Abstract
Background
Attention-deficit hyperactivity disorder (ADHD) affects around 1 in 20 children and is associated with life-long sequelae. Previous studies of the association between Apgar score and ADHD have reported inconsistent findings.
Methods
Record linkage of maternity, prescribing and school pupil census databases was used to conduct a population e-cohort study of singleton children born in Scotland and attending school in Scotland at any point between 2009 and 2013. Binary logistic regression analysis was used to investigate the association between 5-min Apgar score and treated ADHD adjusting for sociodemographic and maternity confounders.
Results
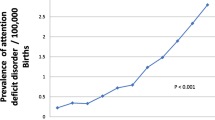
Of the 758,423 children, 7,292 (0.96%) received ADHD medication. The results suggested a potential dose–response relationship between Apgar score and treated ADHD independent of confounders. Referent to an Apgar score of 10, risk of treated ADHD was higher for scores of 0–3 (adjusted OR 1.76, 95% CI 1.32–2.34), 4–6 (adjusted OR 1.50, 95% CI 1.21–1.86) and even 7–9 (adjusted OR 1.26, 95% CI 1.18–1.36) which are traditionally considered within the normal range.
Conclusions
In addition to reinforcing the need to maximise Apgar score through good obstetric practice, the findings suggest that Apgar score may be useful in predicting future risk of ADHD and therefore facilitating early diagnosis and treatment.
Similar content being viewed by others
Introduction
Attention-deficit hyperactivity disorder (ADHD) is thought to affect around 5% of school-aged children globally [1], however estimates vary due to differences in the demographics of study populations and the diagnostic criteria applied [2, 3]. Worldwide, the prevalence of ADHD is between 2 and 7%, with a mean of 5% [4]. Based on administrative data, Holden estimated a childhood UK prevalence of 0.55% (0.93% in boys, and 0.15% in girls [5]. Additionally, a more recent retrospective cohort study in the UK indicated that the childhood prevalence of ADHD was 1.8% among boys and 0.4% among girls [6]. Due to increased knowledge, recognition, and diagnosis, the prevalence of ADHD amongst children in the UK has increased in recent years [5, 6].
The condition is generally characterised by consistent inattention, which may be accompanied with or without hyperactivity or impulsivity, but inattention is not present in a minority of cases [7]. Comorbidity is common and includes: dyslexia, low self-esteem, reading, writing and executive function challenges, mood disorders, learning disability, substance abuse, conduct and oppositional defiant disorders, Tourette syndrome, coordination difficulties, and autism spectrum disorder [8]. Symptoms persist into adulthood for 15–65% of affected children [9] and ADHD is associated with significant long-term social and economic consequences [7], including academic underachievement [10], challenges with careers and relationships [11], and criminality [12].
The Apgar score is routinely recorded on all newborn infants, 1- and 5-min following birth, irrespective of place of delivery. It was originally developed in 1952 to identify those in need of respiratory support [13]. Scores of 0–2 are allocated to each of five domains—colour, heart rate, reflexes, muscle tone, and respiration – and summated. The Apgar score is the sum of the sub-scores for each component, producing an overall score of 0–10. Overall scores in the range 7–10 are considered normal, 4–6 are considered low, and 0–3 very low [14]. Apgar scores taken at 1- minute frequently reflect acute perinatal events affecting oxygen supply, while 5-min Apgar scores measure the effectiveness of resuscitation. The Apgar score quantifies clinical manifestations of neonatal depression, such as cyanosis or pallor, bradycardia (reduced heart rate), impaired reflex response to stimulation, flaccidity of the muscles, and apnoea or gasping respirations [15]. In addition, it offers a reliable and practical way to report on the new-born baby’s condition immediately after birth and the baby's reaction to resuscitation, if necessary. Since its creation five decades ago, the Apgar score has become widely accepted as a measure of all new-born's viability and is widely utilized as an index of all infant's vitality shortly after birth [13].
Whilst not developed as a risk prediction tool, low Apgar score has nonetheless been shown to be associated with adverse neurological outcomes, including cerebral palsy (https://www.ncbi.nlm.nih.gov/books/NBK470569/#article-17763.s2). APGAR score itself is not mechanistically or causally associated with ADHD. Rather, it is a measure of the neonate’s overall status at that point in time and, therefore, a proxy measure of underlying biological mechanisms that may be causal of ADHD. For example, prenatal and intrapartum hypoxia are known to impact both APGAR score [16] and neurodevelopmental outcomes such as cerebral palsy, autism, and cognitive dysfunction [17]. However, the Apgar score in isolation cannot confirm hypoxia or its consequences [18]. Further, evidence of anomalies in neuroimaging [19] and low umbilical arterial blood gas results could be indicative of ADHD [20]. Furthermore, it has been suggested that a low Apgar score may result in neonatal hypoxia [21], which may have an impact on the dopamine-related neurodevelopmental pathways that relate to ADHD. Dopamine, a crucial neurotransmitter involved in memory, emotion, attention, behaviour, cognition, learning, sleep, and arousal, is low in those with ADHD [22]. Dopamine is produced by the basal ganglia of which perinatal anoxia has been demonstrated in animal experiments to alter mesocortical dopamine function and elevate hyperactivity [23]. Also, the dopaminergic pathways, which are thought to be crucial in the pathophysiology of ADHD, depend on the basal ganglia, which are highly susceptible to hypoxia [24]. Although the Apgar score can indicate infant hypoxia, it cannot prove it [18] since other factors, such as prematurity, low birthweight, maternal illnesses, and toxins, can also affect it [25, 26]. Therefore, a number of pathways may be involved in the association between low Apgar scores and ADHD.
Previous studies examining the relationship between 5-min Apgar score and ADHD have been heterogeneous in their methods and have produced conflicting results. Some cohort studies have reported a dose–response relationship between 1-min [27] and 5-min [28] Apgar scores and ADHD in childhood, with ADHD risk being higher even for Apgar scores of 7–8 compared to 9–10. One population cohort study reported a dose–response relationship with increasing risk of ADHD in adulthood associated with Apgar scores 0–3 and 4–6 when compared to scores of 7–9 [29]. Other studies have reported mixed or negative results. In a nested case–control study, 1-min Apgar scores less than 7 were associated with higher odds of ADHD compared with scores of 7–10 [30]. However, there was no association with 5-min Apgar score. Similarly, a population-based study, conducted in Western Australia, found no association between low 5-min Apgar score and ADHD [31]. There is some evidence that Apgar score may also be associated with ADHD severity. In a cohort of children with ADHD, symptom severity, based on the externalising scale of the Child Behaviour Checklist and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) hyperactivity symptoms count, was higher among those who had a 1-min Apgar score of 1–6 than those with Apgar scores of 7–10 [32].
Conflicting results from previous studies exist due to inconsistencies around adjustment for confounders and categorisation of Apgar score. Many previous studies have not adjusted for important factors such as maternal smoking status, sex, ethnicity, gestational age at delivery, birth-weight centile, mode of delivery and socioeconomic deprivation [27, 30] whilst others have used small sample sizes [30, 32, 33]. In this large-scale, retrospective population cohort study, we investigate the association between 5-min Apgar score and treated ADHD in childhood using linked health and education data on all children attending school in Scotland between 2009 and 2013. The study overcomes previous methodological limitations by utilising granular data covering the whole of Scotland and adjusting for a range of sociodemographic and obstetric confounders.
Methods
Data sources
Three Scotland-wide databases were linked at the individual level. The Scottish Exchange of Educational Data (ScotXed) provided data from the School Pupil Census which is conducted annually at the beginning of the academic year (September) and covers all local authority run primary, secondary, and special schools in Scotland. Public Health Scotland (PHS) provided data from the Prescribing Information System (PIS) and Scottish Morbidity Record 02 (SMR02). The PIS database covers all medications prescribed and dispensed in the community in Scotland, by community pharmacies or primary healthcare providers. The SMR02 maternity record collects data on pregnant women receiving inpatient or day case care and includes information at delivery pertaining to mother and child.
Definitions and inclusion criteria
Our cohort comprised all children born from singleton pregnancies in Scotland and attending a local authority run primary, secondary, or special school at some point between 2009 and 2013. The cohort included children born in years 1991 through to 2009. Children could attend school in multiple years across the study period and could enter or leave the cohort at any point during this time. The number of observations per child ranged from a minimum of one observation (if they left school in 2009 or started school in 2013) to five observations (if they attended school across all years). The study was limited to children born from singleton pregnancies since it was impossible to be certain that the correct child had been linked in the case of multiple births of same-sex offspring. Children with no recorded Apgar score and children recorded as attending school at < 4 years of age or > 19 years of age were excluded. The number of pupils and pupil records omitted at each stage of data cleaning is summarised in Fig. 1.
Consistent with our previous work using the same linked cohort [10, 34, 35], treated ADHD was defined as being present in a given academic year if there had been at least one dispensed prescription during that academic year of a medication authorised only for this condition: methylphenidate, dexamphetamine, atomoxetine, or lisdexamphetamine. 5-min Apgar score was obtained from the SMR02 record and categorised as 0–3, 4–6 and 7–9, with 10 as the referent category. Maternal age at delivery, parity, maternal smoking during pregnancy, gestational age at delivery, mode of delivery, sex- and gestation-specific birthweight centiles and postcode of residence at the time of delivery were all obtained from the SMR02 record. History of smoking at any point during the current pregnancy is ascertained by midwives and recorded in the SMR02 record. Mothers are recorded as having smoked during pregnancy even if they stopped before confirmation of pregnancy but after their last menstrual cycle. Postcode of residence was used to derive the Scottish Index of Multiple Deprivation 2012 (SIMD) (http://www.gov.scot/Topics/Statistics/SIMD/BackgroundMethodology) from aggregated Census data (for median populations of 769 residents) on 38 markers across seven domains: income, occupation, health, accommodation, geographic access, offences, education, and abilities and competence. Pupil’s age, sex, and ethnicity were obtained from the School Pupil Census.
Statistical analyses
The characteristics of children were summarised by Apgar score category using frequency and percentage and compared using χ2tests for trend and association and Spearman rank correlation. Binary logistic regression analysis was used to investigate the association between Apgar score and ADHD, using an Apgar score of 10 as the referent category. Because children had repeated outcome observations obtained across multiple census years, generalized estimating equations (GEE) were used to analyse the association between Apgar scores and ADHD within this longitudinal cohort. Using GEE models accounted for relationships between repeated observations relating to the same pupil throughout different census years [36]. Diverse correlation structures were compared using the user-written quasi-likelihood under the independence model criterion (QIC) statistic [37]. The most suitable correlation structure had the lowest trace QIC. For these data, an independent correlation structure was the most appropriate. The association between categorised Apgar score and ADHD was investigated using GEE analyses with an independent correlation structure, a binomial distribution, and a logit link function. Confidence intervals were produced using cluster-robust standard errors to account for correlations in the data.
The model was run univariately, followed by sequential multivariate models adjusting firstly for sociodemographic factors (age, sex, deprivation, and ethnicity) then also for obstetric factors (sex-gestation-specific birthweight centile, parity, mode of delivery, maternal age, gestational age, and maternal smoking status during pregnancy) and year of birth. The latter variable was to account for changes in Apgar score and changes in treatment of ADHD over time. Statistical analyses were undertaken using Stata MP, version 14.1 (StataCorp).
Results
Of the 758,423 eligible children with valid data on Apgar score, 7,292 (0.96%) received treatment for ADHD in the follow up period which ranged from 1 year through to 18 years (median follow up 9 years). Overall, 3,709 (0.49%) children had Apgar scores of 0–3, 7,302 (0.96%) had scores of 4–6, 625,299 (82.45%) had scores of 7–9, and 122,113 (16.10%) scored 10. Children with lower Apgar scores were more likely to be treated for ADHD. Children with the lowest Apgar scores (0–3) were more likely to be male, deprived, of white ethnicity, have a mother aged 25–29 years who smoked and was multiparous, be born preterm, have a lower birthweight centile, and be born via emergency Caesarean section (Table 1). The variables with the greatest amount of missing data were smoking during pregnancy (9.42%), and pupil ethnicity (1.79%). However, missing values for those variables, as well as missing data for mode of delivery (0.02%), were analysed as “unknown” categories and included in all analyses to minimise loss of records. The remaining variables were ordinal categories therefore including missing data as unknown categories did not make sense. However, these variables had lower levels of missing values: parity (0.57%), maternal deprivation quintile (0.14%), birthweight centile (0.09%), gestational age (0.05%) and maternal age (< 0.01%). Therefore, we did not deem multiple imputation to be necessary and instead used complete case analyses. There were no missing data for pupil sex or pupil age. All the children had valid ADHD prescribing, because those who had not linked to healthcare data were omitted at the data cleaning stage (Fig. 1).
After fully adjusting for sociodemographic and maternity confounders, lower Apgar score was associated with higher risk of receiving ADHD medication and, whilst the confidence intervals overlapped, there was evidence of a potential dose–response relationship. Compared to those with an Apgar score of 10, the increase in risk was highest among children with an Apgar score of 0–3 (odds ratio (OR) 1.76, 95% CI 1.32–2.34), and was also significantly higher among children with an Apgar score of 4–6 (OR 1.50, 95% CI 1.21–1.86) and among children with an Apgar score of 7–9 (OR 1.26, 95% CI 1.18–1.36) (Table 2).
Discussion
The study findings demonstrated a significant association between 5-min Apgar score and treated ADHD that was independent of sociodemographic and maternity confounders, with evidence of a potential dose–response relationship. Even children with Apgar scores of 7–9, which are generally interpreted as being within the normal range, had a significantly higher risk of ADHD than children who scored 10.
Previous studies have used varying approaches to categorising Apgar score. We used the categories recommended in the Neonatal Encephalopathy and Neurologic Outcome report, which defines an Apgar score of 0–3 as low and 4–6 as moderately abnormal. Comparison of Apgar scores of 7–9 with 10 enabled us to demonstrate that the potential dose–response relationship extended across the whole spectrum of scores and that even slightly reduced scores carried increased risk. This finding is consistent with previous studies showing that, compared to an Apgar score of 10, Apgar scores of 7–9 are associated with higher risk of neonatal morbidity and mortality, neurodevelopmental sequelae [38], autistic spectrum disorder [39], and problems with emotional and physical health [40, 41].
Previous studies have produced conflicting results as to whether 5-min Apgar score is associated with ADHD [28, 29] or is not [27, 31]. Many previous studies have treated Apgar score as a binary variable or only investigated low Apgar score however a few previous studies have examined whether there is evidence of a dose–response relationship [28, 42] across the spectrum and, consistent with our findings, demonstrated that Apgar scores below 10 but within the ‘normal’ range are nonetheless associated with increased risk of ADHD. Whilst they found a significant relationship, the effect sizes they obtained were inconsistent across studies. These differences can be partly explained by the method of ADHD ascertainment. To ascertain ADHD, Sucksdorff et al. [43] used a discharge register to identify cases. Whilst it was validated, there is still a possibility of misclassification bias and incomplete or incorrect information. It is equally possible that the authors missed some cases not included in the register. Schwenke et al. [30] used questionnaires which can often be affected by high rates of non-responders. Information was obtained from a caregiver or the parent with can also result in recall bias. Li et al. [28] and Halmoy et al. [29] utilised multiple data sources, including discharge registers, medical product statistics, information from clinicians, and informants' ascertainment of cases, potentially reducing information bias. However, like most secondary data, there is still potential for misclassification bias.
Choice of confounding variables, and methods used to adjust for them, varied across previous studies. Sucksdorff et al. [43] used logistic regression analysis to adjust for various confounders, including gestational age, weight for gestational age, maternal age and maternal socioeconomic status. They also adjusted for the mother's smoking status, alcohol intake, substance abuse during pregnancy, and history of maternal psychiatric diagnoses. Adjustments for maternal mental history and substance misuse were based on specialised public health care diagnoses. As a result, the authors could not adjust for ailments or personality traits treated in primary healthcare or private hospitals. This limitation is typical of observational studies where residual confounders are often present. While Sucksdorff et al. [43] adjusted for a wide range of confounders, Chandola et al. [27] only adjusted for sex in their study since most social data were not recorded in the Cardiff births register (CBR). However, the large control group enabled them to detect small but clinically significant differences between groups. Unlike Sucksdorff et al., [43] Chandola et al. [27] were limited by the amount of information in the register and resultant inability to adjust for a wide range of confounders.
Halmoy et al. [29] undertook their study adjusting for sex, mother’s age, maternal and paternal academic attainment, and parity. However, there were missing data on additional confounders, such as the history of psychiatry disorders, parents’ smoking habits, and the presence of other comorbid conditions. The confounders adjusted for were not as wide-reaching as in Surskdorff’s work [43]. Gustaffson et al., [44] like Halmoy et al., [29] used multivariable analyses to adjust for confounders, namely, year of birth, maternal age, maternal smoking, birthweight centile (birthweight for gestational age) and parity. However, the authors still lacked information on socioeconomic status, and like Halmoy et al., [29] did not have data on family history of mental disorders and abuse.
Mikkelsen et al. [16] and Li et al. [28] also used regression models to adjust for confounders. Li et al. [28] adjusted for sex, parity, gestational age, and low birth weight, family history of mental illness, maternal socioeconomic status, and smoking status during pregnancy using Cox regression. Mikkelsen et al. [16] used logistic regression, while Grizenko et al. [32] utilised a step-wise linear regression and adjusted for the same variables as Li et al. [28] except for gestational age and household history of mental ailments. Hanc et al. [33] in a case control study, matched the boys' age, socioeconomic factors, and parents' academic attainment.
The present study has several methodological strengths. The study sample of 758,423 children, was 25 times larger than any previous study addressing the same research question. We were able to adjust for a wide range of potential sociodemographic and maternal confounders including some not included in previous analyses: ethnicity, socioeconomic deprivation, child’s age, and mode of delivery. Inclusion was non-selective and country-wide, reducing the risk of bias and improving the generalisability of the results. Secondary analysis of routine databases obviated the possibility of reporting or recall bias. The grouping of Apgar scores in this study aligns with the recommendation by the Neonatal Encephalopathy and Neurologic Outcome report [19], projecting a more standardised and uniform way of categorising Apgar scores. Sub-categorising Apgar scores of 7–10 into 7–9 and 10, enabled us to examine whether it is safe to assume that any score below 10 is without risk.
As with all observational studies, causality cannot be automatically inferred from association.
However, our finding of a potential dose–response relationship is an important one. As with any observational research, there is potential for residual confounding. Specifically, we did not have access to data on family history of mental health disorders, relevant paternal factors like age, paternal history of ADHD, maternal substance abuse, and intra-uterine exposure to teratogens, and maternal history of ADHD. The annual School Pupil Census does not cover private schools, but these account for fewer than 5% of Scottish schoolchildren. With routine data, controlling completeness and accuracy is more difficult, however the databases used are all subject to regular quality control audits. Probabilistic matching was used to link education and health records however this method has been validated as 99% accurate for singletons [45]. The childhood prevalence of ADHD is around 5% in Scotland [46]. However, only 1% of children were receiving ADHD medication. The difference is likely to reflect our ascertainment method based on prescribed medication. Non-pharmacological treatment consisting primarily of parenting interventions that focuses on behavioural management is generally recommended and treatment is only reserved for those who meet certain criteria and are considered to have severe ADHD. Therefore, our ascertainment method has the limitation of potential misclassification bias whereby children with undiagnosed or milder untreated ADHD would not be ascertained and would be classed as condition free, whilst children classed as having ADHD would likely comprise those with more severe forms of the condition. This may have underestimated the strength of the relationship between Apgar score and ADHD. Unfortunately, we could not determine age of ADHD onset from our data as our observation period only started from 2009 onwards. Therefore, prescriptions for ADHD (our method of ascertainment) administered before this time were not available. Our study enabled us to ascertain children who 1. Were already on treatment for ADHD at the start of the study period (2009) or 2. Went on start treated during the study period (2009–2013). Age at commencement of treatment was only available for those in category 2 and age at formal diagnosis was not available for either.
Conclusions
There was a significant, and potential dose–response, relationship between Apgar score and treated ADHD. In addition to reinforcing the need to maximise Apgar score through good obstetric practice, these findings suggest that Apgar score could be indicative of future risk of ADHD, therefore facilitating earlier diagnosis and treatment.
Availability of data and materials
The datasets generated and analysed during the study are not publicly available. All health data are owned by Public Health Scotland (https://www.publichealthscotland.scot), and all education data are owned by the Scottish Government (www.2.gov.scot/Topics/Statistics/ScotXed). Under the terms of our data access agreements with them we are not permitted to pass the data onto third parties. Interested researchers may apply at these sites for data access to health and education data by emailing phs.edris@phs.scot and ASU_schools_Data_Access@gov.scot respectively. The authors applied for permission to access, link, and analyse these data and undertook mandatory training in data protection, IT security and information governance. The study was approved by the National Health Service (NHS) Public Benefit and Privacy Panel and covered by a data processing agreement between Glasgow University and Public Health Scotland and a data sharing agreement between Glasgow University and ScotXed. The electronic Data Research and Innovation Service (eDRIS) within Public Health Scotland helped the authors obtain approvals, linked the data, and uploaded the final datasets into a secure analytical platform within the National Safe Haven for the researchers to analyse. The researchers did not receive any special privileges or access to the third-party data.
References
Szatmari P. The Epidemiology of Attention-Deficit Hyperactivity Disorders Child Adolesc Psychiatr. Clin N Am. 1992;1(2):361–71.
Møller LR, Sørensen MJ, Thomsen PH. ICD-10 classification in Danish child and adolescent psychiatry–have diagnoses changed after the introduction of ICD-10? Nord J Psychiatry. 2007;61(1):71–8.
Taylor E, Döpfner M, Sergeant JA, Asherson PJ, Banaschewski T, Buitelaar JK, et al. European clinical guidelines for hyperkinetic disorder. Eur Child & Adolesc Psychiatry. 2004;13.
Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175–86.
Holden SE, Jenkins-Jones S, Poole CD, Morgan CL, Coghill D, Currie CJ. The prevalence and incidence, resource use and financial costs of treating people with attention deficit/hyperactivity disorder (ADHD) in the United Kingdom (1998 to 2010). Child Adolesc Psychiatry Ment Health. 2013;7(1):34.
McKechnie DGJ, O’Nions E, Dunsmuir S, Petersen I. Attention-deficit hyperactivity disorder diagnoses and prescriptions in UK primary care, 2000–2018: population-based cohort study. BJPsych Open. 2023;9(4):e121.
Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990-1002.e2.
Albrecht B, Uebel-Von Sandersleben H, Gevensleben H, Rothenberger A. Pathophysiology of ADHD and associated problems—starting points for NF interventions? Front Hum Neurosci. 2015;9(6):1–14.
Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65.
Fleming M, Fitton CA, Steiner MFC, McLay JS, Clark D, King A, et al. Educational and health outcomes of children treated for attention-deficit/hyperactivity disorder. JAMA Pediatr. 2017;171(7):e170691.
Adamou M, Arif M, Asherson P, Aw T-C, Bolea B, Coghill D, et al. Occupational issues of adults with ADHD. BMC Psychiatry. 2013;17(13):59.
Young S, Thome J. ADHD and offenders. World J Biol Psychiatry. 2011;1(12 Suppl 1):124–8.
Apgar V. A Proposal for a New Method of Evaluation of the Newborn Infant. Originally published in July 1953, volume 32, pages 250–259. Anesth Analg. 2015 May;120(5):1056–9.
Zaichkin J WG. Textbook of Neonatal Resuscitation (NRP), 7th Ed. American Academy of Pediatrics; 2016.
Watterberg KL, Aucott S, Benitz WE, Cummings JJ, Eichenwald EC, Goldsmith J, et al. The apgar score. Pediatrics. 2015;136(4):819–22.
Mikkelsen SH, Olsen J, Bech BH, Wu C, Liew Z, Gissler M, et al. Birth asphyxia measured by the pH value of the umbilical cord blood may predict an increased risk of attention deficit hyperactivity disorder. Acta Paediatr. 2017;106(6):944–52.
Ma Q, Xiong F, Zhang L. Gestational hypoxia and epigenetic programming of brain development disorders. Drug Discov Today. 2014;19:1883–96.
Ruth VJ, Raivio KO. Perinatal brain damage: predictive value of metabolic acidosis and the Apgar score. BMJ. 1988;297(6640):24–7.
Burd I, Andrikopoulou M, Farzin A, Bienstock J, Graham E. Neonatal Encephalopathy and Neurologic Outcome. Postgrad Obstet Gynecol. 2014;34:1–5.
Getahun D, Rhoads GG, Demissie K, Lu S-E, Quinn VP, Fassett MJ, et al. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. 2013;131(1):e53-61.
Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcomes: analysis of 51,519 consecutive validated samples. BJOG. 2012;119(7):824–31.
Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid Med Cell Longev. 2016: 9730467.
Brake W, Sullivan R, Gratton A, Brake WG, Sullivan RM, Gratton A. Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci. 2000;20:5538–43.
Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96(9):1269–74.
Catlin EA, Carpenter MW, Brann BS 4th, Mayfield SR, Shaul PW, Goldstein M, et al. The Apgar score revisited: influence of gestational age. J Pediatr. 1986;109(5):865–8.
Freeman JM, Nelson KB. Intrapartum asphyxia and cerebral palsy. Pediatrics. 1988;82(2):240–9.
Chandola CA, Robling MR, Peters TJ, Melville-Thomas G, McGuffin P. Pre- and Perinatal Factors and the Risk of Subsequent Referral for Hyperactivity. J Child Psychol Psychiatry. 1992;33(6):1077–90.
Li J, Olsen J, Vestergaard M, Obel C. Low Apgar scores and risk of childhood attention deficit hyperactivity disorder. J Pediatr. 2011;158(5):775–9.
Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71(5):474–81.
Schwenke E, Fasching PA, Faschingbauer F, Pretscher J, Kehl S, Peretz R, et al. Predicting attention deficit hyperactivity disorder using pregnancy and birth characteristics. Arch Gynecol Obstet. 2018;298(5):889–95.
Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;133(1):e14-22.
Grizenko N, Eberle ML, Fortier M-E, Côté-Corriveau G, Jolicoeur C, Joober R. Apgar scores are associated with attention-deficit/hyperactivity disorder symptom severity. Can J Psychiatry / La Rev Can Psychiatr. 2016;61(5):283–90.
Hanć T, Szwed A, Słopień A, Wolańczyk T, Dmitrzak-Węglarz M, Ratajczak J. Perinatal Risk Factors and ADHD in Children and Adolescents: A Hierarchical Structure of Disorder Predictors. J Atten Disord. 2018;22(9):855–63.
Fleming M, Salim EE, Mackay DF, Henderson A, Kinnear D, Clark D, et al. Neurodevelopmental multimorbidity and educational outcomes of Scottish schoolchildren: A population-based record linkage cohort study. PLoS Med. 2020. 17(10)
Fleming M, Bandyopadyay A, McLay JS, Clark D, Kind A, Mackay DF, Lyons RA, Sayal K, Brophy S, Pell JP. Age within schoolyear and attention deficit hyperactivity disorder in Scotland and Wales. BMC Public Health. 2022;22:1070.
Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. 2nd ed. Cambridge University Press; 2013.
Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–20.
Razaz N, Cnattingius S, Joseph KS. Association between Apgar scores of 7 to 9 and neonatal mortality and morbidity: population based cohort study of term infants in Sweden. BMJ. 2019;365:l1656.
Modabbernia A, Sandin S, Gross R, Leonard H, Gissler M, Parner E, et al. Apgar score and risk of autism. Eur J Epidemiol. 2019;1:34.
Razaz N, Boyce WT, Brownell M, Jutte D, Tremlett H, Marrie RA, et al. Five-minute Apgar score as a marker for developmental vulnerability at 5 years of age. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F114–20.
Razaz N, Cnattingius S, Persson M, Tedroff K, Lisonkova S, Joseph KS. One-minute and five-minute Apgar scores and child developmental health at 5 years of age: A population-based cohort study in British Columbia. Canada BMJ Open. 2019;9(5):1–9.
He H, Yu Y, Wang H, Obel CL, Li F, Li J. Five-Minute Apgar Score and the Risk of Mental Disorders During the First Four Decades of Life: A Nationwide Registry-Based Cohort Study in Denmark. Front Med. 2022;8(January):1–9.
Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Gissler M, Sourander A. Lower Apgar scores and Caesarean sections are related to attention-deficit/hyperactivity disorder. Acta Paediatr. 2018;107(10):1750–8.
Gustafsson P, Källén K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: Results from a population-based study utilizing the Swedish Medical Birth Register. Dev Med Child Neurol. 2011;53(3):263–8.
Wood R, Clark D, King A, Mackay D, Pell JP. Novel cross-sectoral linkage of routine health and education data at an all-Scotland level: a feasibility study. Lancet. 2013;382.
Edinburgh UNS. Healthcare Improvement Scotland. Attention deficit and hyperkinetic disorders: services over Scotland; Final report 2012. 2008.
Acknowledgements
The study was sponsored by Health Data Research UK (www.hdruk.ac.uk) which is a joint investment led by the Medical Research Council, together with the National Institute for Health Research (England), the Chief Scientist Office (Scotland), Health and Care Research Wales, Health and Social Care Research and Development Division (Public Health Agency, Northern Ireland), the Engineering and Physical Sciences Research Council, the Economic and Social Research Council, the British Heart Foundation and Wellcome (grant reference number MR/S003800/1). The sponsor and funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Funding
Health Data Research UK (grant reference number MR/S003800/1).
Medical Research Council,MR/S003800/1
Author information
Authors and Affiliations
Contributions
MF had the original concept. All authors agreed the study design. JJB and JDB undertook the literature search. JJB and MF undertook the statistical analyses. All authors interpreted the results. JJB, JPP and MF drafted the manuscript and all authors contributed to its revisions. All authors reviewed and approved the final version of the manuscript. MF is guarantor for the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Linkage studies of Scottish routine data are covered by generic ethics approval. The NHS West of Scotland Research Ethics Service confirmed that formal NHS ethics approval was not required since the study involved linkage of routinely collected data with an acceptably negligible risk of identification. This study was approved by the Public Benefit and Privacy Panel (reference number 1920–0144) and covered by a data sharing agreement between the University of Glasgow and Public Health Scotland. The linked data were anonymised then analysed remotely in a national safe-haven environment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bala, J.J., Bala, J.D., Pell, J.P. et al. Association between 5-min Apgar score and attention deficit hyperactivity disorder: a Scotland-wide record linkage study of 758,423 school children. BMC Psychiatry 23, 794 (2023). https://doi.org/10.1186/s12888-023-05217-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05217-6