Abstract
Background
Although anemia is one of the leading causes of the global burden of disease, information about its prevalence in Russia is mostly missing. We therefore assessed its prevalence and associated factors in a Russian population.
Methods
The population-based Ural Eye and Medical Study included 5899 (80.5%) out of 7328 eligible individuals (mean age:59.0 ± 10.7 years;range:40–94 years) who underwent a standardized interview and detailed general examination. The definition of anemia was based on the hemoglobin concentration (definition #1:hemoglobin concentration < 140 g/L in men,< 130 g/L in women; definition #2:hemoglobin concentration < 130 g/L in men,< 120 g/L in women [World Health Organization definition]).
Results
Higher hemoglobin concentration (mean:142.6 ± 14.8 g/L; range:80-171 g/L) was associated (multivariable analysis) with male gender (P < 0.001; standardized regression coefficient beta:-0.20), higher waist-hip circumference ratio (P < 0.001;beta:0.05), higher prevalence of car ownership (P < 0.001;beta:0.05), higher blood concentrations of bilirubin (P < 0.001;beta:0.05) and triglycerides (P < 0.001;beta:0.06), lower erythrocyte sedimentation rate (P < 0.001;beta:-0.32), and shorter blood clotting time (P < 0.001;beta:-0.39). Using definition #1 and #2, anemia was detected in 1385 individuals (23.6%;95% confidence interval CI)CI:22.5,24.7) and in 453 individuals (7.7%;95%CI:7.0,8.4), respectively. Prevalence of moderate anemia (hemoglobin concenttration:110 g/L-80 g/L), detected in 165 individuals (2.8%;95%CI:2.4,3.2), increased with younger age (P = 0.008;odds ratio (OR):0.98;95%CI:0.96,0.99), female gender (P < 0.001;OR:2.52;95%CI:1.47,4.33), higher erythrocyte sedimentation rate (P < 0.001;OR:1.08;95%CI:1.06,1.09), longer blood clotting time (P < 0.001;OR:8.56;95%CI:5.68,12.9), and marginally significantly, with a lower waist-hip circumference ratio (P = 0.058;OR:0.13;95%CI:0.02,1.07). In women, it was significantly (P < 0.001) higher before menopause (8.8%;95%CI:6.4,11.1) than after menopause (3.5%;95%CI:2.8,4.3).
Conclusions
In this Russian population as compared to populations from countries with a similar socio-demographic index, anemia prevalence was relatively low. As in other populations, higher anemia prevalence was strongly and inversely associated with menopause, and to a minor degree, with lower waist-hip circumference ratio and lower socio-economic background.
Similar content being viewed by others
Background
The Global Burden of Diseases, Injuries and Risk Factors (GBD) Study estimated in 2010 that the global prevalence of anemia was 32.9%, and that anemia caused 68.4 million YLDs (years lived with disability) or 8.8% of all YLDs [1, 2]. That figure was higher than the figures for major depression (63.2 million YLD; 8.2%), chronic respiratory diseases (49.3 million YLDs, 6.3%) and the whole of all injuries (47.2 million YLDs; 6.3%). The anemia-associated YLDs increased in all ages between 1990 and 2010, driven by a combination of increased prevalence and population growth in the young age groups. In adulthood, the increase in the anemia-related YLDs was attributed to population growth and enhanced survival of chronic diseases associated with anemia, such as chronic kidney disease. In elderly groups, the increase in the anemia-associated YLDs was mainly due to population aging while the anemia prevalence in the elderly age groups decreased from all causes except for malaria. In most regions and age groups, anemia prevalence was higher in females than in males [1,2,3].
Despite the general importance of anemia for public health and although Russia is by area the largest, and by population one of the most populous countries worldwide, information about the prevalence of anemia in Russia and factors associated with the occurrence of anemia in Russia has been mostly missing so far. We therefore conducted this study to assess the prevalence of anemia in a population in Russia and to explore its associations with other factors [4, 5].
Methods
Designed as a population-based investigation, the Ural Eye and Medical Study (UEMS) was carried out in the Ufa (urban region of Kirovskii), the capital of the Russian Republic of Bashkortostan, and in villages of the Karmaskalinsky District located in a distance of 65 km from Ufa [6]. Bashkortostan (total population: 4.07 million) is located in the west of the southern Ural Mountains. The population of Ufa with 1.1 million inhabitants includes Russians, Tatars, Bashkirs, Ukrainians and other ethnicities. While there were no exclusion criteria, the only inclusion criteria for the participation in the study were an age of 40 or more years and living in the study region.
The series of examinations started with a standardized interview which was performed by trained social workers. It consisted of more than 250 questions about socioeconomic variables (e.g., educational level), life style including diet, smoking and alcohol consumption, depression and suicidal ideation, physical activity, and medical history including known diagnosis and therapy of major systemic diseases including presence or history of previous iron-deficiency related anemia. The data were reported using the Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement guidelines [7].
The physical examinations included measurement of blood pressure, pulse rate and body height, body weight and hip and waist circumference. Using a dynamometer, we determined the handgrip strength. We measured hearing loss by hearing loss-related questions in the interview and by conducting Rinne’s test and Weber’s test. The assessment of blood samples, which were taken under fasting conditions, included a complete blood cell count and determinations of the serum concentrations of glucose, blood lipids, C-reactive protein, bilirubin, urea, creatinine, and other substances. The pulmonary function was tested by spirometry. Diabetes mellitus was defined by a glucose concentration of ≥7.0 mmol/L or a self-reported history of a physician-related diagnosis of diabetes or a history of drug treatment of diabetes. Using the Center for Epidemiologic Studies Depression Scale (CES-D) Scoresheet, we assessed the presence of depression. We applied the State-Trait Anxiety Inventory (STAI) to determine trait and state anxiety.
Anemia was defined by a hemoglobin concentration of less than 140 g/L in men and of less than 130 g/L in women (definition #1), and it was defined by a hemoglobin concentration of less than 130 g/L in men and of less than 120 g/L in women (definition #2). The reason to use two definitions of anemia was that anemia has not yet been generally defined in a consensus meeting or corresponding article. While the second anemia definition applied in our study accorded with the WHO (World Health Organization) definition of anemia, the first definition was based on the several previous population-based studies which addressed the prevalence of anemia and which used different definitions of anemia [1,2,3,4,5, 8, 9]. Anemia was classified into mild anemia (definition #1: hemoglobin concentration of 139 g/L to 110 g/L in men and of 129 g/L to 110 g/L in women; and definition #2: hemoglobin concentration of 129 g/L to 110 g/L in men and of 119 g/L to 110 g/L in women), and into moderate anemia (both definitions #1 and #2) with a hemoglobin concentration ranging between less than 110 g/L and 80 g/L in both gender. Severe anemia was defined by a hemoglobin concentration of less than 80 g/L.
We used a commercially available statistical software program (Statistical Package for Social Science, SPSS, version 25.0; IBM-SPSS Inc., Chicago, USA) for the statistical analysis. In a first step, we determined the mean hemoglobin concentration, expressed as mean ± standard deviation, and the mean prevalence of anemia, presented as mean and its 95% confidence intervals (CI). In a second step, we searched for associations in univariate analysis between the prevalence of anemia and other parameters. In a third step, we conducted a multivariable binary regression analysis with the prevalence of anemia as dependent variable and as independent variables all those parameters which were significantly associated with the prevalence of anemia in the univariate analysis. Finally, we assessed associations between the blood hemoglobin concentration and other parameters in a multivariable linear regression analysis. Odds ratios (OR) and their 95% CIs, the standardized regression coefficient beta and the non-standardized regression coefficient B were calculated. All P-values were two-sided and considered statistically significant when the values were less than 0.05.
Results
Out of 5889 individuals primarily participating in the Ural Eye and Medical Study, the present study included 5864 (99.4%) individuals with biochemical blood examinations. The mean age of the study population (2559 (43.6%) men) was 59.0 ± 10.7 years (Table 1). The composition of the study population with respect to gender and age corresponded to the gender and age distribution in the Russian population according to the most recent census carried out in 2010 [9]. It showed two constrictions for the birth year groups from 1940 to 1946 and for the birth year groups from 1962 to 1970, directly and indirectly caused by the consequences of World War II.
The group of individuals with information about the hemoglobin concentration and the group of subjects without blood examination data did not differ significantly in age (59.0 ± 10.7 years versus 61.1 ± 12.9 years; P = 0.33), gender (2259 (43.6%) men / 3305 (56.4%) women versus 21 (60.0%) men / 14 (40.0%) women; P = 0.06), and level of education (P = 0.98).
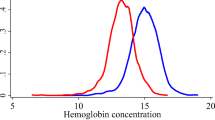
The mean hemoglobin concentration was 142.6 ± 14.8 g/L (median: 144 g/L; range: 80–171 g/L) (Fig. 1). It was significantly higher in men than in women (150.4 ± 12.6 g/L versus 136.6 ± 13.6 g/L; P < 0.001). Applying definition #1, anemia as a whole was detected in 1385 individuals (23.6%; 95%CI: 22.5, 24.7), and mild anemia was present in 1220 individuals (20.8%; 95%CI: 19.8, 21.8). The age-standardized prevalence rates based on the WHO world standard population aged 40+ years were 23,5% for anemia as a whole (definition #1) and 20,2% for mild anemia (definition #1). Using definition #2, anemia as a whole was found in 453 individuals (7.7%; 95%CI: 7.0, 8.4), and mild anemia was present in 288 individuals (4.9%; 95%CI: 4.4, 5.5). The age-standardized prevalence rates based on the WHO world standard population aged 40+ years were 8.2% for anemia as a whole (definition #2) and 5.1% for mild anemia (definition #2). Among the 288 individuals with mild anemia, 35 (12.2%) participants reported about the presence of an iron-deficiency anemia. Moderate anemia (both definitions #1 and #2) was diagnosed for 165 individuals (2.8%; 95%CI: 2.4, 3.2), out of whom 38 (23%) participants reported about the presence of an iron-deficiency anemia. The age-standardized prevalence rate for moderate anemia as based on the WHO world standard population aged 40+ years was 3.2%. None of study participants had severe anemia.
Within the Russian group (n = 1182; 506 (42.8%) men) with a mean age of 60.1 ± 11.1 years, the prevalence of anemia (definition #1) as a whole was 266 / 1182 (22.5%; 95%CI: 20.1, 24.9), the prevalence of mild anemia (definition #1) was 230 / 1182 (19.5%; 95%CI: 17.2, 21.7), and the prevalence of moderate anemia was (36 / 1182; 3.0%; 95%CI: 2.1, 4.0) (Table 2). Applying the anemia definition #2, the anemia prevalence as a whole was 79 / 1182 (6.7%; 95%CI: 5.3, 8.1), and the prevalence of mild anemia was 43 / 1182 (3.6%; 95%CI: 2.6, 4.7). The prevalences of anemia as a whole (definition #1), of mild anemia (definition #1) and moderate anemia did not differ significantly between the Russian group and the non-Russian group (P = 0.44, P = 0.27, and P = 0.55, respectively). In a similar manner, the prevalence of anemia as a whole (definition #2) and of mild anemia (definition #2) did not vary significantly between the Russian group and the non-Russian group (P = 0.26 and P = 0.052, resp.). As a corollary, the hemoglobin concentration did not vary significantly (P = 0.06; beta: 0.02) between both ethnic groups after adjusting for gender.
In univariate analysis, a higher prevalence of anemia (definition #1) was associated with older age (P < 0.001) and female gender (P < 0.001) (Fig. 2). For exploring any other associations, we adjusted the analysis for age and gender. After adjusting for age and gender, a higher prevalence of anemia was associated with lower prevalence of married family status (P = 0.01), higher prevalence of Muslima religion (P = 0.07), lower body weight (P < 0.001) and lower body mass index (P < 0.001), shorter waist circumference (P < 0.001), shorter hip circumference (P = 0.001), and lower waist-to-hip circumference ratio (P < 0.001), lower monthly income (P = 0.04), lower frequency of an ownership of a car (P = 0.001), lower prevalence of a history of arterial hypertension (P = 0.002), tumbling (P = 0.08) and menopause (P < 0.001), higher prevalence of a history of iron-deficiency anemia (P < 0.001), lower blood concentration of alanine transferase (P = 0.003), aspartate aminotransferase (P = 0.02), bilirubin (P < 0.001), low-density lipoproteins (P = 0.02), triglycerides (P < 0.001), cholesterol (P < 0.001), higher blood concentration of the rheumatoid factor (P = 0.001), creatinine (P = 0.001), urea (P = 0.001), residual nitrogen (P = 0.09), faster erythrocyte sedimentation rate (P < 0.001), longer blood clotting time (P < 0.001), lower prothrombin time (P = 0.05), lower blood count of leukocytes (P < 0.001), any type of granulocytes (P < 0.001), lymphocytes and monocytes (P < 0.001), lower systolic (P = 0.01) and diastolic (P < 0.001) blood pressure, higher ankle-brachial index (P = 0.07), higher prevalence of not able to stop drinking alcohol once started (P = 0.003), of failing to do what was normally expected due to alcohol consumption (P = 0.02) and of feeling guilty after drinking (P = 0.01), higher total hearing loss score (P = 0.02), and lower manual dynamometric force (P < 0.001) (Table 3).
The multivariable regression analysis included the prevalence of anemia (definition #1) as dependent variable and as independent variables all those parameters which were significantly associated with the prevalence of anemia in the univariate analysis. Due to collinearity we first dropped the parameters of body weight, and hip and waist circumference. Due to a lack of statistical significance, we dropped the parameters of alcohol consumption-related parameters (“feeling guilty after drinking alcohol” (P=0.73); “failing to do what was normally expected due to alcohol consumption” (P = 0.89); “not able to stop drinking alcohol once started” (P = 0.10)), religion (P = 0.97), blood concentrations of rheumatoid factor (P = 0.99), low-density lipoproteins (P = 0.79), urea (P = 0.83), alanine transferase (P = 0.95), aspartate aminotransferase (P = 0.39), creatinine (P = 0.45) and prothrombin time (P = 0.49), family status (married versus non-married) (P = 0.43), history of tumbling (P = 0.78) and arterial hypertension (P = 0.58), manual dynamometric force (P = 0.42), count of lymphocytes (P = 0.21) and monocytes (P = 0.24), ankle-brachial index (P = 0.34), systolic blood pressure (P = 0.30), body mass index (P = 0.28), self-reported income (P = 0.19), blood residual nitrogen (P = 0.16), hearing loss score (P = 0.17), blood concentration of cholesterol (P = 0.11), diastolic blood pressure (P = 0.08) and age (P = 0.33). In the final model, a higher prevalence of anemia (definition #1) was associated with male gender (P < 0.001), lower waist-hip circumference ratio (P = 0.002), lower prevalence of an ownership of a car (P = 0.005), lower blood concentrations of bilirubin (P < 0.001) and triglycerides (P = 0.003), higher erythrocyte sedimentation rate (P < 0.001), longer blood clotting time (P < 0.001) and lower count of leukocytes (P = 0.03) (Table 4). If the parameter of car ownership was replaced by the parameter of level of education, lower educational level was significantly associated with higher anemia prevalence (P = 0.001; OR: 0.93; 95%CI: 0.89, 0.97). There was a tendency for higher anemia prevalence in the urban versus rural region of habitation (P = 0.10; OR: 0.84; 95%CI: 0.68, 1.03).
In a similar manner, a higher prevalence of anemia as defined by definition #2 was associated with male gender (P < 0.001), lower waist-hip circumference ratio (P = 0.01), lower prevalence of an ownership of a car (P = 0.006), lower blood concentrations of bilirubin (P = 0.006) and triglycerides (P = 0.003), higher erythrocyte sedimentation rate (P < 0.001), longer blood clotting time (P < 0.001) and lower count of leukocytes (P = 0.03), and additionally with younger age (P = 0.045) (Table 5). In women, it was significantly higher before menopause (16.6%; 95%CI: 13.5, 19.7) than after menopause (7.1%; 95%CI: 6.0, 8.1).
Higher prevalence of moderate anemia was correlated with younger age (P = 0.008), female gender (P < 0.001), higher erythrocyte sedimentation rate (P < 0.001), longer blood clotting time (P < 0.001), and marginally significantly, with a lower waist-hip circumference ratio (P = 0.058) (Table 6). In women, it was significantly higher before menopause (8.8%; 95%CI: 6.4, 11.1) than after menopause (3.5%; 95%CI: 2.8, 4.3).
In multivariable linear regression analysis, the blood hemoglobin concentration was associated (regression coefficient r2 = 0.50) with male gender (P < 0.001), lower waist-hip circumference ratio (P < 0.001), lower prevalence of an ownership of a car (P < 0.001), lower blood concentrations of bilirubin (P < 0.001) and triglycerides (P < 0.001), higher erythrocyte sedimentation rate (P < 0.001) and longer blood clotting time (P < 0.001) (Table 7). If the parameter of car ownership was replaced by the parameter of level of education, higher educational level was significantly associated with higher hemoglobin concentration (P = 0.007; beta: 0.03).
Similar results were obtained if the parameters of erythrocyte sedimentation rate and blood clotting time as factors secondary to anemia were dropped from the model,
Discussion
In our population-based study in Russia, higher hemoglobin concentration was associated with male gender, higher waist-hip circumference ratio, higher level of education, higher blood concentrations of bilirubin and triglycerides, lower erythrocyte sedimentation rate and shorter blood clotting time. The prevalence of anemia defined by a hemoglobin concentration < 140 g/L in men and < 130 g/L in women was 23.6% (95% CI: 22.5, 24.7), and the prevalence of anemia defined by a hemoglobin concentration of < 130 g/L in men and < 120 g/L in women was 7.7% (95%CI: 7.0, 8.4). The prevalence of moderate anemia (hemoglobin concentration: 110 g/L to 80 g/L) was 2.8% (95%CI: 2.4, 3.2) and increased with younger age, female gender, higher erythrocyte sedimentation rate, longer blood clotting time, and marginally significantly, with a lower waist-hip circumference ratio. In women, it was significantly (P < 0.001) higher before menopause (8.8%; 95%CI: 6.4, 11.1) than after menopause (3.5%; 95%CI: 2.8,4.3) (Fig. 2). These prevalence figures for this study population in Russia were relatively low as compared with other populations. As in other populations, the prevalence of anemia was strongly associated with menopause, and to a minor degree, with a decreased lower waist-hip circumference ratio and lower socio-economic and educational background. There was a tendency for a higher anemia prevalence in the urban region of habitation (P = 0.10).
Anemia as a major public health problem has been addressed in numerous studies before, estimating the total number of individuals affected by anemia to be 1.62 billion people globally [10]. The prevalence of anemia ranged between 9% in high-income countries and 43% in countries with a relatively low degree of development [10]. Anemia prevalence was usually higher in pregnant women, women of reproductive age and in children than in men or elderly women. Global estimates for the prevalence of anemia were as high as 47% for children with an age of less than 5 years, 42% for pregnant women, and 30% for non-pregnant women with an age of 15 to 49 years [10]. Africa and Asia were the continents bearing the load of about 85% of the absolute anemia burden in the high-risk groups. It has been estimated that globally and per year, anemia contributed to more than 591,000 perinatal deaths and 115,000 maternal deaths [11]. The figures found for our study population from Russia show that the prevalence of anemia in Russia is similar to the anemia prevalence found in high-income countries, despite the marked difference between Russia and high-income countries in the social development index (SDI) as calculated by the Global Burden of Disease Study [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Using the WHO definition of anemia, the prevalence of anemia in our study population was 7.7% (95%CI: 7.0, 8.4). Correspondingly, none of the study participants had a severe anemia defined by a hemoglobin concentration of less than 80 mg/L. These results are in contrast to anemia prevalences found in countries with a similar SDI as Russia but which show a markedly higher anemia prevalence including severe anemia [10, 11, 13,14,15,16,17,18,19,20,21,22,23,24,25]. It suggests that the public health care system in Russia is better than it would be expected based on its SDI. It agrees with the finding of a relatively low unawareness rate for diabetes of 25% in the Russian population (Ural Eye and Medical Study; own data), a figure similar or even lower than the rate found in high-income countries.
The results obtained in our study on associations of anemia with other systemic parameters are in agreement with findings obtained in previous investigations. The main factor associated with anemia was female gender in the reproductive age (Fig. 2), in addition to a low waist-hip circumference ratio or low body mass index and a low socio-economic and educational background [1,2,3, 10, 11, 13,14,15,16,17,18,19,20,21,22,23,24,25].
The univariate analysis included many diverse parameters which were either the determinants of anemia or the sequels of anemia. Subsequently, the multivariate analysis consisted of causes and of consequences of anemia. To cite examples, the parameters of male gender, higher waist-hip circumference ratio, higher prevalence of car ownership and status before menopause were likely variables associated with the causation of anemia, while the variable of a higher blood concentration of bilirubin was probably a consequence of anemia. The diverse nature of the independent parameters in the multivariable analysis should therefore be taken into account when the results of the statistical analysis are discussed.
When the results of our study are discussed, its limitations should be taken into account. First, as for any population-based study, the participation rate is critical to assure the representativeness of the study population. With more than 80% of the eligible population taking part in our investigation, a pronounced bias in the inclusion of participants might have been unlikely. Second, our study population was composed of various ethnicities. While this multi-ethnic composition was typical for Southern Russia, the population of North-Western Russia and Central Russia usually shows a higher percentage of Russians. To overcome this potential limitation of our study, we assessed the prevalence of anemia in dependence of the ethnic background and found that the prevalence did not differ significantly between the Russian group and the non-Russian group. The age and gender distribution in our study population was comparable to the results of the Russian census 2010 [12]. Third, there were several definitions of anemia, and we used the anemia definition based on the blood hemoglobin concentration. It lacks, however, specificity for establishing the iron status. Measurement of the concentrations of serum ferritin and transferrin receptor in combination with determinations of the hemoglobin concentrations would have been better measures. Fourth, in the statistical analysis we did not clearly differentiate between biomarkers which were probably due to anemia and determinants of anemia.
Conclusions
In conclusion, in this ethically mixed urban and rural Russian population aged 40+ years, the prevalence of anemia (defined by a hemoglobin concentration of < 130 g/L in men and of < 120 g/L in women) was relatively low (7.7%;95%CI:7.0,8.4), and it was similar to the anemia prevalence in high-income countries. As in other populations, the anemia prevalence was strongly associated with menopause, and to a minor degree, with a lower waist-hip circumference ratio and lower socio-economic and educational background. There was a tendency for a higher anemia prevalence in the urban region of habitation (P = 0.10). These data may be of interest for assessing the burden of anemia in Russia.
Availability of data and materials
Available on request from the corresponding author.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- Fig:
-
Figure
- GATHER:
-
Guidelines for Accurate and Transparent Health Estimates Reporting
- GBD:
-
Global Burden of Diseases, Injuries and Risk Factors
- OR:
-
Odds ratio
- SDI:
-
Social development index
- SPPS:
-
Statistical Package for Social Science
- UEMS:
-
Ural Eye and Medical Study
- YLD:
-
Years lived with disability
References
Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24.
Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–35.
Kassebaum NJ. GBD 2013 Anemia Collaborators. The Global Burden of Anemia Hematol Oncol Clin North Am. 2016;30:247–308.
Turusheva A, Frolova E, Hegendoerfer E, Degryse JM. Predictors of short-term mortality, cognitive and physical decline in older adults in Northwest Russia: a population-based prospective cohort study. Aging Clin Exp Res. 2017;29:665–73.
Global Burden of Disease Pediatrics Collaboration, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, Coffeng LE, Dandona L, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming TD, Forouzanfar MH, Graetz N, Guinovart C, Haagsma J, Higashi H, Kassebaum NJ, Larson HJ, Lim SS, Mokdad AH, Moradi-Lakeh M, Odell SV, Roth GA, Serina PT, Stanaway JD, Misganaw A, Whiteford HA, Wolock TM, Wulf Hanson S, Abd-Allah F, Abera SF, Abu-Raddad LJ, FS AB, Amare AT, Antonio CA, Artaman A, Barker-Collo SL, Barrero LH, Benjet C, Bensenor IM, Bhutta ZA, Bikbov B, Brazinova A, Campos-Nonato I, Castañeda-Orjuela CA, Catalá-López F, Chowdhury R, Cooper C, Crump JA, Dandona R, Degenhardt L, Dellavalle RP, Dharmaratne SD, Faraon EJ, Feigin VL, Fürst T, Geleijnse JM, Gessner BD, Gibney KB, Goto A, Gunnell D, Hankey GJ, Hay RJ, Hornberger JC, Hosgood HD, Hu G, Jacobsen KH, Jayaraman SP, Jeemon P, Jonas JB, Karch A, Kim D, Kim S, Kokubo Y, Kuate Defo B, Kucuk Bicer B, Kumar GA, Larsson A, Leasher JL, Leung R, Li Y, Lipshultz SE, Lopez AD, Lotufo PA, Lunevicius R, Lyons RA, Majdan M, Malekzadeh R, Mashal T, Mason-Jones AJ, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mock CN, Murray J, Nolte S, Oh IH, Olusanya BO, Ortblad KF, Park EK, Paternina Caicedo AJ, Patten SB, Patton GC, Pereira DM, Perico N, Piel FB, Polinder S, Popova S, Pourmalek F, Quistberg DA, Remuzzi G, Rodriguez A, Rojas-Rueda D, Rothenbacher D, Rothstein DH, Sanabria J, Santos IS, Schwebel DC, Sepanlou SG, Shaheen A, Shiri R, Shiue I, Skirbekk V, Sliwa K, Sreeramareddy CT, Stein DJ, Steiner TJ, Stovner LJ, Sykes BL, Tabb KM, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Ukwaja KN, Vasankari T, Venketasubramanian N, Vlassov VV, Vollset SE, Weiderpass E, Weintraub RG, Werdecker A, Wilkinson JD, Woldeyohannes SM, Wolfe CD, Yano Y, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, El Sayed Zaki M, Naghavi M, Murray CJ, Vos T. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170:267–87.
Bikbov MM, Fayzrakhmanov RR, Kazakbaeva GM, Zainullin RM, Salavatova VF, Gilmanshin TR, Arslangareeva II, Nikitin NA, Panda-Jonas S, Mukhamadieva SR, Yakupova DF, Khikmatullin RI, Aminev SK, Nuriev IF, Zaynetdinov AF, Uzianbaeva YV, Jonas JB. Frequency and associated factors of bone fractures in Russians: the Ural eye and medical study. Sci Rep. 2018;8:7483.
Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, Grove JT, Hogan DR, Hogan MC, Horton R, Lawn JE, Marušić A, Mathers CD, Murray CJL, Rudan I, Salomon JA, Simpson PJ, Vos T, Welch V (The GATHER Working Group). Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet 2016;388:e19–e23.
WHO. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: World Health Organization; 2008. http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf. Assessed 4 June 2019.
Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–50.
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12:444–54.
Ezzati M, Lopez AD, Rodgers AA, Murray CJL. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization; 2004.
https://en.wikipedia.org/wiki/Demographics_of_Russia. Assessed 4 June 2019.
el-Sahn F, Sallam S, Mandil A, Galal O. Anaemia among Egyptian adolescents: prevalence and determinants. East Mediterr Health J. 2000;6:1017–25.
Schellenberg D, Schellenberg JR, Mushi A, Savigny DD, Mgalula L, Mbuya C, Victora CG. The silent burden of anaemia in Tanzanian children: a community-based study. Bull World Health Organ. 2003;81:581–90.
Semba RD, Guralnik JM, Chaves P, Ricks MO, Fried LP. Women's Health and Aging Studies Iron status and anemia in a population-based study of women with and without disability living in the community: the Women's Health and Aging Studies. Haematologica. 2004;89:357–8.
Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8.
Skjelbakken T, Langbakk B, Dahl IM, Løchen ML. Tromsø study. Haemoglobin and anaemia in a gender perspective: the Tromsø study. Eur J Haematol. 2005;74:381–8.
Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51.
Tettamanti M, Lucca U, Gandini F, Recchia A, Mosconi P, Apolone G, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Savoia L, Fasolo G, Ponchio L, Della Porta MG, Riva E. Prevalence, incidence and types of mild anemia in the elderly: the “health and Anemia” population-based study. Haematologica. 2010;95:1849–56.
Plante C, Blanchet C, Rochette L, O'Brien HT. Prevalence of anemia among Inuit women in Nunavik, Canada. Int J Circumpolar Health. 2011;70:154–65.
Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M. Nutrition impact model study group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–25.
Eisele L, Dürig J, Broecker-Preuss M, Dührsen U, Bokhof B, Erbel R, Moebus S, Jöckel KH, Heinz Nixdorf Recall Study Investigative Group. Prevalence and incidence of anemia in the German Heinz Nixdorf recall study. Ann Hematol. 2013;92:731–7.
Bowling CB, Muntner P, Bradbury BD, Kilpatrick RD, Isitt JJ, Warriner AH, Curtis JR, Judd S, Brown CJ, Allman RM, Warnock DG, McClellan W. Low hemoglobin levels and recurrent falls in U.S. men and women: prospective findings from the REasons for geographic and racial differences in stroke (REGARDS) cohort. Am J Med Sci. 2013;345:446–54.
Silla LM, Zelmanowicz A, Mito I, Michalowski M, Hellwing T, Shilling MA, Friedrisch JR, Bittar CM, Albrecht CA, Scapinello E, Conti C, Albrecht MA, Baggio L, Pezzi A, Amorin B, Valim V, Fogliatto L, Paz A, Astigarraga C, Bittencourt RI, Fischer G, Daudt L. High prevalence of anemia in children and adult women in an urban population in southern Brazil. PLoS One. 2013;8:e68805.
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59.
Acknowledgements
None.
Financial disclosures
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Design and conception: MMB, GMK, JBJ; Data assessment: MMB, GMK, RMZ, VFS, TRG, DFY, YVU, IIA, SPJ, SRM, RIK, SKA, IFN, AFZ, JBJ; Statistical analysis: JBJ; Writing of the manuscript: JBJ; Editing and final approval of the manuscript: MMB, GMK, RMZ, VFS, TRG, DFY, YVU, IIA, SPJ, SRM, RIK, SKA, IFN, AFZ, JBJ.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
According to the Declaration of Helsinki, the Ethics Committee of the Academic Council of the Ufa Eye Research Institute approved the study and all participants gave informed written consent. The ethics committee confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bikbov, M.M., Kazakbaeva, G.M., Zainullin, R.M. et al. Prevalence and associated factors of anemia in a Russian population: the Ural eye and medical study. BMC Public Health 19, 762 (2019). https://doi.org/10.1186/s12889-019-7016-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-019-7016-6