of the children suffered from pale conjunctiva, followed by Bitot’s spots (19.8%), red inflamed tongue (18.3%), spongy bleeding gums (16.3%), wasted appearance (13.8%), dry and infected cornea (13.2%), loss of hair pigment (10.7%), sub-dermal haemorrhage (4.6%), oedema (2.7%), bowed legs (2.6%), and goitre (0.6%) (Table 1).
Children > 5 years old had twice the odds, all else constant, of having nutritional deficiencies compared to their younger counterparts (AOR = 1.84; 95% CI: 1.30–2.62; P = 0.01). Children whose caregivers washed their hands after cleaning a baby’s bottom were at lower odds of having nutritional deficiencies (AOR = 0.60; 95% CI = 0.40–0.92; P = 0.02) compared to children whose caregivers did not follow such practice. Children living in houses where animals were kept inside overnight had 1.71 times higher odds of having signs of nutritional deficiencies (AOR = 1.71; 95% CI = 1.17–2.51; P = 0.01) than their counterparts. Children from households producing their own food were significantly better protected against nutritional deficiencies (AOR = 0.51; 95% CI: 0.35–0.76; P = 0.01) than were children without their own food production. Children from households in the category of high latrine hygiene were at lower odds of nutritional deficiencies (AOR = 0.61; 95% CI: 0.41–0.91; P < 0.001) than those living in households with low latrine hygiene. Similarly, a high level of kitchen hygiene decreased children’s odds of nutritional deficiencies compared to low kitchen hygiene (AOR = 0.4; 95% CI 0.22–2.76; P = 0.008). Being in the intermediate or lower category of personal hygiene increased a child’s odds for clinical signs of nutritional deficiencies by 1.84 and being in the higher category by 1.9 (AOR = 1.84; 95% CI: 1.22–2.76; P = 0.005 and AOR = 1.9; 95% CI: 1.17–3.1; P = 0.005). Children from households with coliform bacteria in their drinking water sources had 10.4 times higher odds of having symptoms of nutritional deficiencies (AOR = 10.4; 95% CI: 1.61–67.42; P = 0.01) than children from households with an uncontaminated water source (Table 2).
Prevalence of undernutrition and associated risk factors
Table 1 shows the percentage distribution of undernutrition in the study sample by sex, age group, and study sites. The prevalence of undernutrition was 55.5%, while the prevalence of stunting was 44.5%, thinness 11.2%, and underweight 29.9%.
Table 3 provides an overview of the association between undernutrition and associated risk factors in univariate and multivariate regression analysis. A higher and intermediate socioeconomic status had a significant negative association with undernutrition with odds ratios of 0.70 (95% CI: 0.43–1.11) and 0.43 (95% CI: 0.25–0.75), respectively. Children of caregivers with sound knowledge about the importance of regular deworming had lower odds of undernutrition (AOR = 0.44; 95% CI = 0.20–0.94; P = 0.03) than children having caregivers lacking such knowledge. Children receiving supplementary food were at significantly lower odds of being undernourished (AOR = 0.57; 95% CI: 0.38–0.84; P = 0.01) than children without supplementary food. Similarly, children from households producing their own food were at significantly lower odds of being undernourished (AOR = 0.67; 95% CI: 0.46–0.97; P = 0.03) than those from households without agricultural production.
Prevalence of intestinal parasites and associated risk factors
Tables 4 and 5 show the prevalence of intestinal parasitic infections in the study population and associated risk factors. The overall prevalence of intestinal parasitic infection is 51.1%. The predominant helminth species infecting the children were Ascaris lumbricoides (21.1%), followed by Hymenolepsis nana (4.6%), Ancylostoma duodenale (3.2%), Enterobius vermicularis (2.7%), and Trichuris trichiura (0.7%). Polyparasitism and co-infection were not common. About 23.4% of the children were infected with Giardia intestinalis.
Multivariate analysis showed that children in households with a simple pit latrine for defecation had seven times higher odds of being infected with intestinal parasites than those in households with a pour flush pit latrine (AOR = 7.47; 95% CI:1.91–29.3; P = 0.006). Children with caregivers having clean hands had significantly better odds of protection from intestinal parasitic infection (AOR = 0.61; 95% CI: 0.42–0.89; P = 0.01) than those with caregivers having dirty hands (Table 5).
Water handling, water quality, sanitation, hygiene, and WASH promotion
Table D in the supplementary materials and Table 6 describes water handling, water quality, sanitation, hygiene practices and WASH promotion in the four study sites. Some 75.5% of the respondents depend on a communal village tap for drinking purposes and 20.7% had access to piped water in the house or yard. More than half (54.4%) of the respondents were confident about the safety of their available drinking water. 16.5% of the households reported treating their water at the point of use and one third (33.7%) reported disliking the taste of treated water.
We found that the majority of water samples from the point of collection and point of use were contaminated with E. coli (93.6 and 95.3%, respectively) and total coliform bacteria (99.4 and 98.8%, respectively). Five percent of water samples at the point of consumption met the WHO’s guidelines for microbial safety of drinking water (< 1 CFU E. coli/100 mL), 16.0% were in the low risk category (1–10 CFU E. coli/100 mL), 51.0% in the intermediate risk category (10–100 CFU E. coli/100 mL), and 28% in the high and very high risk categories (> 100 CFU E. coli/100 mL) [36].
We found that 6.3% of the households did not have latrines, and 93.7% had pit latrines. Almost half of the latrines (48.7%) were in a poor hygienic state. Three quarters (76.0%) of the respondents reported having washed their hands with soap less than five times per day prior to the day of the survey. The overall hygiene conditions were very low/ low in 64.0% of the surveyed households. Around 10% of the respondents reported having received information on water treatment and hygiene. Among those, 89.7% reported that the information changed their WASH behaviour, such as using soap more often for washing hands (Table 6).
Prevalence of diarrhoea and associated risk factors
Table 7 presents the association of risk factors with diarrhoea. A total of 16.5% of children < 5 years suffered from diarrhoea within 7 days prior to the survey. The results from the multivariate regression analysis showed that children > 5 years old had significantly lower odds of diarrhoea (AOR = 0.39; 95% CI: 0.27–0.58; P = 0.01) than their younger counterparts. Children from the households experiencing a service interruption at the collection point of their main drinking water supply scheme of more than 1 week at the time of the visit had 2.87 higher odds of diarrhoea (AOR = 2.72; 95% CI: 1.18–6.31; P = 0.02) than children not experiencing such an interruption. Children of caregivers who were aware of the need for handwashing during critical times, such as when they looked dirty, were significantly better protected against diarrhoea (AOR = 0.47; 95% CI: 0.32–0.71; P = 0.01) than children of unaware caregivers. Children from households with clean latrines were significantly better protected against diarrhoea (AOR = 0.68; 95% CI: 0.47–0.98; P = 0.04) than those from other households. Similarly, children with visually clean hands were significantly better protected against diarrhoea than those with dirty hands (AOR = 0.62; 95% CI: 0.40–0.96; P = 0.03). Children living in households with a floor made of mud painted with animal dung had 2.29 times higher odds of suffering from diarrhoea than children living in households with a cement floor (AOR = 2.29; 95% CI: 1.20–4.37; P = 0.01).
Child health and health-seeking behaviours
Table E in the supplementary materials shows the percentage distribution of child health records, health-seeking behaviours, and knowledge, attitude and practices related to health and hygiene. A total of 49.9% of the children from < 6 months to 5 years were reported to have been sick within 7 days prior to the survey. Respiratory illnesses and fevers were most common (both 40.4%), followed by diarrhoea (16.5%).
A majority (82.7%) of the respondents knew that contaminated water can cause diarrhoea. However, a majority (78.9%) had never heard about intestinal parasites. The proportions of caregivers who were aware that handwashing with soap might prevent intestinal parasitic infections were 11.1%, wearing shoes 5.0%, drinking clean water 16.6%, and undergoing regular deworming treatment 11.1%.
Discussion
Our findings highlight alarming health conditions among children in the remote areas of rural Nepal where the study took place. While more than half of the surveyed children were infected with parasites and suffered from undernutrition and nutritional deficiencies, the prevalence of diarrhoea was slightly lower. Our analysis identified specific risk factors for each of these health outcomes.
Undernutrition
The high prevalence of undernutrition in our study sites could be explained by high poverty rates. Undernutrition was linked less to hygiene-related risk factors and more to the low socioeconomic status of the household and poor nutrition. These findings are in line with the results of recent randomised evaluations of WASH and nutrition interventions, which found that nutritional interventions significantly reduced child stunting or thinness, whereas WASH interventions, delivered either separately or in a combined fashion, showed no such effects on child health outcomes [37,38,39]. Although the relationship between undernutrition and intestinal parasitic infections is not well understood, undernutrition may be caused by recurring infections in the gut, which limit the proper absorption of calories and nutrients [40, 41]. Our findings, which identified an association between undernutrition and intestinal parasitic infection, are in agreement with studies conducted elsewhere [41, 42]. However, in contrast to a previous study conducted in Bangladesh, our study did not identify diarrhoea infection as a risk factor for undernutrition [43]. Because this study relies on a cross-sectional design, we do not have any longitudinal information on the frequency and severity of diarrhoea cases occurring in the study population. We expect that chronic diarrhoea and environmental enteropathy are likely linked with undernutrition; however, this hypothesis cannot be confirmed in the present study [39, 44, 45].
We observed that unsafe water was used to wash feeding and storage containers, unhygienic kitchen cloths were used to dry children’s utensils, caregivers did not wash their hands with soap while preparing and feeding children and food was not hygienically stored. In addition, 76.8% of the households had flies indoors and in their surroundings. The recurrent food-borne infections are likely to have contributed to nutritional deficiency, environmental enteropathy, and consequent undernutrition [39, 41, 46, 47]. Similar observations of unsafe WASH practices and inadequate food hygiene were reported in a study conducted elsewhere in Nepal [48].
Clinical signs of nutritional deficiencies
The prevalence of 63.9% of children having at least one clinical sign for a nutritional deficiency was high. Due to the dearth of studies conducted on children with clinical signs of nutritional deficiencies in Nepal and other similar contexts, it is difficult draw meaningful comparisons with other studies. The most frequently encountered sign of a nutritional deficiency, pale conjunctiva indicates iron-deficiency and anaemia and, thus, can be related to the lack of animal protein in the diet.
In contrast to our findings on the risk factors associated with undernutrition, clinical signs of nutritional deficiencies were significantly associated with water quality and various hygiene factors. Our analysis identified a significant protective association with handwashing, improved latrine cleanliness, better hygiene in the kitchen, and household’s own production of food. A higher risk for a nutritional deficiency was associated with poor water quality at the source, keeping animals inside the house overnight, and the low personal hygiene of caregivers and of children. Further in-depth research is required to provide more insight into these issues.
Intestinal parasitic infections
The high prevalence of intestinal parasitic infections among children in our study is similar to or higher than the rate reported in studies conducted in other areas of Nepal [16, 18, 49]. The higher infection rates may be explained by the fact that our study areas were located in extremely remote and hilly areas with difficult road access and a lack of infrastructure, which together results in a low level of access to basic health and WASH services [16, 18, 49]. Our analysis showed that children from households with simple pit latrines had higher odds of developing an intestinal parasitic infection than did those with water sealed latrines. The effect of inadequate sanitary conditions on intestinal parasitic infections was also documented in a systematic review and meta-analysis conducted by Ziegelbauer et al. (2012) [50].
The cleanliness of caregivers’ hands was identified as a significant risk factor for children’s parasitic infections, suggesting that caregivers’ hands play a critical role in transferring parasites from the household environment to their children. We observed poor handwashing conditions and a limited presence of soap and water at the handwashing stations. The importance of clean hands to preventing parasitic infections is in agreement with previous studies conducted in eastern Nepal [16, 51]. There is strong evidence that a high load of pathogens in the household environment and inadequate handwashing increase the density of pathogens on caregivers’ hands [52]. The association between inadequate sanitation, insufficient hygiene and infections with intestinal parasites has also been documented by studies conducted in other parts of Nepal [16, 49, 53].
Diarrhoea
We observed a very strong association between diarrhoea prevalence and the children living in a house with a mud floor, similar to studies conducted in Bangladesh [54, 55]. The cultural practice of painting mud floors in the home with animal dung remains widespread in the study area, indicating a need for this potential driver of exposure to be given increased attention. We hypothesize that the practice of painting floors with cow dung leads to a high load of diarrhoea causing pathogens in the household environment at orders of magnitude higher than concentrations found in drinking water, thus masking the impact of clean drinking water on children’s health. Children playing on the floor inside or around their houses are at high risk of ingesting pathogens [52, 56]. This assumption is confirmed by several studies that report an association between E. coli contamination of the floor with the disposal of faeces and the presence of animals close to the household. Kwong et al. reported that 35% of children put their hands in their mouths after touching soil particles, putting them at risk of contamination [55]. Additionally, we observed that animals were often kept in or near the home and brought indoors overnight. Such practices have been shown to increase exposure to faecal contamination in the household environment in other rural settings [15, 57,58,59]. Other studies conducted in India and Bangladesh highlighted the importance of faecal contamination of animal origin in the domestic environment, including source and stored drinking water, hands, and soil [43, 44].
A strong association was also found between diarrhoea incidence and reported interruptions of the water supply. Underlying reasons for this might be the subsequent lack of water for hygienic purposes. In addition, intermittent water services present an increased need for storage at the household level and, therefore, the potential for recontamination. The risk of pathogen infiltration into the piped network, might also be greater during such low-pressure events [58, 59]. Similar results were reported in a study conducted in low- and middle-income countries, which reported that the provision of high-quality piped water, sewer connections, and the use of water filters were associated with considerable reductions in diarrhoea [60].
Conclusion
In our study, more than half of the children living in the remote hilly areas of Nepal suffered from impaired nutritional status, nutritional deficiencies, intestinal parasitic infections, and to a lesser degree, diarrhoea disease. A better nutritional status of children was only indirectly linked to WASH factors. The odds of children having parasitic infections and diarrhoea incidence were both highly associated with poor hand hygiene and inadequate sanitation. Keeping animals in the household overnight and painting mud floors with animal dung were identified as important risk factors for child diarrhoea and nutritional deficiencies. Consequently, interventions to reduce the load of pathogens transmitted by animals into the household environment could be promising for improving children’s health and require further investigation. To reduce diarrhoea risk, our findings also highlight the importance of having access to a safe, reliable and continuous supply of water, which is necessary for adequate hygiene practices.
Availability of data and materials
The dataset and the questionnaire supporting the conclusions are available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- BMIZ:
-
Body Mass Index Z score
- EPG:
-
Eggs per gram
- FAO:
-
Food and Agricultural Organisations
- HAZ:
-
Height for age Z score
- KAP:
-
Knowledge, attitude and practices
- SDC:
-
Sustainable development goals
- WAZ:
-
Weight for age Z score
- WASH:
-
Water, sanitation, and hygiene
- WARM-P:
-
Water resource management programme
- WHO:
-
World Health Organisation
References
Grimes JET, Tadesse G, Gardiner IA, Yard E, Wuletaw Y, Templeton MR, et al. Sanitation, hookworm, anemia, stunting, and wasting in primary school children in southern Ethiopia: baseline results from a study in 30 schools. PLoS Negl Trop Dis. 2017;11:e0005948.
Waage J, Banerji R, Campbell O, Chirwa E, Collender G, Dieltiens V, et al. The millennium development goals: a cross-sectoral analysis and principles for goal setting after 2015: lancet and London international development Centre commission. Lancet. 2010;376:991–1023.
Adelo B, Temesgen S. Undernutritional status of children in Ethiopia: application of partial proportional odds model. Etiyopyadaki Çocukların Yetersiz Beslenme Durumu Kısmi Oransal Odds Model Uygulaması. 2015;7:77–89.
Mshida HA, Kassim N, Mpolya E, Kimanya M. Water, sanitation, and hygiene practices associated with nutritional status of under-five children in semi-pastoral communities Tanzania. Am J Trop Med Hyg. 2018;98:1242–9.
WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis : report of a WHO expert committee. Geneva: World Health Organization; 2002.
Gelaw A, Anagaw B, Nigussie B, Silesh B, Yirga A, Alem M, et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13:304.
Freeman MC, Chard AN, Nikolay B, Garn JV, Okoyo C, Kihara J, et al. Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasit Vectors. 2015;8:412.
Nokes C, Bundy DAP. Does helminth infection affect mental processing and educational achievement? Parasitol Today. 1994;10:14–8.
Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DAP. Parasitic helminth infection and cognitive function in school children. Proc R Soc Lond B. 1992;247:77–81.
Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–71.
Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34.
Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–81.
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51.
Marks S, Diener A, Bhatta M, Sihombing D, Meierhofer R. Researching water quality, consumer preference and treatment behavior. Water Supply and Treatment. 2015;16:26–7.
Meierhofer R, Bänziger C, Deppeler S, Kunwar BM, Bhatta M. From water source to tap of ceramic filters-factors that influence water quality between collection and consumption in rural households in Nepal. Int J Environ Res Public Health. 2018;15:2439.
Sah RB, Bhattarai S, Yadav S, Baral R, Jha N, Pokharel PK. A study of prevalence of intestinal parasites and associated risk factors among the school children of Itahari, Eastern Region of Nepal. Trop Parasitol. 2013;3:140–4.
Ministry of Health and Population [Nepal], New ERA, and ICF international Inc. Nepal Demographic and Health Survey 2016. Claverton: Ministry of Health and Population, New ERA, and ICF International; 2016.
Tandukar S, Sherchand JB, Xue J, Uprety S, Sherchan SP, Bhandari D, et al. Prevalence and associated risk factors of Giardia duodenalis infection among school-going children in Nepal. Parasitol Res. 2018;117:287–93.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60.
UNICEF and WHO. Core questions on drinking-water and sanitation for household surveys. Joint Monitoring Programme updates 2006. New York; Geneva: United Nations Children’s fund; World Health Organisation; 2006.
Marias Y, Glasauer P. Guidelines for assessing nutrition-related knowledge, attitudes and practices. Rome: Food and Agriculture Organization of the United Nations; 2014.
Open Data Kit. 2018. https://opendatakit.org/. Accessed 20 Oct 2019.
Miller LC, Joshi N, Lohani M, Singh R, Bhatta N, Rogers B, et al. Head growth of undernourished children in rural Nepal: association with demographics, health and diet. Paediatr Int Child Health. 2016;36:91–101.
Webb GP. Nutrition. maintaining and improving health, 4th edition. Baco Raton: CRC Press; 2012.
De Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1_suppl_1):S27–36.
De Onis M. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7.
Cheesbrough M. District Laboratory Practice in Tropical Countries. 2nd edition. Cambridge: University Press; 2006.
Neimeister R, Logan AL, Egleton JH, Kleger B. Evaluation of direct wet mount parasitological examination of preserved fecal specimens. J Clin Microbiol. 1990;28:1082–4.
Utzinger J, Botero-Kleiven S, Castelli F, Chiodini PL, Edwards H, Köhler N, et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin Microbiol Infect. 2010;16:267–73.
Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400.
WHO. Soil-transmitted helminthiases: eliminating as public health problem soil-transmitted helminthiases in children: progress report 2001–2010 and strategic plan 2011-2020. Geneva: World Health Organization; 2012.
WHO. Guidelines for drinking-water quality: fourth edition incorporating first addendum. Geneva: World Health Organization; 2017.
Bartholomew DJ. The foundations of factor analysis. Biometrika. 1984;71:221–32.
Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, Shrestha A, et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the plateau central and Centre-Ouest regions of Burkina Faso. Parasit Vectors. 2016;9:554.
WHO. Guidelines for drinking water quality: surviellance and control of community supplies. 2nd ed. Geneva: World Health Organisation; 1997.
Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health. 2018;6:e302–15.
Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2018;6:e316–29.
Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–5.
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60.
Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505.
USAID. WASH and nutrition-water and development strategy and multi-sectoral nutrition strategy implementation brief. Washington, D.C: United states Agency for International Development; 2018.
Ferdous F, Das SK, Ahmed S, Farzana FD, Latham JR, Chisti MJ, et al. Severity of diarrhea and malnutrition among under five-year-old children in rural Bangladesh. Am J Trop Med Hyg. 2013;89:223–8.
Guerrant RL, Schorling JB, McAuliffe JF, Souza MAD. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg. 1992;47(1_Suppl):28–35.
George CM, Oldja L, Biswas S, Perin J, Sack RB, Ahmed S, et al. Unsafe child feces disposal is associated with environmental enteropathy and impaired growth. J Pediatr. 2016;176:43–9.
Ngure FM, Reid BM, Humphrey JH, Mbuya MN, Pelto G, Stoltzfus RJ. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann N Y Acad Sci. 2014;1308:118–28.
Pan-American Health Organization, World Health Organisation. Guiding principles for complementary feeding of the breastfed child. Washington, DC: PAHO/WHO; 2003.
Gautam OP, Schmidt W-P, Cairncross S, Cavill S, Curtis V. Trial of a novel intervention to improve multiple food hygiene behaviors in Nepal. Am J Trop Med Hyg. 2017;96:1415–26.
Shakya B, Shrestha S, Madhikarmi NL, Adhikari R. Intestinal parasitic infection among school children. J Nepal Health Res Counc. 2012;10:20–3.
Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162.
Okyay P, Ertug S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health. 2004;4:64.
Julian TR. Environmental transmission of diarrheal pathogens in low and middle income countries. Environ Sci Process Impacts. 2016;18:944–55.
Shrestha A, Narayan KC, Sharma R. Prevalence of intestinal parasitosis among school children in Baglung districts of Western Nepal. Kathmandu Univ Med J KUMJ. 2012;10:3–6.
Ercumen A, Pickering AJ, Kwong LH, Arnold BF, Parvez SM, Alam M, et al. Animal feces contribute to domestic fecal contamination: evidence from E. coli measured in water, hands, food, flies, and soil in Bangladesh. Environ Sci Technol. 2017;51:8725–34.
Kwong LH, Ercumen A, Pickering AJ, Unicomb L, Davis J, Luby SP. Hand- and object-mouthing of rural Bangladeshi children 3–18 months old. Int J Environ Res Public Health. 2016;13:563.
Pickering AJ, Julian TR, Marks SJ, Mattioli MC, Boehm AB, Schwab KJ, et al. Fecal contamination and diarrheal pathogens on surfaces and in soils among Tanzanian households with and without improved sanitation. Environ Sci Technol. 2012;46:5736–43.
Daniel D, Diener A, van de Vossenberg J, Bhatta M, Marks SJ. Assessing drinking water quality at the point of collection and within household storage Containers in the Hilly Rural Areas of mid and far-Western Nepal. Int J Environ Res Public Health. 2020;17(7):2172. https://doi.org/10.3390/ijerph17072172.
Kumpel E, Nelson KL. Mechanisms affecting water quality in an intermittent piped water supply. Environ Sci Technol. 2014;48:2766–75.
Kumpel E, Nelson KL. Intermittent water supply: prevalence, practice, and microbial water quality. Environ Sci Technol. 2016;50:542–53.
Wolf J, Prüss-Ustün A, Cumming O, Bartram J, Bonjour S, Cairncross S, et al. Systematic review: assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: systematic review and meta-regression. Tropical Med Int Health. 2014;19:928–42.
Acknowledgements
We would like to thank all caregivers in the households for their participation and their commitment. We would like to thank Prof. Dr. Christian Schindler from the Swiss Tropical and Public Health Institute for checking the statistical analysis and providing input to the manuscript. We also would like to thank the team of the Helvetas Swiss Intercooperation Nepal especially Rubika Shrestha and Madan Bhatt for their support and technical assistance during the field work. Lastly, we would like to thank our field team for their efforts in data collection.
Funding
This work was supported by the Swiss Agency for Development and Cooperation and the REACH project. The donors had no role in study design or data collection or analysis or the preparation of the manuscript or the decision to publish the manuscript.
Author information
Authors and Affiliations
Contributions
The following authors contributed to the study design: RM, AS and SM. RM, AS, JS and DD coordinated the field and laboratory work, and supervised the research assistants. AS, RM, JS, DD performed the statistical analysis and AS, RM and SM drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Kantonale Ethikkommission, Zurich in Switzerland (KEK, reference no. 2018–00089) and the Nepal Health Research Council, Kathmandu in Nepal (NHRC, reference no. 2956). Primary caretakers of the children provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Questionnaire for the caretakers.
Additional file 2: Table A
. characteristics of the study population.
Additional file 3: Table B
. Detailed information on Children’s nutritional status in the four study sites.
Additional file 4: Table C
. Nutrition provided to children between 6 months and 10 years.
Additional file 5: Table D
. Water supply, water handling and water quality.
Additional file 6: Table E
. Child health, health seeking behaviour and awareness on health protecting behaviours.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shrestha, A., Six, J., Dahal, D. et al. Association of nutrition, water, sanitation and hygiene practices with children’s nutritional status, intestinal parasitic infections and diarrhoea in rural Nepal: a cross-sectional study. BMC Public Health 20, 1241 (2020). https://doi.org/10.1186/s12889-020-09302-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-020-09302-3