Abstract
Background
Choosing an antipsychotic medication is an important medical decision in the treatment of schizophrenia. This decision requires risk-benefit assessments of antipsychotics, and thus, shared-decision making between physician and patients is strongly encouraged. Although the efficacy and side-effect profiles of antipsychotics are well-established, there is no clear framework for the communication of the evidence between physicians and patients. For this reason, we developed an evidence-based shared-decision making assistant (SDM-assistant) that presents high-quality evidence from network meta-analysis on the efficacy and side-effect profile of antipsychotics and can be used as a basis for shared-decision making between physicians and patients when selecting antipsychotic medications.
Methods
The planned matched-pair cluster-randomised trial will be conducted in acute psychiatric wards (n = 14 wards planned) and will include adult inpatients with schizophrenia or schizophrenia-like disorders (N = 252 participants planned). On the intervention wards, patients and their treating physicians will use the SDM-assistant, whenever a decision on choosing an antipsychotic is warranted. On the control wards, antipsychotics will be chosen according to treatment-as-usual. The primary outcome will be patients’ perceived involvement in the decision-making during the inpatient stay as measured with the SDM-Q-9. We will also assess therapeutic alliance, symptom severity, side-effects, treatment satisfaction, adherence, quality of life, functioning and rehospitalizations as secondary outcomes. Outcomes could be analysed at discharge and at follow-up after three months from discharge. The analysis will be conducted per-protocol using mixed-effects linear regression models for continuous outcomes and logistic regression models using generalised estimating equations for dichotomous outcomes. Barriers and facilitators in the implementation of the intervention will also be examined using a qualitative content analysis.
Discussion
This is the first trial to examine a decision assistant specifically designed to facilitate shared-decision making for choosing antipsychotic medications, i.e., SDM-assistant, in acutely ill inpatients with schizophrenia. If the intervention can be successfully implemented, SDM-assistant could advance evidence-based medicine in schizophrenia by putting medical evidence on antipsychotics into the context of patient preferences and values. This could subsequently lead to a higher involvement of the patients in decision-making and better therapy decisions.
Trial registration
German Clinical Trials Register (ID: DRKS00027316, registration date 26.01.2022).
Similar content being viewed by others
Background
A large number of antipsychotics are available for the treatment of schizophrenia with substantial differences in their side-effect profiles and smaller in efficacy [1]. This makes the selection of an antipsychotic for the individual more difficult, given also that several factors should be taken into consideration, such as patient preferences, prior experiences, side-effect profiles, comorbidities, age and sex [2,3,4,5]. This “preference-sensitive” decision requires shared-decision-making (SDM) between patients and their treating physicians [4, 6]. In addition, physician decision-making behaviours are often not evidence-based, but influenced by irrational factors [7,8,9], cognitive biases [10, 11], and lack of statistical knowledge [12]. Furthermore, decisions in the treatment of schizophrenia are perceived by many patients as “paternalistic” or dominated by the physician, without patients really having a choice about which drug to be prescribed [13, 14]. These aspects could lead to less optimised drug selection, poor therapeutic relationship, lower adherence and increased relapse rates [15].
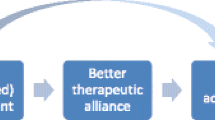
In order to overcome the above defects in decision-making, we developed an evidence-based shared-decision-making assistant (SDM-assistant) for choosing antipsychotics. This newly developed tool provides a framework of shared-decision-making illustrating evidence from a network meta-analysis on the efficacy and side-effects of antipsychotics [1]. It may also overcome limitations of previous tools with limited capabilities in shared-decision-making or presenting evidence of lower quality [16]. Patients and practitioners can use the SDM-assistant as a basis of share-decision-making by discussing and navigating differences of antipsychotics. This could facilitate “better” therapy decisions (e.g., fewer adverse events) and increase patient involvement, and thus, could subsequently lead to greater patient satisfaction and, potentially, fewer rehospitalisations due to improved adherence [17, 18]. Figure 1 shows the potential outcomes of using the SDM-assistant for choosing antipsychotics, as adapted by our previous work on shared-decision making in patients with an acute exacerbation of schizophrenia [19].
The planned cluster-randomized-controlled trial aims to compare the effects of SDM-assistant versus treatment-as-usual (TAU) in inpatients with schizophrenia. We will investigate whether the SDM-assistant can improve perceived shared-decision-making, lead to fewer side-effects and better adherence at discharge and three-months after discharge. We will also explore factors that could have an impact on the use and effects of the SDM-assistant tool.
Methods
Trial design
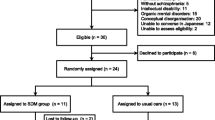
The planned study is a cluster-randomized-controlled trial evaluating the use of an evidence-based shared-decision making assistant (SDM-assistant) for choosing antipsychotics in patients with schizophrenia hospitalised in acute psychiatric wards in three hospitals in Munich, Germany (Klinikum rechts der Isar of the Technical University of Munich, Isar-Amper-Klinikum München-Ost and München-Schwabing). The protocol is registered in the German Clinical Trials Register (ID: DRKS00027316, registration date 26.01.2022). The protocol is reported according the SPIRIT [20] and SUNDAE [21] statements.
Participants
Participants should have a diagnosis of a schizophrenia spectrum disorder (ICD-10 F20-F9) and be hospitalized in an acute psychiatric ward at the time of examination. They should be aged between 18 and 65 years at the time of inclusion and be able to give their informed consent to participate in the study. There are no other exclusion criteria, e.g., in terms of sex, ethnicity, illness stage.
Intervention and control group
Antipsychotic drugs are recommended for the treatment of acute episodes of schizophrenia [4]. Therefore, decisions on choosing which antipsychotic drug to be used are necessary in acute psychiatric wards. In the intervention group, whenever a decision on choosing an antipsychotic is warranted (e.g., initiation, continuation or change of antipsychotic drugs), patients and their treating physicians will use the SDM-assistant in the context of a shared-decision-making process for the selection of an antipsychotic, in addition to the usual care. The SDM-assistant can be used more than once in different sessions during the trial. In the control group, patients and their treating physicians will not use the SDM-assistant, and antipsychotics will be chosen according to treatment-as-usual (TAU). Changes in the treating physicians will be discouraged, yet, when necessary, they will be recorded. In addition, we will use cluster randomization at the ward level, and therefore, a possible switch to a physician from the other arm of the trial would be unlikely. We will consider as protocol violations when patients do not receive at least one session of the SDM-assistant or when patients switch from the control to the intervention group or vice versa.
SDM-assistant for choosing antipsychotics
The SDM-assistant is a browser-based-application developed by our team in accordance to the IPDASi v4.0 [22] (eAppendix 1) and provides an interactive framework of shared-decision-making between patients and physicians for the selection of antipsychotics. The SDM-assistant provides a basis for a decision; the final decision of the selection of the drug will be taken in a shared-decision-making process between clinician and patients, and there is no direct treatment recommendation provided by the SDM-assistant tool. Here, reference is made to the three-talk model of SDM, and the work with the decision aid is located in the “option talk” [23]. We included patients and relatives in the development and design of the SDM-assistant, and its final format and way of presenting the data was determined in pre-tests and focus groups.
Each session of the SDM-assistant is multi-step (Fig. 2). At the very beginning, treating physicians will receive training in the use of the SDM-assistant and will be introduced in the methods of evidence-based-medicine and shared-decision-making. In a first step, users (patients and physicians) work on the SDM-assistant separately. Patients get information from text descriptions and video presentations by health practitioners and ex-users about schizophrenia, aspects of drug treatment with antipsychotics, and shared-decision-making. Patients are then guided to think about their own goals and preferences for the treatment with antipsychotics (e.g., prior experience with antipsychotics, side-effects to avoid, antipsychotic formulation). Physicians, on the other side, enter clinically relevant patient information (e.g., age, sex, duration of illness, comorbidities).
In a second step, patients and physicians can jointly consult forest plots presenting evidence on antipsychotics (Fig. 3), and subsequently make a shared treatment decision.
Forest plot interface of the SDM-assistant for choosing antipsychotics. Forest plots presenting effect-sizes (point estimates and their 95% credible intervals) for the comparisons of antipsychotics with placebo. Relative risks are used as the effect-size, except for mean differences in kg for weight gain. The figure was taken as a screenshot from the SDM-assistant for choosing antipsychotics
In the context of the study, evidence on twelve antipsychotics (i.e., amisulpride, aripiprazole, cariprazine, clozapine, haloperidol, olanzapine, paliperidone, perphenazine, quetiapine, risperidone, sertindole, and ziprasidone) licensed and commonly used in Germany is considered. Other antipsychotics were not considered, e.g. not yet licensed (e.g., brexpiprazole or lurasidone), old and not widely used (e.g., many first-generation antipsychotics) [24] and/or based on limited evidence from RCTs (e.g., fluphenazine or sulpiride) [1]. Published evidence from a large network meta-analysis conducted by our group is used [1]. Network meta-analysis is considered the highest level of evidence in treatment guidelines [25], and thus, can be informative for the shared-decision-making process. Both efficacy and side-effects are important for the selection of an antipsychotic [2, 3], therefore, we consider overall efficacy (reduction of overall symptoms as measured by validated scales) and six important side-effects of antipsychotics, i.e., weight gain, use of antiparkinsonian medications as a proxy of extrapyramidal symptoms, akathisia, sedation, anticholinergic side-effects (e.g., blurred vision, constipation, dry mouth or hyposalivation and urinary retention), and hyperprolactinaemia that could also be used a weak proxy of sexual side-effects [26, 27].
Forest plots present effect-sizes and their 95% credible intervals of the comparisons between antipsychotics and placebo. Relative risks are used as the effect-size in the forest plots, since they can be more intuitive than other measures such as odds ratios, standardized-mean-differences, or even mean-differences (e.g., in scale scores for overall efficacy and extrapyramidal symptoms or ng/ml for prolactin levels) [28]. However, mean-differences are presented for weight gain (in kg) because of their straightforward understanding for this outcome. When relative risks are not originally reported in the network meta-analysis [1], they are converted from other effect-sizes indices [29] (eAppendix 2). We prefer relative over absolute measures (e.g., absolute risk of a patient to have a side-effect) because they are generally constant across different baseline risks and definitions of the outcome [30], which are common in meta-analysis, especially for the side-effects of antipsychotics [31]. In addition, absolute measures require a baseline risk that can be difficult to be estimated for the individual. Nevertheless, the risk in the placebo group can be presented and discussed, i.e., the weighted percentage of patients in the placebo group with a positive response to treatment or a side-effect.
Patients and their physicians see first the forest plot of efficacy, in which antipsychotics are ranked from the best to the worst efficacy according to the ranking in the network meta-analysis [1]. Then, they can add or remove forest plots for the different side-effects. In that way, they can simultaneously look at effect-sizes of antipsychotics across different outcomes and change the ranking of antipsychotics based on one of these outcomes. They can remove antipsychotics from the list and provide reasons for exclusion. In the end of this step, patients and physicians select one (or more) antipsychotics as potential candidates to be used.
In order to facilitate the process, there are help buttons. In addition, there are short text descriptions for each outcome, and short summaries for each antipsychotic based on the findings of the network meta-analysis [1], treatment guidelines [4], reference textbooks [32] and the respective product leaflets (Fig. 4). The short summaries for each antipsychotic consist of a text description that provides information about the unique indications, contraindications, dosing, pharmacokinetics, and side-effect profile of the selected antipsychotic (also considering side-effects not presented in the forest plots, such as QTc prolongation, myocarditis and agranulocytosis etc.), and a plot that illustrates the relationship of the relative risk of the antipsychotic in comparison to the average relative risk of all antipsychotics with z-scores (eAppendix 2).
Summary description and summary plot for amisulpride. The short summary for each antipsychotic (e.g., amisulpride) consists of a text description and a plot that presents Z-scores of relative risks across outcomes, which can show the relationship of the relative risk of amisulpride in comparison to the average relative risk of all medications. The figure was taken as a screenshot from the SDM-assistant for choosing antipsychotics
In a third step, patients and their physicians look at an overview of patient’s goals and preferences, clinically relevant patient characteristics, selected and excluded antipsychotic medications. They have the chance to further discuss and modify their selections.
In a fourth step, they finalize the antipsychotic selection and save the session.
Outcomes
Recruitment and data collection will take place at the participating wards. Baseline data will be measured at inclusion and before using the SDM-assistant (T0). Then, the aim is that patients and physicians will use the SDM-assistant at least once during inpatient stay whenever a decision on choosing an antipsychotic is warranted, but no later than three weeks after admission. Both will also be encouraged to use the SDM-assistant as often as possible. At the time of discharge from the hospital, outcomes will be measured (T1). Three months after the discharge, we will contact each patient and the treating outpatient psychiatrist via telephone and/or post in order to obtain follow-up outcome data (T2). Table 1 presents a schematic diagram of participant timeline and data collection. Scales (German versions) could be filled accordingly by patients, their treating physicians or the study personnel (e.g., clinical psychologists not involved in the treatment of the patients and trained to administer the scales). Physicians and study personnel will receive training on the use of individual scales.
Baseline parameters
We will record at baseline (T0) socio-demographics (i.e., age, sex, nationality, language, education, family and occupation status, digital literacy), anamnesis (ICD-10 diagnoses, duration of illness, number of previous hospitalizations, previously prescribed medications), and information about the current admission (legal status, voluntary or involuntary treatment with antipsychotics).
We will also record patient-related factors that could influence the shared-decision making for selecting treatments, i.e. patients’ participation preferences for decision making using the corresponding subscale of the Autonomy Preference Index [33], insight and perceived necessity of treatment using the Birchwood Insight Scale [34] and perception of the current admission using the MacArthur Admission Experience Survey [35].
Primary outcome
The primary outcome will be patients’ perceived involvement in decision-making using the Shared-Decision-Making Questionnaire (SDM-Q-9) [36] at T1. The SDM-Q-9 is a self-report scale consisting of nine items that describe different parts of the SDM process and reflecting the inpatient decision-making, e.g., here on the selection of antipsychotic medication. Each item will be scored from 0 “completely disagree” to 5 “completely agree”, and a total score will be calculated ranging from 0 to 45, with a higher score indicated a better-perceived involvement [36]. We expect that the use of the SDM-assistant would substantially improve patients’ perceived involvement, thus identifying a clinically meaningful mean difference of at least 15 points in SDM-Q-9 between the intervention and control group [37, 38].
Secondary outcomes and other variables
In addition to the patient’s perceived involvement, we will also investigate physicians’ perceived involvement in decision-making as a secondary outcome using the physician version of the SDM-Q-9 (SDM-Q-Doc) [39] at T1. This will refer to the perceived involvement of the inpatient physician, and any change of the treating physician during the inpatient stay will be recorded.
Apart from the potential improvement in shared-decision-making, we will investigate whether the SDM-assistant could facilitate a more appropriate selection of antipsychotic, as reflected by lower symptom severity and fewer adverse events, higher quality of life and functioning, higher treatment satisfaction and improved adherence.
We will record information about the prescribed antipsychotic medications (e.g., dose schedule, formulation, monotherapy or polypharmacy, concomitant medications) at T0, T1 and T2.
Symptom severity and improvement will be measured at T0 and T1, using the clinician-rated Positive and Negative Syndrome Scale 6-item version (PANSS-6) [40]. PANSS-6 is a recently validated abbreviated version of the original PANSS scale [41], consisting of six items of core schizophrenia symptoms (P1 = delusions, P2 = conceptual disorganization, P3 = hallucinations, N1 = blunted affect, N4 = social withdrawal, N6 = lack of spontaneity/flow of conversation) [40, 42,43,44]. We will also investigate the clinician-rated Clinical Global Impression-Severity and Improvement (CGI-S, CGI-I) [45], and the patient-rated Clinical Global Impression (CGI-P) [46]. In addition, we will record the length of inpatient stay at T1, and the outpatient psychiatrist will be asked at T2 to document any readmission to the hospital of the patient.
Side-effects will be recorded using items of the clinician version of the Udvalg for kliniske undersøgelser scale (UKU-SERS) [47] at T0, T1 and T2. The UKU scale consists of 48 items covering a comprehensive list of side-effects grouped in four categories, i.e., psychic, neurologic, autonomic and other side-effects. We will also evaluate two additional items about suicidality and oedema. Each item is scored from 0 to 3 based on the severity with a higher score indicating more severity [47]. It will be administered via a semi-structured interview by the study personnel. The weight of patients will be measured at T0 and T1, and we will ask the patients to report their weight at T2. Furthermore, we developed the patient-reported antipsychotic and antidepressant side-effect scale (ANTISIDES) that will be administered at T0, T1 and T2. The scale consists of 41 items covering a broad spectrum of side effects that can be caused by psychotropic drugs. Each item will be rated on a visual analogue scale from 0 “not at all” to 10 “extremely strong”, supplemented by an assessment of the impairment experienced due to the respective side effect (eAppendix 3).
We will address global functioning using the clinician-rated Social and Occupational Functioning Assessment Scale (SOFAS) [48] at T0, T1 and T2, and quality of life using the patient-reported EUROHIS-QOL [49] at T2.
Treatment satisfaction, attitudes and adherence will be measured using the patient-reported scales Questionnaire on Patients’ Treatment Satisfaction (ZUF8) [39], Medication Satisfaction Questionnaire (MSQ) [50], Drug Attitude Inventory (DAI) [51] and Medication Adherence Rating Scale (MARS) [52] at T0, T1 and T2. Physicians will also rate adherence to the medication treatment at T1 and T2 using a single question scored on a 4-point Likert scale from 1 “very good” to 4 “poor”.
Last, data about the use of the SDM-assistant (e.g., number of sessions, duration of use) will be recorded within the SDM-assistant and evaluated at T1. This information would also be helpful in assessing treatment integrity.
Qualitative data
We will also obtain qualitative data in order to gain insights into the treatment integrity and mode of action of using the SDM-assistant for choosing antipsychotics, as well as identify further potential influencing factors. The combination of quantitative (hypothesis-driven) and qualitative approach would increase the validity and interpretation of the findings of the trial. For this purpose, we will use purposive sampling in order to identify individual cases of successful/unsuccessful joint decisions or antipsychotic selection. We will conduct interviews with about 15 patients from the intervention group until no fundamentally new aspects will be added. Possible inclusion criteria are as follows: 1) patient or physician are dissatisfied with the use of SDM-assistant and/or do not want to use it in the future, 2) patient or physician discontinue the use of SDM-assistant, 3) patient or physician disagree with the outcome of the SDM-assistant. 4) patient or physician satisfaction with the SDM-assistant is far above average, 5) the use or the outcome of the SDM-assistant triggers unusual or unexpected reactions in the patient or physician. Physicians will be interviewed separately and in terms of their experience across several uses of the app with their patients. The interviews will be guideline-based and conducted by the study personnel, recorded acoustically, then transcribed and qualitatively analysed using content analysis [53].
Sample size and recruitment
The target sample size is N = 252 patients from n = 14 clusters (126 participants from 7 intervention wards and 126 participants from 7 control wards). Power calculation estimated N = 180 participants (n = 12 clusters) to be analysed in order to detect an expected Cohen’s d of 0.45–0.5 in the primary outcome of SDM-Q-9 with a power of at least 80% at two-sided alpha of 0.05 assuming ICC = 0.02. These assumptions are based on data from a comparable study (SDM-PLUS), which involved a similar population and the same main outcome parameter [38]. Due to an assumed drop-out rate of patients of 15–25% and to account for a potential drop out of clusters, we aim at recruiting n = 14 clusters and N = 252 patients.
Patient recruitment will be conducted by a trained study staff and will take place on the 14 selected wards at three hospitals in Munich, Germany (Klinikum rechts der Isar of the Technical University of Munich, kbo-Isar-Amper-Klinikum München-Ost and München-Schwabing). These hospitals with more than 1500 beds in total can facilitate the enrolment of the planned number of patients (N = 252) in a timely manner, as in our previous trials [38, 54].
Randomization and blinding
Randomization will take place at the cluster (i.e., ward) level, in order to minimize contamination effects inherent to the nature of the intervention in case the randomization was performed at the patient or physician level [55]. The investigators will determine seven pairs of comparable wards (regarding the treatment spectrum, the size of the ward, etc.) and then randomize one ward of each pair either to the intervention or to the control group. While the principal investigators will determine the paired wards, the randomization will be conducted by a blinded investigator independent to the study using the coin toss method. However, due to the nature of the intervention (use of the SDM-assistant vs. TAU), there will be no blinding between intervention and control wards.
Data analysis
The effect-size for continuous outcomes will be the mean difference (MD) and for dichotomous outcomes will be the odds ratio (OR), presented with their 95% confidence intervals. Per-protocol analysis will be conducted, i.e., by excluding patients with no outcome data, patients that do not receive at least one session of SDM-assistant, patients who moved from a ward belonging to the control group to a ward belonging to the intervention group or vice versa. There will be no imputation of missing outcome data, and observed cases will be used.
The primary outcome (SDM-Q-9 at T1) and secondary continuous outcomes will be analysed with mixed-effects linear regression models fitted with ward (cluster) as a random-effects term and with intervention/control group as a fixed-effects term. In a similar vein, dichotomous outcomes will be analysed with logistic regression models using the generalised estimating equations (GEEs).
The robustness of the results of the primary outcome will be investigated in a sensitivity analysis by adjusting for baseline covariates such as severity of illness, admission status and variables with a large baseline imbalance.
In addition, we plan exploratory subgroup analyses in order to investigate if there is potential effect-modification of patient characteristics (e.g., age, sex, symptom severity, duration of illness) on the primary outcome.
Alpha will be set at two-sided alpha of 0.05.
Discussion
Choosing an antipsychotic medication is an important medical decision that should require benefit-risk assessments and the active involvement of patients whenever possible (i.e., shared-decision making) [4, 5]. In order to facilitate shared-decision making for choosing antipsychotics, we developed an evidence-based shared-decision making assistant (SDM-assistant). In the planned cluster-randomised trial, we will examine the use of SDM-assistant for choosing antipsychotics in hospitalized patients with an acute exacerbation of schizophrenia. We will evaluate the perceived involvement of patients in the decision-making as primary outcome, and therapeutic alliance, symptom severity, side-effects, treatment satisfaction, adherence, quality of life, functioning and rehospitalizations as secondary outcomes.
There are potential methodological limitations and challenges.
First, the SDM-assistant aims to present high-quality evidence from a network meta-analysis on the efficacy and side-effects of antipsychotics [1]. However, not every important side-effect is covered, for example cognitive and sexual side-effects (yet prolactin elevation used as a proxy of sexual side-effects, as discussed above) [56, 57], since they have not been well examined in network meta-analyses. In addition, the current version of SDM-assistant is focusing on the selection of antipsychotics in the acute phase, and other aspects of antipsychotic treatment are not considered, long-term treatment, management of adverse events, switching antipsychotics, dosing etc. Moreover, we focused on medication issues, yet we acknowledge that the treatment of schizophrenia is multimodal, e.g., psychological treatments have also a major role [58, 59]. In future updates, SDM-assistant could implement new data from network meta-analysis (or other sources of high-quality evidence), expand the list of side-effects and antipsychotics, as well as consider additional treatment modalities (e.g., psychological treatments and non-invasive brain stimulation) and extend the framework to facilitate a broader range of decisions. Additional features could also be added such as giving the option to select different effect-size indices (e.g., absolute percentages) [29] or different plots for presentation (e.g., Cates or Kilim plots, etc.) [60, 61]. Furthermore, recent meta-analytic methods that have been developed to individualise treatment recommendations, e.g., [62, 63] were not yet implemented. The use of such complicated methods in shared-decision making requires caution, since they could swift decision-making to a computerised paternalistic process that is heavily dependent on statistical assumptions [16].
Third, there are certain difficulties in the implementation of the intervention. Shared-decision making can be successful, yet challenging, in acutely ill inpatients with schizophrenia, potentially due to reduced decisional capacity, high severity psychotic symptoms, cognitive impairment, lack of insight, and negative attitudes towards the treatment [64,65,66]. The use of the SDM-assistant could make the process even more demanding due to the higher attention and cooperation requirements. For these reasons, we will use a mixed-methods approach to evaluate barriers and facilitators for the implementation of the intervention. SDM-assistant aims to enable patients to get familiar with the evidence and participate in the decision-making, without forcing them to be involved. In those cases, a more paternalistic decision-making approach with the physician choosing a suitable medication might be still acceptable [18]. Nevertheless, the early involvement of patients in the selection of antipsychotics can be very important in order to prevent burdensome side-effects, since at least some of them could be subjectively realized only later after the improvement of symptoms, e.g. rapid weight gain [67]. Third, treatment-as-usual will be used in the control group, instead of an active control group (e.g., using paper forms to present evidence on antipsychotics or unspecific group training) in order to avoid contamination. However, it is possible that the increased attention in the intervention group could inflate any differences found.
In conclusion, we developed SDM-assistant, an SDM assistant that presents high-quality evidence on the efficacy and side-effect profile of antipsychotics and can be used as a framework for shared-decision making between physicians and patients with schizophrenia when selecting antipsychotic medications. The major aim of the planned trial is to show that the use of SDM-assistant leads to increased patients’ perceived involvement in the decision-making process as well as to a more suitable selection of antipsychotics, as reflected by our secondary outcomes (e.g., symptom severity, side-effects, adherence, treatment satisfaction, rehospitalizations). The implementation of this intervention is challenging in acutely ill patients with schizophrenia, and potential barrier and facilitators will be examined. Last, according to one of the principles of evidence-based medicine [68], we hope that SDM-assistant can successfully advance evidence-based medicine in schizophrenia by putting medical evidence on antipsychotics into the context of patient preferences and values.
Availability of data and materials
Not applicable.
Abbreviations
- ANTISIDES:
-
Antipsychotic and antidepressant side-effect scale
- API:
-
Autonomy Preference Index
- BIS:
-
Birchwood Insight Scale
- CGI-I:
-
Clinical Global Impression Improvement scale
- CGI-P:
-
Clinical Global Impression Patient scale
- CGI-S:
-
Clinical Global Impression Severity scale
- CRF:
-
Case Report Form
- DAI:
-
Drug Attitude Inventory
- GEE:
-
Generalised estimating equations
- ICD:
-
International Classification of Diseases
- IPDAS:
-
International Patient Decision Aids Standards instrument
- kg:
-
kilogram
- MAES:
-
MacArthur Admission Experience Survey
- MARS:
-
Medication Adherence Rating Scale
- MD:
-
Mean difference
- MSQ:
-
Medication Satisfaction Questionnaire
- OR:
-
Odds ratio
- PANSS-6:
-
Positive and Negative Syndrome Scale
- SDM:
-
Shared-Decision-Making
- SDM-assistant:
-
Shared-Decision Making Assistant for choosing antipsychotics
- SDM-Q-9:
-
Shared Decision Making Questionnaire 9-tem patient version
- SDM-Q-9-Doc:
-
Shared-Decision Making Questionnaire 9-item physician version
- SOFAS:
-
Social and Occupational Functioning Assessment Scale
- TAU:
-
Treatment-As-Usual
- UKU-SERS:
-
Udvalg for kliniske undersøgelser Side Effect Rating scale
References
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51.
Edlinger M, Hofer A, Rettenbacher MA, Baumgartner S, Widschwendter CG, Kemmler G, et al. Factors influencing the choice of new generation antipsychotic medication in the treatment of patients with schizophrenia. Schizophr Res. 2009;113(2–3):246–51.
Achtyes E, Simmons A, Skabeev A, Levy N, Jiang Y, Marcy P, et al. Patient preferences concerning the efficacy and side-effect profile of schizophrenia medications: a survey of patients living with schizophrenia. BMC Psychiatry. 2018;18(1):292.
Gaebel W, Hasan A, Falkai P, editors. S3-Leitlinie Schizophrenie. Springer-Verlag; 2019. https://link.springer.com/book/10.1007/978-3-662-59380-6.
Leucht S, Heres S, Kissling W, Davis JM. Evidence-based pharmacotherapy of schizophrenia. Int J Neuropsychopharmacol. 2011;14(2):269–84.
Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140(1):54–9.
Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–9.
Hamann J, Adjan S, Leucht S, Kissling W. Psychiatric decision making in the adoption of a new antipsychotic in Germany. Psychiatr Serv. 2006;57(5):700–3.
Hamann J, Langer B, Leucht S, Busch R, Kissling W. Medical decision making in antipsychotic drug choice for schizophrenia. Am J Psychiatry. 2004;161(7):1301–4.
Hamann J, Mendel R, Heres S, Leucht S, Kissling W. How much more effective do depot antipsychotics have to be compared to oral antipsychotics before they are prescribed? Eur Neuropsychopharmacol. 2010;20(4):276–9.
Mendel R, Traut-Mattausch E, Jonas E, Leucht S, Kane JM, Maino K, et al. Confirmation bias: why psychiatrists stick to wrong preliminary diagnoses. Psychol Med. 2011;41(12):2651–9.
Hamann J, Mendel R, Bühner M, Leucht S, Kissling W. Drowning in numbers-what psychiatrists mean when talking to patients about probabilities of risks and benefits of medication. Eur Psychiatry. 2011;26(2):130–1.
Hamann J, Kohl S, McCabe R, Bühner M, Mendel R, Albus M, et al. What can patients do to facilitate shared decision making? A qualitative study of patients with depression or schizophrenia and psychiatrists. Soc Psychiatry Psychiatr Epidemiol. 2016;51(4):617–25.
Hamann J, Mendel RT, Fink B, Pfeiffer H, Cohen R, Kissling W. Patients' and psychiatrists' perceptions of clinical decisions during schizophrenia treatment. J Nerv Ment Dis. 2008;196(4):329–32.
Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62.
Schuster F, Müller K, Rodoligco A, Siafis S, Leucht S, Hamann J. How should patient decision aids for schizophrenia treatment be designed? - a scoping review. under review; 2022.
Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):CD001431-CD.
Hamann J, Langer B, Winkler V, Busch R, Cohen R, Leucht S, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr Scand. 2006;114(4):265–73.
Hamann J, Holzhüter F, Stecher L, Heres S. Shared decision making PLUS - a cluster-randomized trial with inpatients suffering from schizophrenia (SDM-PLUS). BMC Psychiatry. 2017;17(1):78.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Sepucha KR, Abhyankar P, Hoffman AS, Bekker HL, LeBlanc A, Levin CA, et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: the development of SUNDAE Checklist. BMJ Qual Saf. 2018;27(5):380–8.
Joseph-Williams N, Newcombe R, Politi M, Durand MA, Sivell S, Stacey D, et al. Toward minimum standards for certifying patient decision aids: a modified delphi consensus process. Med Decis Mak. 2014;34(6):699–710.
Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. Bmj. 2017;359:j4891.
Leucht S, Davis JM. Which first-generation antipsychotics should be “repurposed” for the treatment of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2022;272(1):1–3.
Leucht S, Chaimani A, Cipriani AS, Davis JM, Furukawa TA, Salanti G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016;266(6):477–80.
Knegtering H, van den Bosch R, Castelein S, Bruggeman R, Sytema S, van Os J. Are sexual side effects of prolactin-raising antipsychotics reducible to serum prolactin? Psychoneuroendocrinology. 2008;33(6):711–7.
Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. 2011;26(3):130–40.
Johnston BC, Alonso-Coello P, Friedrich JO, Mustafa RA, Tikkinen KAO, Neumann I, et al. Do clinicians understand the size of treatment effects? A randomized survey across 8 countries. CMAJ. 2016;188(1):25–32.
Leucht S, Siafis S, Engel RR, Schneider-Thoma J, Bighelli I, Cipriani A, et al. How Efficacious Are Antipsychotic Drugs for Schizophrenia? An Interpretation Based on 13 Effect Size Indices. Schizophr Bull. 2021.
Furukawa TA, Akechi T, Wagenpfeil S, Leucht S. Relative indices of treatment effect may be constant across different definitions of response in schizophrenia trials. Schizophr Res. 2011;126(1–3):212–9.
Pope A, Adams C, Paton C, Weaver T, Barnes TR. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. Br J Psychiatry. 2010;197(1):67–72.
Benkert O, Hippius H. Kompendium der Psychiatrischen Pharmakotherapie: Springer; 2021.
Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients' desire for autonomy: decision making and information-seeking preferences among medical patients. J Gen Intern Med. 1989;4(1):23–30.
Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89(1):62–7.
O'Donoghue B, Roche E, Ranieri VF, Shannon S, Crummey C, Murray J, et al. Service users’ perceptions about their hospital admission elicited by service user-researchers or by clinicians. Psychiatr Serv. 2013;64(5):416–22.e1–3.
Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80(1):94–9.
Rodenburg-Vandenbussche S, Pieterse AH, Kroonenberg PM, Scholl I, van der Weijden T, Luyten GP, et al. Dutch Translation and Psychometric Testing of the 9-Item Shared Decision Making Questionnaire (SDM-Q-9) and Shared Decision Making Questionnaire-Physician Version (SDM-Q-Doc) in Primary and Secondary Care. PLoS One. 2015;10(7):e0132158.
Hamann J, Holzhüter F, Blakaj S, Becher S, Haller B, Landgrebe M, et al. Implementing shared decision-making on acute psychiatric wards: a cluster-randomized trial with inpatients suffering from schizophrenia (SDM-PLUS). Epidemiol Psychiatr Sci. 2020;29:e137.
Scholl I, Kriston L, Dirmaier J, Buchholz A, Härter M. Development and psychometric properties of the Shared Decision Making Questionnaire--physician version (SDM-Q-Doc). Patient Educ Couns. 2012;88(2):284–90.
Østergaard SD, Lemming OM, Mors O, Correll CU, Bech P. PANSS-6: a brief rating scale for the measurement of severity in schizophrenia. Acta Psychiatr Scand. 2016;133(6):436–44.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76.
Hieronymus F, Kølbæk P, Correll CU, Østergaard SD. Antipsychotic-placebo separation on the PANSS-6 subscale as compared to the PANSS-30: a pooled participant-level analysis. NPJ Schizophr. 2021;7(1):41.
Lin CH, Lin HS, Lin SC, Kuo CC, Wang FC, Huang YH. Early improvement in PANSS-30, PANSS-8, and PANSS-6 scores predicts ultimate response and remission during acute treatment of schizophrenia. Acta Psychiatr Scand. 2018;137(2):98–108.
Østergaard SD, Foldager L, Mors O, Bech P, Correll CU. The Validity and Sensitivity of PANSS-6 in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Study. Schizophr Bull. 2018;44(2):453–62.
Guy W. Clinical global impression. Assessment manual for. Psychopharmacology. 1976:217–22.
Hermes ED, Sokoloff D, Stroup TS, Rosenheck RA. Minimum clinically important difference in the Positive and Negative Syndrome Scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry. 2012;73(4):526–32.
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand. 1987.
Morosini PL, Magliano L, Brambilla LA, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatr Scand. 2000;101(4):323–9.
Brähler E, Mühlan H, Albani C, Schmidt S. Teststatistische prüfung und normierung der deutschen versionen des EUROHIS-QOL lebensqualität-Index und des WHO-5 wohlbefindens-index. Diagnostica. 2007;53(2):83–96.
Vernon MK, Revicki DA, Awad AG, Dirani R, Panish J, Canuso CM, et al. Psychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients. Schizophr Res. 2010;118(1–3):271–8.
Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177–83.
Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. 2000;42(3):241–7.
Mayring P, Fenzl T. Qualitative inhaltsanalyse. Handbuch Methoden der empirischen Sozialforschung: Springer; 2019. p. 633–48.
Hamann J, Parchmann A, Sassenberg N, Bronner K, Albus M, Richter A, et al. Training patients with schizophrenia to share decisions with their psychiatrists: a randomized-controlled trial. Soc Psychiatry Psychiatr Epidemiol. 2017;52(2):175–82.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008;337.
Doane MJ, Sajatovic M, Weiden PJ, O'Sullivan AK, Maher S, Bjorner JB, et al. Antipsychotic Treatment Experiences of People with Schizophrenia: Patient Perspectives from an Online Survey. Patient Prefer Adherence. 2020;14:2043–54.
Moncrieff J, Cohen D, Mason JP. The subjective experience of taking antipsychotic medication: a content analysis of Internet data. Acta Psychiatr Scand. 2009;120(2):102–11.
Bighelli I, Rodolico A, García-Mieres H, Pitschel-Walz G, Hansen W-P, Schneider-Thoma J, et al. Psychosocial and psychological interventions for relapse prevention in schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2021;8(11):969–80.
Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2018;17(3):316–29.
Veroniki AA, Bender R, Glasziou P, Straus SE, Tricco AC. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J Clin Epidemiol. 2019;111:11–22.
Seo M, Furukawa TA, Veroniki AA, Pillinger T, Tomlinson A, Salanti G, et al. The Kilim plot: A tool for visualizing network meta-analysis results for multiple outcomes. Res Synth Methods. 2021;12(1):86–95.
Chalkou K, Steyerberg E, Egger M, Manca A, Pellegrini F, Salanti G. A two-stage prediction model for heterogeneous effects of treatments. Stat Med. 2021;40(20):4362–75.
Mavridis D, Porcher R, Nikolakopoulou A, Salanti G, Ravaud P. Extensions of the probabilistic ranking metrics of competing treatments in network meta-analysis to reflect clinically important relative differences on many outcomes. Biom J. 2020;62(2):375–85.
Becher S, Holzhüter F, Heres S, Hamann J. Barriers and facilitators of shared decision making in acutely ill inpatients with schizophrenia-Qualitative findings from the intervention group of a randomised-controlled trial. Health Expect. 2021;24(5):1737–46.
Hamann J, Mendel R, Cohen R, Heres S, Ziegler M, Bühner M, et al. Psychiatrists' use of shared decision making in the treatment of schizophrenia: patient characteristics and decision topics. Psychiatr Serv. 2009;60(8):1107–12.
Hamann J, Leucht S, Kissling W. Shared decision making in psychiatry. Acta Psychiatr Scand. 2003;107(6):403–9.
Waite F, Langman A, Mulhall S, Glogowska M, Hartmann-Boyce J, Aveyard P, et al. The psychological journey of weight gain in psychosis. Psychol Psychother. 2022.
Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet. 2017;390(10092):415–23.
Acknowledgements
We would like to thank Judith Schulze (Department of Psychiatrie and Psychotherapy, TUM), Susanne Stier (Einbindung von Psychiatrie-Erfarhenen, EX-IN), Elfriede Scheuring and Wulf-Peter Hansen (Bündnis gegen Stigma, BASTA), Holger Aurich and Lukas Nikol (Fantomas™) for their help, comments and feedback in the design of the SDM-assistant for choosing antipsychotics.
Funding
Financing for this study is provided by the Innovation Fund of the Federal Government (funding code: 01VSF19022, funding amount: € 699,625.00). There are no further contributions from third parties. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JH and SL supervise the study and obtained funding. JH, SL, SS, NB, KM, LS, PB, MB, SH, and FS contributed to the design of the study. JH, SL, SS, NB, KM, TH, FM, LS, FS, AR and JW developed, designed and pre-tested the intervention. SS, NB, KM, LS, JH and SL drafted the manuscript. All authors read, contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics for approval and consent to participate
The study was approved by the review board of the ethics committee of the Technical University of Munich. This scientific study is aiming to examine whether better information for patients has positive effects on patient-relevant outcomes. The evidence-based information will be presented in a computer-based format. However, the SDM-assistant should only enhance the process of shared-decision making and is not intended to be developed as a medical product or to pursue a commercial interest.
Patients meeting inclusion criteria will be asked to provide informed consent (template of the informed consent form available upon request) for study participation, and their capability to provide informed consent will be ensured by trained study staff. Furthermore, we will pay special attention to how many patients decline to participate in the study and ask about the underlying reasons. Participation in the study is voluntary. Consent can be withdrawn at any time, without giving reasons and without disadvantages for further medical care. Should the study be terminated by the physician or patient, the data stored up to this point will continue to be used unless the patient or caregiver explicitly withdraws the release of their patient data. This procedure is pointed out verbally and in the consent form and, in the event of a cancellation, this is recorded accordingly in writing.
Data will be saved and exchanged exclusively in pseudonymised forms. Neither the initials nor the date of birth will therefore appear in the encryption code. A study file will be kept for each patient-physician dyad (Case Report Form, CRF), which will be transferred to an electronic study file (eCRF) at the end of the study. After the finalisation of data collection (close out visit) of each patient, copies of both study files, CRF and eCRF, are sent to the managing study centre (AG Patient Orientation and Health Services Research, RDI). The names of the patients and all other confidential information are subject to medical confidentiality and the provisions of the Federal Data Protection Act, i.e., the consent form and personal data remain in the recruiting centre.
The transfer of the pseudonymised data to the study management, as well as information on data protection are included in the consent form. In the event of withdrawal of consent, the stored data will be destroyed at the patient’s request.
Consent for publication
Not applicable.
Competing interests
In the past 3 years Stefan Leucht has received honoraria as a consultant and/or advisor and/or for lectures from Alkermes, Angelini, Eisai, Gedeon Richter, Janssen, Lundbeck, Lundbeck Institute, Merck Sharpp and Dome, Otsuka, Recordati, Rovi, Sanofi Aventis, TEVA, Medichem, Mitshubishi. The other authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
eAppendix.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Siafis, S., Bursch, N., Müller, K. et al. Evidence-based Shared-Decision-Making Assistant (SDM-assistant) for choosing antipsychotics: protocol of a cluster-randomized trial in hospitalized patients with schizophrenia. BMC Psychiatry 22, 406 (2022). https://doi.org/10.1186/s12888-022-04036-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-04036-5