Abstract
Background
Concerns about postcessational weight gain might hamper rather than encourage smokers to quit smoking.
Methods
We conducted a comprehensive multi-institutional smoking cessation program for health care and industrial workers (n = 654) employed at University Hospital Basel (Switzerland) and two local health industry companies (Novartis International AG, F. Hoffmann-La Roche AG). The program contained counselling with an option of pharmacological support. Changes in body weight were observed during 24 months of follow-up. Factors associated with longitudinal weight gain (>5 % of baseline weight) were identified by cox-regression analysis.
Results
In 51 % of permanent quitters no significant changes of mean body weight were observed after 12 (0.52 kg, SD ±2.87 kg) and 24 months (0.40 kg, SD ± 2.99 kg). Marked weight gain following smoking cessation was characterized by a wide margin of changes. In more than a half of former smokers (58 %) weight increases were moderate (<5 kg), whereas excessive increases (>10 kg) were seen in only 10 % of quitters. Lower baseline BMI (HR 0.60, 95 % CI 0.40- 0.80, p = 0.03), daily consumption of less than ten cigarettes (HR 0.53, 95 % CI 0.27- 0.63, p = 0.04) and ischemic cardiopathy (HR 0.21, 95 % CI 0.07-0.62; p < 0.01) were associated with a lower risk for weight gain. Employees with lower educational levels (HR 2.60, 95 % CI 1.60-5.50, p < 0.01), diabetes mellitus (HR 3.05, 95 % CI 2.20-8.06, p = 0.02) and those smoking to reduce boredom in life (HR 1.68, 95 % CI 1.21-2.33, p < 0.01) were at highest risk.
Conclusion
Marked postcessational weight gain occurs less often than expected, but remains difficult to be predicted. Our findings might be helpful to alleviate weight concerns in the average smoker willing to quit.
Similar content being viewed by others
Background
Cigarette smoking is estimated to cause over five millions deaths per year worldwide making it the leading preventable major cause of disease and premature death with a high burden of costs for health care systems [1, 2]. Although smoking cessation is obviously associated with individual health benefits, several barriers addressing physiologic and psychosocial aspects of nicotine addiction often prevent willingness to quit and successful smoking cessation. One of the most important causes of failure in smoking cessation is the fear of excessive weight gain after successful quitting [3, 4]. In comparison with their non-smoking counterparts a lower body weight, a deceleration of age-related weight increase and an 80 percent probability of weight gain occurring within 6 months after quitting is commonly described for smokers [5–8].
Several factors have been examined to identify determinants of postcessational weight gain. These include, although partly controversially discussed, younger age [9], female gender [10], ethnicity, low socioeconomic status [11], baseline weight, determinants of tobacco dependence, a sedentary lifestyle [12] and poor eating habits [13]. In contrast, there is some evidence that both nicotine replacement therapy (NRT) and bupropion alone or prescribed in combination with NRT can at least partly prevent weight gain following smoking cessation [14–16]. Success rates of most smoking cessation interventions remain modest, but may be increased in future through prematurely identifying determinants of weight gain. The main objective of the present study was to explore postcessational weight changes in a first step and thereafter searching for predictors of marked weight gain in an employed population of health care and industrial workers. For this purpose, longitudinal, prospective data for analysis were taken from our comprehensive smoking cessation program [17].
Methods
Study design
The study was designed as an investigator- initiated and investigator- driven, open multicentre project which aimed to implementate a comprehensive smoking cessat-ion program in a cohort of employees. It was conducted between May 2005 and January 2009 in Basel, Switzerland. Smoking employees at the University Hospital of Basel (UHBS, Basel, Switzerland) and two local pharmaceuticals companies (Novartis Pharma AG, Basel, F. Hofmann-La Roche AG, Basel) were recruited on a voluntary basis from a total of 17500 employees. All subjects were invited to take part through local advertising such as posters, newsletters and flyers. An additional, standardized and confidential pre-screening questionnaire was sent to all employees of University Hospital Basel. Returned questionnaires were analysed and smokers interested in quitting were contacted by phone through an occupational healthcare advisor. Main criteria of inclusion were willingness to quit smoking, employment at one of the three worksites, a minimum age of 17 years and a daily consumption of at least five cigarettes. All employees provided their written informed consent to a scientific analysis of their data. Prior to the initiation of the program, occupational healthcare advisors of the three study centres received specific training in smoking cessation counselling skills. For this, a standardized manual of smoking cessation training for healthcare professionals was used [18]. Aims, methodology and study design of our smoking cessation intervention are described more in detail elsewhere [17].
The program was designed in accordance with the Swiss and international clinical guidelines for smoking cessation [19, 20]. The local joint ethical committee of the Canton of Basel, Switzerland, approved the project in January 2005 (EKBB 34/05). Participation was on a voluntary basis and written informed consent for scientific evaluation of the program was required from employees prior to enrolment.
Smoking cessation intervention
The primary intervention consisted of several in site visits within the first 3 months following enrolment. Each visit contained individual face-to-face counselling of at least 15 minutes duration. Counselling support covered standard cognitive- behavioural techniques, such as enhancing motivation, highlighting health benefits, identifying barriers, coping with cravings and relapse prevention. Additionally pharmacological support was provided on a voluntary basis for a total period of 3 months and followed common recommendations of manufacturers and literature regarding dose and delivery systems [21, 22]. Employees were given the choice between either monotherapy or different combinations of nicotine replacement therapy (NRT). Furthermore, bupropion could be added to NRT or was prescribed as monotherapy [17].
After 3 months the primary intervention was completed and all employees had an in-site follow- up at 12 and 24 months, respectively. In addition, telephone contacts asking for smoking status were provided at 6 and 18 months.
Subjects were categorized as abstinent from cigarette smoking by asking self- reported abstinence over the last 30 days. Self-reported abstinence had to be biochemically confirmed by an expired carbon monoxide level of less than 6 ppm measured with a Micro Smokerlyser (Bedfont, Kent, UK). Employees who reported smoking by themselves, had an exhaled carbon monoxide concentration equal or greater than 6 ppb or were lost of follow- up were categorized as relapsed smokers.
Data collection
Data were collected using a web-based standardized and structured electronic case report form (eCRF) at all three worksites. Clinical relevant variables were assessed at in-site visits. Body weight (in kilograms) was measured for each participant under control of study staff. Employees were weighed with their clothes on, but without wearing shoes, jackets or heavy outer garnets. Height was measured to the nearest 0.5 cm using a tape measure without shoes using a standardized scale. Body weight was followed up by using a standardized spring scale at every in-site visit. The Body mass index (BMI) was calculated as body weight (in Kg) divided by the body height (in m2). Weight change was calculated using baseline weight (taken at the individual quit date at visit 3) subtracted from the body weight measured at each consecutive visit.
Statistical analysis
Baseline characteristics of the whole population and long- term quitters separated in those with and without a marked weight gain following smoking cessation were summarized with means, standard deviations (SD) or proportions. Proportions and frequencies between different groups were compared using the χ2- test or the Wilcoxon-rank sum test for not normally distributed variables. Differences in continuous variables were analysed with the two- sample t- test, or with the Mann- Whitney test as appropriate. Marked weight gain was defined as an increase of more than 5 % in reference to the baseline body weight for predictor analysis. Absolute weight chances over times were illustrated in kilograms including means, standard deviations and 95 % confidence intervals. Thereby, quantitative changes of body weight gain were categorized as excessive (more than 10 kg), severe (5–10 kg) and moderate (less than 5 kg) for descriptive statistics (see section results). Longitudinal weight analysis was carried out separately for the whole study population, part- time quitters (abstainers for at least two consecutive visits) and those who remained permanently (over 24 months) abstinent.
In order to detect the effect of nicotine abstinence (versus non- abstinence) on body weight, linear mixed effect models were created. An uni- and multivariable cox- regression analysis to predict the risk of marked weight increase was calculated for several variables of clinical interest. Results are presented as hazard ratios (HR) and 95 % confidence intervals with corresponding p-values. For continuous or ordinal variables hazard ratios had to be based on a meaningful difference of the predicting variable. They were expressed as the ratio from the odds from the 3rd to the 1st quartile representing a typical above average to a typical below average value. All models were adjusted by age, gender and other confounding variables. Due to the exploratory character of the study, no adjustments for multiple comparisons were done. The method for selection of a best subset of variables was based on a stepwise procedure by Akaike Information Criterion (AIC). AIC is a measure of quality of statistical models. Variables were deleted from the whole model until the value of the AIC significantly increased, as previously described [23]. Harrel’s concordance index (C-Index) was calculated with corresponding 95 % confidence intervals to evaluate the concordance between predicted and observed weight gain. All analysis were done using R version 3.0.1. [24].
Results
Postcessational weight gain
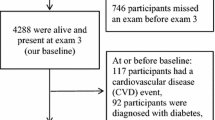
Assuming a smoking prevalence of about 25 % in the overall swiss population, at least 4250 employees from a total of 17500 employees at the three study centres were estimated to be active smokers [25, 26]. Within our program an estimated 20 % or 885 smoking employees could be recruited initially to quit smoking. 2,555 out of 4449 (57 %) pre-screening questionnaires were returned from employees of University Hospital of Basel. 1,455 (57 %) employees were categorized as non-smokers, 791 employees (31 %) as former smokers and 333 employees (13 %) as active smokers. 253 (76 %) out of 333 active smokers were eligible for enrolment and signed the informed consent. Furthermore, 344 smokers out of 9000 employees and 288 smokers out of 4000 employees entered the program from the worksites F. Hoffman- La Roche AG (Basel, Switzerland) and Novartis Pharma AG (Basel, Switzerland) respectively. Participation rates of recruited smokers were in both centres about 70 %. During the follow-up phase three study subcentres had to be closed due to internal restructuring of the two health companies leading to exclusion of 182 employees. 49 further cases had to be excluded due to missing weight data at several time points in 24, invalid data entries in 3 and missing of relevant covariates in 22 cases. Finally, data from 654 employees were eligible for postcessational weight analysis.
The study population consisted of slightly more male, mid-aged smokers with an average consumption of 11–20 cigarettes day and moderate to severe nicotine dependence on FTND. Descriptive characteristics at baseline for the whole study population and separately illustrated for long-term quitters with or without a 5 % increase of body weight are depicted in Table 1. There were no significant baseline differences of demographic data, prevalence of comorbidities, smoking related variables and different types of pharmacological cessation support with the exception of a slightly lower prevalence of heart disease and higher values of FTND in quitters with marked weight gain. At 24 months of follow- up, 229 employees (35 %) from 654 employees were continuously abstinent. 268 employees (41 %) were categorized as lost-of follow- up. 156 employees (24 %), who were categorized as ongoing smokers or not permanent quitters absolved the whole follow-up of 24 months.
Mean weight gain over time in the whole study population (n = 654) was 0.80 kg (±1.20 kg) at 1 month, 1.84 kg (±1.50 kg) at 3 months, 2.04 kg (±1.90 kg) at 12 months and 1.98 kg (±2.20 kg) after 24 months, respectively. At the time of 12 and 24 months, weight changes from permanent abstainers were significantly different from baseline as compared with relapsed smokers (p < 0.01). Weight differences over time splitted for abstinence vs. non- abstinence are illustrated in Fig. 1.
In about a half of permanent quitters (n = 114, 49 %) marked weight gain occurred. Average longitudinal increase of body weight in these 114 subjects was 1.30 kg (SD ±2.40 kg) at 1 month, 2.90 kg (SD ±2.91 kg) at 3 months, 5.07 kg (SD ±3.50 kg) after 1 year and remained stable thereafter with 5.15 kg (±4.14 kg) at 2 year follow-up (p < 0.01). Excessive weight gain (>10 kg) occurred in 11 (10 %), severe (5–10 kg) in 36 (32 %) and moderate gain (less than 5 kg) in 67 (58 %) out of 114 permanent quitters. By comparison, in 51 % of long-term quitters (n = 115) no significant changes of mean body weight were observed [0.07 kg (±1.69 kg) at 1 month, 0.68 (±2.23 kg) at 3 months, 0.52 kg (±2.87 kg) at 12 months and 0.40 kg (±2.99 kg)] after 24 months. The risk of smoking relapse was significantly lower for smokers with an weight gain between 0 and 10 kg as compared to the subgroup with excessive weight gain (>15 kg) ( HR 0.32; 95 % CI 0.25-0.42, p < 0.01).
Predictors of postcessational weight gain
Univariate- and multivariate Cox regression analysis assessing characteristics potentially associated with marked weight gain (defined as 5 % increase of baseline weight) after smoking cessation are summarized in Table 2. Taken demo-graphic characteristics into account, only a lower baseline weight (BMI 20 vs. 30) was associated with a lower chance of postcessational weight gain (HR 0.60, 95 % CI 0.40-0.80, p = 0.03). In contrast to univariate analysis (HR 1.42, 95 % CI 1.07-1.87, p = 0.01), female gender was not an independent predictor for marked weight gain on multivariate analysis (HR 1.40, 95 % CI 0.93-2.10, p = 0.11). Similarly, age of employees (30 vs. 50 years) was also not predictive for weight gain (HR 1.00, 95 % CI 0.97-1.03, p = 0.94). In contrast, a lower level of education, namely basic education or apprenticeship, were strongly associated with an increase of body weight (basic education HR 2.90 95 % CI 1.39-4.83, p < 0.01, apprenticeship HR 2.60, 95 % CI 1.60-5.50, p < 0.01). From present comorbidities a concomitant diagnosis of diabetes mellitus or ischemic cardiopathy was found to be independently associated with weight changes. Thereby, diabetes mellitus was a strong risk factor (HR 3.05, 95 % CI 2.20-8.06, p = 0.02), whereas employees with coronary disease seemed to have a lower risk for weight gain (HR 0.21, 95 % 0.07-0.62, p < 0.01). Smoking related variables of nicotine dependency, namely FTND or Craving scores, revealed no differences in weight changes, although at least a daily consumption of less than ten cigarettes per day appeared to be protective for excessive weight gain (HR 0.53, 95 % CI 0.27-0.63, p = 0.04). The association of cigarette smoking to reduce feelings of boredom resulted in significant higher body weight after quitting (HR 1.68, 95 % CI 1.21-2.33, p < 0.01).
Differences in weight gain could not be observed when different types of nicotine replacement therapy with or without bupropion were assessed individually in comparison with the counselling only option. A total of 9 variables were selected for a best subset regression model and a nomogram to estimate one and two year probability for marked weight gain was constructed (c- index. 0.68, 95 % CI [0.63; 0.72]) (see Fig. 2).
“Best subset regression” model for weight gain predicting variables. To use the nomogram, locate the first variable. Draw line straight up to the points axis to determine the number of points received for the variable. This has to be repeated for all other variables and points achieved for each variable have to be summarized. The sum of these numbers is located on the total point axis and a line drawn has to be drawn down to determine the likehood of 1- and 2- probability of postcessational weight gain. BMI = Body Mass Index, FTND = Fagerstroem Test for Nicotine Dependence
Discussion
To our knowledge, the present study is one of the first of its kind to examine long-term weight gain in order to identify predicting factors in a cohort of healthy mid-aged workers participating in a comprehensive worksite related health intervention.
As a main finding of the present study, we observed no significant weight changes following smoking cessation in more than a half of long-term quitters. In the subgroup of long-term with marked weight gain we revealed a mean weight increase of 5 kg after quitting with a high variability between individuals. We were also able to identify some determinants which are potentially useful to prematurely identify smokers at greatest risk.
The relationship between smoking cessation and consecutive weight gain is considered to be complex in nature and most likely multifactorially conditioned. In line with previous research, smoking cessation was clearly associated with substantial weight gain in many smokers of our population. Thereby it should be mentioned that the frequency, the amount and pattern of weight gain differed widely between investigated populations in the past [27, 28]. In general, assessment of postcessational weight changes is made complex by several facts. The majority of healthy adults in developed countries tend to gain weight with age as it has be shown in several large population studies [10, 29]. More recent data have also confirmed this observation in a smoking population [30]. Furthermore, the individual body weight might also be relevant influenced by different cultural and nutritionals factors [31, 32]. In addition, studies in homozygotic twins have demonstrated that the postcessational weight gain might be in large a part of compensation for underweight in the smoking twin compared to the non-smoking twin [33]. Therefore, a genetic component of individual body weight might also affect postcessational weight gain.
Finally, contrary reports about weight gain following smoking cessation could also be explained –at least to some extent- by several methodological reasons including the use of only self- reported height and weight, failure to biochemically confirm smoking status, missing control- groups, using more often point- versus continuous abstinence and limited follow-up periods [34].
Relevant weight gain might both negatively affect the outcome of a quit attempt and at least party attenuate the health benefits of smoking cessation due to an increased probability for metabolic and cardio-vascular disorders in high-risk subjects [35, 36].
We found a mean weight gain of 5 kg occurring in the first year after quitting, which is in line with various earlier reports describing an increase between 4 to 6 kilograms occurring within the first year in continuously abstinent outpatient smokers [4, 37–40]. As one might expect, we observed greater weight gain over time in permanent abstainers as compared to non-sustained quitters and ongoing smokers. Significant gain of body weight occurred mostly within the first year post quitting and showed thereafter a tendency to stabilize until 24 months. Although evidence whether weight increase beyond one year continues is conflicting, our observation stands in agreement with some newer investigations [41–43].
Some randomized clinical studies postulated that both NRT and bupropion might prevent or at least attenuate weight gain [39, 44, 45]. However, other investigations could not confirm this finding [40, 46]. In the present study, we found no differences in weight changes post- quitting in regards to several modalities of nicotine replacement with or without bupropion as compared to counselling advice only. Nevertheless, it should be mentioned, that our finding was made in a non- randomized, “real-life” setting with unmatched treatment groups. The non-effect of NRT on weight might be therefore due to a self-selection of NRT by heavier smokers with an overall higher risk for weight gain. Furthermore, we did not provide pharmacological support with varenicline, which might be more effective in attenuating weight gain than NRT based on latest findings [47].
Postcessational weight gain is considered both to negatively affect the outcome of a quit attempt and at least party attenuate the health benefits of smoking cessation due to an increased probability for metabolic and cardio-vascular disorders in high- risk subjects [35, 36]. Data from the present study showed that smokers with excessive postcessational weight gain (>10 kg) had a significant higher rate of smoking relapse. Therefore, meaningful predictor variables would be helpful to identify smokers at risk for postcessational weight gain. Predictors identified by existing reports have a number of limitations including restricted generality (data were often collected in clinical populations), which probably differ in weight changes as compared to outpatient, “healthy” smokers [48–51]. In addition, results of population-based investigations often relied on retrospective reports without any objective assessment of weight and smoking status. Additionally, long-term success rates of cessation interventions remained often low. In the present study weight increases revealed a broad range of variation with moderate weight gain (<5 kg) in 58 % and excessive weight gain (>10 kg) in only 10 % of former smokers respectively [11, 37]. Therefore many tested predictor variables for a 5 % increase of baseline weight might be therefore inaccurate in the individual case.
A lower educational level was the strongest risk factors for weight gain after quitting in the present study. Although data describing the influence of socioeconomic variables on body weight gain are scarce, adopted health habits including limited resources for changing nutritional intake and a more sedentary lifestyle in less educated smokers may contribute to relevant weight gain following smoking cessation [11]. Addressing motivational factors, employees who reported to smoke for reducing boredom in their life were significant more vulnerable for weight increase. This may represent an increased caloric intake as vicarious satisfaction. In contrast, employees who reported to utilize smoking as weight control practice had surprisingly neither an increased risk to gain weight after smoking cessation nor lower abstinence rates, although this subgroup of smokers was associated with higher nicotine dependence and lower resources of self- efficacy to control body weight [52]. This observation can’t be fully explained, because data about important confounding variables like restraint eating or increased physical activities during quit attempts were not collected in the present study. Furthermore, the association between individual weight concerns and weight gain after cessation remains controversially discussed in literature [53, 54].
The subgroup of subjects suffering from diabetes had a greater change for weight gain. This seems to be particularly relevant due to the potentially elevated risk for uncontrolled metabolic situation, morbidity and mortality following smoking cessation [55, 56].
The lower risk of weight gain in subjects with coronary heart disease seems surprising at first glance. We assume that employable subjects with coronary heart disease might display a higher alertness for healthier lifestyles. We didn’t found any related articles in order to scientifically substantiate this observation. Additionally, the sample size participants with coronary heart disease (n = 28) was quite low, so the strength of this observation needs further proof due to a low statistical power.
In accordance with previous investigations, smokers with a lower amount of cigarettes smoked per day at baseline seem to be less vulnerable for weight gain suggesting that lower levels of nicotine dependence may be protective against body weight increase [40, 57]. Importantly, it should be mentioned, that our population consisted mainly of low and medium dependent smokers (FTND mean: 4.42 pts, SD ±2.30) and therefore other determinants of nicotine dependence, namely FTND, carbon monoxide and intensity of craving might have failed to independently predict weight gain. The observation, that individual characteristics like age and gender showed no association with postcessational weight gain are in line with some, but not all earlier reports [11, 12, 58, 59]. Our findings are also in accordance with some earlier investigations showing that smokers with normal baseline BMI (20 kg/m2) are less vulnerable for marked gain than those with higher baseline weight (BMI 30 kg/m2) [58, 60].
The present study has some strengths and limitations. The strengths consist of its community based approach and a high rate of successful long-term quitters (over 35 % at 24 months). In line with a more recent published study we also analysed weight changes for continuous abstainers, part-time quitters and ongoing smokers at different points of time through a longitudinal model [40]. The later aspect ensured that weight changes of all participants were taken into account and not only weight changes from those who remained permanently abstinent or completed the whole follow-up.
One of our most important limitations is the absence of a control group of lifelong non- smokers that would certainly have added to the strength of the study. Nevertheless, in contrast to many similar studies a relevant number of 156 unsuccessful smokers was followed up for 24 months. These participants represented a kind of internal control group enabling us to better estimate the impact of permanent smoking cessation on long-term body weight.
In summary marked weight gain following smoking cessation occurred in less than a half of smokers following smoking cessation. The majority of smokers intending to quit smoking has concerns about postcessational weight gain and the experience of a minority of quitters who gain large amounts of weight may discourage them from quitting. Therefore, it seems important that health care providers alleviate weight concerns and encourage smokers for a quit attempt. Numerous demographic, socioeconomic, smoking related, cultural and even genetic factors were associated with postcessational weight gain. Therefore, it seems improbable that a single or even a subset of variables as demonstrated by the moderate performance of our multidimensional model can predict individual weight gain following smoking cessation. Nevertheless, early recognition of those smokers at risk for excessive weight gain could enhance the success rate of smoking cessation interventions. Further longitudinal well- designed studies are needed to further characterize the determinants of smokers who are particularly vulnerable for weight gain.
Conclusion
The present study is one of the first of its kind investigating postcessational weight changes in a large community of health care and industrial workers participating on a comprehensive smoking cessation intervention. One of the main findings of our investigation is the observation that marked weight gain following smoking cessation occurs less often than expected. Consistent with previous reports, long- term abstinence was associated with a mean weight gain of 5 kilograms, which occurred in continuous quitters within 1 year. Although some determinants of weight gain could be identified, accurate prediction using a clinical set of characteristics remains difficult in the individual case.
Abbreviations
- CO:
-
Carbon monoxide
- COPD:
-
Chronic obstructive pulmonary disease
- eCRF:
-
Electronic case report form
- FTND:
-
Fagerstroem test for nicotine dependence
- VAS:
-
Visual analogue scale
- NRT:
-
Nicotine replacement therapy
- HR:
-
Hazard ratio
- 95 % CI:
-
95 % confidence interval
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
References
Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45.
Zarocostas J. WHO report warns deaths from tobacco could rise beyond eight million a year by 2030. BMJ. 2008;336:299.
Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychol. 2000;19:242–6.
Perkins KA. Weight gain following smoking cessation. J Consult Clin Psychol. 1993;61:768–77.
Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med. 2007;44:290–5.
Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–8.
Lycett D, Munafo M, Johnstone E, Murphy M, Aveyard P. Weight change over eight years in relation to alcohol consumption in a cohort of continuing smokers and quitters. Nicotine Tob Res. 2011;13:1149–54.
Samet JM. The 1990 report of the surgeon general: the health benefits of smoking cessation. Am Rev Respir Dis. 1990;1990(142):993–4.
Wane S, van Uffelen JG, Brown W. Determinants of weight gain in young women: a review of the literature. J Womens Health (Larchmt). 2010;19:1327–40.
Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–45.
Swan GE, Carmelli D. Characteristics associated with excessive weight gain after smoking cessation in men. Am J Public Health. 1995;85:73–7.
Hall SM, Ginsberg D, Jones RT. Smoking cessation and weight gain. J Consult Clin Psychol. 1986;54:342–6.
Munafo MR, Tilling K, Ben-Shlomo Y. Smoking status and body mass index: a longitudinal study. Nicotine Tob Res. 2009;11:765–71.
Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202.
Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med. 2001;135:423–33.
Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Cochrane Database Syst Rev 2009;(1):CD006219. doi: 10.1002/14651858.CD006219.pub2. Review. Update in: Cochrane Database Syst Rev. 2012;1:CD006219.
Stolz D, Scherr A, Seiffert B, Kuster M, Meyer A, Fagerstrom KO, et al. Predictors of success for smoking cessation at the workplace: a longitudinal study. Respiration. 2014;87:18–25.
Humair JP, Cornuz J. A new curriculum using active learning methods and standardized patients to train residents in smoking cessation. J Gen Intern Med. 2003;18:1023–7.
Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45:1196–9.
Cornuz J, Humair JP, Seematter L, Stoianov R, van Melle G, Stalder H, et al. Efficacy of resident training in smoking cessation: a randomized, controlled trial of a program based on application of behavioral theory and practice with standardized patients. Ann Intern Med. 2002;136:429–37.
Roddy E. Bupropion and other non-nicotine pharmacotherapies. BMJ. 2004;328:509–11.
Molyneux A. Nicotine replacement therapy. BMJ. 2004;328:454–6.
Venables WN, Ripley BD, Venables WN. Modern applied statistics with S. 4th ed. New York: Springer; 2002.
Venables WN, Smith DM. R Development Core Team.: An introduction to R: notes on R: a programming environment for data analysis and graphics, version 1.4.1. Bristol: Network Theory; 2002.
Marques-Vidal P, Cerveira J, Paccaud F, Cornuz J. Smoking trends in Switzerland, 1992–2007: a time for optimism? J Epidemiol Community Health. 2011;65:281–6.
Bolliger CT. Tobacco abuse: background and countermeasures. Praxis (Bern 1994). 1998;87:76–82.
Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. J Fam Pract. 1998;46:460–4.
John U, Meyer C, Rumpf HJ, Schumann A, Dilling H, Hapke U. No considerable long-term weight gain after smoking cessation: evidence from a prospective study. Eur J Cancer Prev. 2005;14:289–95.
Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch NM, Schindler C, Felber Dietrich D, Stutz EZ, et al. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991–2003: methods and characterization of participants. Soz Praventivmed. 2005;50:245–63.
Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring). 2007;15:1311–8.
Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34:404–12.
Steffen A, Sorensen TI, Knuppel S, Travier N, Sanchez MJ, Huerta JM, et al. Development and validation of a risk score predicting substantial weight gain over 5 years in middle-aged European men and women. PLoS One. 2013;8:e67429.
Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking–a study of male twins. N Engl J Med. 1992;327:829–33.
Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. J Consult Clin Psychol. 1997;65:286–91.
Alberg AJ, Carter CL, Carpenter MJ. Weight gain as an impediment to cigarette smoking cessation: a lingering problem in need of solutions. Prev Med. 2007;44:296–7.
Stein JH, Asthana A, Smith SS, Piper ME, Loh WY, Fiore MC, et al. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: three-year outcomes after a quit attempt. PLoS One. 2014;9:e98278.
Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439.
Chatkin R, Chatkin JM. Smoking and changes in body weight: can physiopathology and genetics explain this association? J Bras Pneumol. 2007;33:712–9.
Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103.
Prod’hom S, Locatelli I, Giraudon K, Marques-Vidal P, Clair C, Bize R, et al. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine Tob Res. 2013;15:910–6.
Reas DL, Nygard JF, Sorensen T. Do quitters have anything to lose? Changes in body mass index for daily, never, and former smokers over an 11-year period (1990–2001). Scand J Public Health. 2009;37:774–7.
Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9.
Cairella G, Ciaralli F, Longo P, Rebella V, Molino N, D’Urso A. Smoking cessation and weight gain. Ann Ig. 2007;19:73–81.
Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2012;1:CD006219.
McVay MA, Copeland AL. Smoking cessation in peri- and postmenopausal women: a review. Exp Clin Psychopharmacol. 2011;19:192–202.
Allen AM, Kleppinger A, Lando H, Oncken C. Effect of nicotine patch on energy intake and weight gain in postmenopausal women during smoking cessation. Eat Behav. 2013;14:420–3.
Taniguchi C, Tanaka H, Nakamura N, Saka H, Oze I, Ito H, et al. Varenicline is more effective in attenuating weight gain than nicotine patch 12 months after the end of smoking cessation therapy: an observational study in Japan. Nicotine Tob Res. 2014;16:1026–9.
Grandi SM, Filion KB, Gervais A, Joseph L, O’Loughlin J, Paradis G, et al. Weight change in patients attempting to quit smoking post-myocardial infarction. Am J Med. 2014;127:641–9.
Cropsey KL, McClure LA, Jackson DO, Villalobos GC, Weaver MF, Stitzer ML. The impact of quitting smoking on weight among women prisoners participating in a smoking cessation intervention. Am J Public Health. 2010;100:1442–8.
Filia SL, Baker AL, Gurvich CT, Richmond R, Kulkarni J. The perceived risks and benefits of quitting in smokers diagnosed with severe mental illness participating in a smoking cessation intervention: gender differences and comparison to smokers without mental illness. Drug Alcohol Rev. 2014;33:78–85.
Filia SL, Baker AL, Gurvich CT, Richmond R, Lewin TJ, Kulkarni J. Gender differences in characteristics and outcomes of smokers diagnosed with psychosis participating in a smoking cessation intervention. Psychiatry Res. 2014;215:586–93.
Pinto BM, Borrelli B, King TK, Bock BC, Clark MM, Roberts M, et al. Weight control smoking among sedentary women. Addict Behav. 1999;24:75–86.
Aubin HJ, Berlin I, Smadja E, West R. Factors associated with higher body mass index, weight concern, and weight gain in a multinational cohort study of smokers intending to quit. Int J Environ Res Public Health. 2009;6:943–57.
Thorner-Bantug E, Jaszyna-Gasior M, Schroeder JR, Collins CC, Moolchan ET. Weight gain, related concerns, and treatment outcomes among adolescent smokers enrolled in cessation treatment. J Natl Med Assoc. 2009;101:1009–14.
Feldstein AC, Nichols GA, Smith DH, Rosales AG, Perrin N. Weight change and glycemic control after diagnosis of type 2 diabetes. J Gen Intern Med. 2008;23:1339–45.
Schauer GL, Bush T, Cerutti B, Mahoney L, Thompson JR, Zbikowski SM. Use and effectiveness of quitlines for smokers with diabetes: cessation and weight outcomes, Washington State Tobacco Quit Line, 2008. Prev Chronic Dis. 2013;10:E105.
Komiyama M, Wada H, Ura S, Yamakage H, Satoh-Asahara N, Shimatsu A, et al. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS One. 2013;8:e72010.
Dale LC, Schroeder DR, Wolter TD, Croghan IT, Hurt RD, Offord KP. Weight change after smoking cessation using variable doses of transdermal nicotine replacement. J Gen Intern Med. 1998;13:9–15.
Nides M, Rand C, Dolce J, Murray R, O’Hara P, Voelker H, et al. Weight gain as a function of smoking cessation and 2-mg nicotine gum use among middle-aged smokers with mild lung impairment in the first 2 years of the Lung Health Study. Health Psychol. 1994;13:354–61.
Lycett D, Munafo M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106:188–96.
Acknowledgement
D. Stolz was supported by grants of the Swiss National Foundation, the Liechtenstein Foundation, the Freiwillige Akademische Gesellschaft Basel and the European Respiratory Society for Educational Research. Additional funding was granted by the Employee’s Health Department of the University Hospital of Basel. The sponsors of this investigator- driven project had no involvement in the design and conduct of the study, data collection, management, analysis and interpretation of the data nor in the preparation, review, approval of the manuscript or the decision to submit the manuscript. We thank Schötzau und Müller, Eudox Basel, Switzerland) for advice on statistical matters.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Author’s contribution
All authors had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. AS contributed to data analysis, interpretation of results and drafted the manuscript. BS, MK and AM contributed substantially to recruitment and data acquisition, KF gave critical revision for important intellectual content, MT contributed to study conception, interpretation of data and gave critical revision for the manuscript, DS was the primary investigator of the study and contributed to study conception, design, data analysis, interpretation and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Scherr, A., Seifert, B., Kuster, M. et al. Predictors of marked weight gain in a population of health care and industrial workers following smoking cessation. BMC Public Health 15, 520 (2015). https://doi.org/10.1186/s12889-015-1854-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-015-1854-7