Abstract
Background
Individuals with major depressive disorder (MDD) have a high suicide risk. Some evidence suggests that uric acid (UA) may be involved in the pathophysiology of MDD. The purpose of this study was to evaluate whether serum UA levels were associated with suicide risk in MDD patients.
Methods
One hundred four female patients with MDD (52 patients with suicide risk and 52 patients without suicide risk) and 52 healthy individuals were included in this study. The suicide risk was evaluated by Mini International Neuropsychiatric Interview (M.I.N.I.). Fasting serum levels of UA, as well as glucose, lipid and renal function indicators were measured.
Results
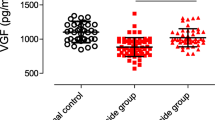
Serum UA levels in MDD patients with suicide risk (245.01 ± 55.44 μmol/L) were significantly lower than those in MDD patients without suicide risk (274.17 ± 72.65 μmol/L) (p = 0.017) and healthy controls (271.42 ± 55.25 μmol/L) (p = 0.030). There was no difference in serum UA levels between the MDD patients without suicide risk and healthy controls (p = 0.821). Binary logistic regression analysis revealed a significant relationship between suicide risk and decreased serum UA levels (OR = 0.989, p = 0.010) in MDD patients.
Conclusion
Decreased serum UA levels were associated with suicide risk in MDD patients. Purinergic system dysfunction may be involved in the neurobiological basis of suicide risk in these patients.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is one of the most common and serious neuropsychiatric disorders with a current prevalence rate of approximately 2.2% in men and 3.3% in women in the general population of China [1]. Among individuals suffering from MDD, an estimated 15% have suicide ideation while as many as 6% is attempt suicide [2]. Most of the individuals with suicide attempts may meet the diagnostic criteria for MDD [3]. Although there are several dedicated suicide risk assessment instruments in clinical practice, such as Beck Hopelessness Scale and Suicide Ideation Scale, it is very difficult to predict and distinguish the individuals who are vulnerable to suicide [4]. An improved understanding of the pathophysiology of suicide risk in patients with MDD is critical for developing specific targeted strategies for prevention and intervention.
Strong evidence has indicated that there are biochemical and morphological abnormalities in certain brain regions of MDD patients and suicide victims [5,6,7]. The purinergic system, which includes not only extracellular nucleosides and nucleotides, such as adenosine and adenosine triphosphate (ATP), but also various receptor subtypes, such as adenosine A1 and A2A receptors, is widely expressed in the central nervous system (CNS) [8]. Several experimental studies have indicated that dysregulation of the purinergic system might play an important role in the pathophysiology of suicide [9,10,11,12] .
Uric acid (UA), as an easily detectable marker, is the final compound generated from the catabolism of purines in humans [13]. As a powerful endogenous antioxidant, UA accounts for more than 60% of free radical scavenging capacity in human blood [14, 15]. Notably, the CNS is considered to be one of the main sites of UA antioxidation [16]. Recently, numerous clinical data suggest that UA might be one of the potential contributing factors in behavioural and neuronal responses. For example, abnormal serum UA levels have been reported in individuals with cognitive dysfunction, dementia, schizophrenia, bipolar disorder, and sleep disorder [17,18,19,20]. The potential involvement of oxidative/antioxidant function in MDD has been demonstrated [21]. Unfortunately, investigations about the causal relationship between UA and MDD have produced inconsistent results and there is still no conclusive evidence [22,23,24]. To date, few studies have been designed to explore the association between UA and suicide. Recently, promising findings from Bartoli and his team have shown an inverse relationship between UA levels and severity of suicidal ideation in individuals with major affective disorders [25, 26] . However, some confounding factors such as glucose and lipid metabolism, renal function and prescribed medications were not controlled in these studies.
In consideration of the relationship between UA levels and MDD or suicide risk remains poorly understood, we carried out this study aimed at investigating and comparing serum UA levels in MDD patients with or without suicide risk. It is well known that females are more likely to suffer from depression and to attempt suicide [27, 28] than males, and sex is one of the most important factors affecting serum UA levels. Previous evidence indicates that UA levels in men are higher than those in women in both the normal population and MDD patients [26]. Moreover, antidepressant treatment may significantly affect serum UA levels [29]. Therefore, only untreated female subjects were allowed to participate in the present study. Based on existing data, we generated the following hypotheses: 1) serum UA levels in the patient groups would be significantly lower than those in the healthy control group [2]; there would be a significant relationship between decreased serum UA levels and suicide risk in patients with MDD.
Methods
Subjects
All patients were consecutively recruited from January 2016 to December 2017 in Beijing HuiLongGuan Hospital, a major psychiatric hospital with over 1000 psychiatric hospital beds in Beijing, China. The study was approved by the Ethics Committee of Beijing HuiLongGuan Hospital, and all participants provided written informed consent in accordance with National Health and Medical Research Council guidelines.
Eligible newly admitted patients were female, aged 18–65 years, Han ethnicity, non-medicated in the past month, and had a Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnosis of MDD, the scores of 17 item Hamilton Depression Rating Scale (HAMD-17) ≥17. The exclusion criteria were as follows: (1) patients in a remission state; (2) patients with other diagnoses such as organic mental disorders, alcohol use disorders, psychotic disorders, mental retardation, neurological illness, renal disease and endocrinal dysfunction or another medical illness which would affect UA levels; and (3) patients who had undergone treatment with electroconvulsive therapy (ECT) within the last month. (4) being pregnant or breastfeeding.
Patients with suicide risk were classified as the suicide risk group and those without suicide risk were classified as the non-suicide risk group. Both were age-matched and selected from the whole eligible patients. In addition, the control group of healthy individuals matched for age to patient groups were enrolled; healthy participants included Han Chinese individuals without a personal or a positive family history for any mental illness, who were recruited from the community near the hospital. Neither patients nor healthy subjects presented drug or alcohol abuse/dependence, pregnancy or breastfeeding, or the use of any medication that may have influenced UA levels. Smoking has been shown to affect UA levels [30], so smokers were excluded from the study. This was confirmed based on the criterion: a smoker was an individual who smoked either daily or occasionally in the 1 month preceding the time of survey.
Mental-status assessment
The DSM-IV diagnosis of MDD was established according to the Chinese version of Mini International Neuropsychiatric Interview (M.I.N.I.), Version 5.0 [31, 32]. All patients were native in the language of interview. M.I.N.I. is a short-structured interview that consists of six questions about past and current suicidality. Scores of the suicide risk section ranged from 0 to 33 and were classified into four subgroups: 0 score index, non-suicide risk; 1–5 score index, low suicide risk; 6–9 score index, medium suicide risk; and ≥ 10 score index, high suicide risk. In this study, all the patients with MDD were classified as having an absence (low or none) or presence of suicide risk (moderate or high) based on previous studies [32,33,34]. Severity of depression symptoms were assessed by the 17 item Hamilton Depression Rating Scale (HAMD-17). Item 3 (suicide) was excluded when we evaluated the association between suicide risk and severity of depression. To ensure consistency and reliability of ratings across the study, four psychiatrists with over 5 years of experiences in clinical practice simultaneously attended a training session on the use of the M.I.N.I. and HAMD-17 before starting this study.
Biochemical measurements
Blood samples (5 ml) were taken from all the subjects by venepuncture and collected into an anticoagulant-free vacuum tube between the hours of 6:00 AM and 7:00 AM after a 12 h overnight fast. The blood was centrifuged at 4000 g for 10 min within 1 h, and resulting serum was withdrawn and analysed immediately. Serum UA levels were measured with a sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (DRG kit Cat#: EIA-2925, Germany). Each sample was run in duplicate. All the samples were performed a quality analysis and assayed by the same technician, who was blinded to the clinical situation. The sensitivity was 0.25 μmol/L, and the intra-assay and inter-assay variation coefficients were 2.1 and 2.7%, respectively. We also collected blood glucose, lipid metabolism and kidney function indicators, which are considered potential confounders of serum UA levels [35, 36].
Statistical analysis
All the continuous variables are summarized as means and standard deviation or medians and interquartile ranges, as appropriate. The categorical variables are presented as raw numbers and percentages (%). For the comparison of the continuous variables among groups, we used one-way ANOVA or independent sample t-tests for parametric variables and the Mann-Whitney test for non-parametric variables. When significant differences were detected by ANOVA, post hoc multiple comparison analyses with Bonferroni adjustment were performed. In addition, UA levels and total cholesterol were compared among groups after controlling for the potentially confounding effects of variables that significantly differed among groups in univariate analyses using analysis of covariance (ANCOVA). A binary logistic regression model was used for the multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All statistical tests were two tailed and a p-value < 0.05 was considered statistically significant. SPSS (Ver. 17.0) was used for the statistical analyses.
Results
A total of 198 eligible patients suffering from MDD were enrolled, of which 52 patients (26.3%) presented a risk of suicide. Two patients who were smokers were excluded. Control data of 52 MDD patients without suicide risk and 52 healthy subjects were included in this study. The demographic characteristics of healthy controls and patients with MDD are presented in Table 1. Among the three groups, there were significant differences in marital status (χ2 = 9.424, p = 0.009), employment rates (χ2 = 6.721, p = 0.035) and BMI (F = 3.408, p = 0.036), but not in education (p > 0.05). Among the subjects with MDD, the proportion of married subjects in the suicide risk group was lower than that in the non-suicide risk group (χ2 = 5.786, p = 0.016). As shown in Table 2, there were no statistically significant differences between the two patient groups in most of their clinical characteristics including the age of onset, number of episodes, duration of illness, rate of family history of psychiatric disorder and first-episode of depression (all p > 0.05), while HAMD-17 total score in the suicide risk group was higher than that in the non-suicide group (t = 4.423, p < 0.001).
Table 3 shows the routine biochemical markers including total cholesterol (CHO), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), creatinine, urea and UA in the three groups. Significant differences were found only in the serum levels of CHO (F = 3.219, p = 0.043), creatinine (F = 4.577, p = 0.012) and UA (F = 3.546, p = 0.031). Post hoc analyses showed that the CHO levels between the two control groups were not significantly different (p > 0.05), whereas both were higher than those in the patients with suicide risk group (patients without suicide risk: p = 0.045; healthy controls: p = 0.021). Creatinine levels were significantly lower in both patient groups when compared with healthy individuals (patients without suicide risk: p = 0.005; patients with suicide risk: p = 0.020); however, no significant differences were detected between the two patient groups (p > 0.05). Serum UA levels were significantly lower in patients with suicide risk than in patients without suicide risk (p = 0.017) and healthy individuals (p = 0.030), while there were no differences between the two latter groups (p = 0.852).
Analysis of covariance showed that serum UA level differences among the three groups remained statistically significant after adjusting for marital status, employment status and BMI (F (5,150) = 3.709, p = 0.027). Further pairwise comparisons indicated that patients with suicide risk had lower serum UA levels than patients without suicide risk (p = 0.010) and healthy individuals (p = 0.046), respectively. However, there was no significant difference with respect to serum levels of CHO among the three groups after adjusting for confounding factors (F (5,150) = 2.731, p = 0.068).
The results of the binary logistic regression analysis are listed in Table 4. We used the significant independent variables from the comparisons between patients with suicide risk and patients without suicide risk including marital status, the levels of CHO, HAMD-17 total score and UA. This regression analysis showed that unmarried status, more severe depressive symptoms, decreased levels of UA increased the odds of suicide risk.
Discussion
The principal finding of this study was that serum UA levels among MDD subjects with suicide risk were significantly lower than those in patients without suicide risk, indicating possible decreased function of the purinergic system-in comparison with MDD patients without suicide risk. It could be inferred that serum UA levels in the CNS of those patients with suicide risk were also reduced, since there was a positive linear correlation between peripheral and central UA levels [16]. In this study, we also found that being unmarried was a risk factor for suicide, consistent with the results of previous studies in the general population [37].
To the best of our knowledge, this study is first to assess the association between the concentration of serum UA and suicide risk in drug-naïve patients with MDD. Of note, the sample of our comparative cross-sectional study consisted of only female subjects and included an appropriate group of depressive patients without suicide risk and healthy individuals. Moreover, several potential confounders, such as variables associated with metabolism and renal function, were taken into account. Specifically, previous evidence showed altered UA levels are influenced by lipid metabolism [38], univariate analysis without adjusting for confounding factors, showed significant differences of serum levels of CHO existed between patients with suicide risk and patients without suicide risk. However, further analysis imply it is not an independent risk factor for suicide risk. On the other hand, there is also evidence that the possible interaction exists between UA metabolism and lipid metabolism [39]. Further investigation is warranted to validate this finding. Our results were in general agreement with previous research that revealed an inverse relationship between UA levels and suicidal ideation severity in patients with major affective disorders [25, 26]. In addition, a decrease in the output of UA was also found in the urine of nonchemical suicide victims, although only 12 patients were included [40]. Based on the available data, we speculate from our results that UA levels might act as a candidate biological marker to discriminate MDD patients with and without suicide risk.
Several hypotheses were proposed to explain the biological mechanism by which low UA levels may influence suicide risk, although the mechanism is far from clear. The first theory is related to the antioxidant properties of UA. Low UA levels lead to the overproduction of free radicals which in turn may cause oxidative damage to brain neuron integrity and function. In fact, oxidative stress is a notorious major underlying mechanism responsible for suicide independent of the effects of depression and classical risk factors, such as marital status [41]. Second, low UA levels imply that other purine metabolism may also be dysregulated, which might contribute to suicide risk. Adenosine, a purine cycle nucleoside, is an important endogenous inhibitory neuromodulator. The activation of adenosine A1 receptors inhibits neurotransmitter release such as glutamate release and suppresses the production of pro-inflammatory cytokines [42, 43]. Therefore, UA may play an important role in neuroregeneration and neuroprotection [42], while neurodegeneration and neuroplasticity have been suggested as part of the neurobiology of suicide [6, 42].
Over the past few years, decreased UA levels have been reported in individuals with current, but not remitted, MDD of both sexes and could be reversed after treatment with certain antidepressant drugs [22, 24, 44, 45]. Moreover, UA levels in patients presenting MDD were also significantly lower than those in patients presenting other mental disorders such as dementia, substance-related disorders, schizophrenia and bipolar disorder [24, 46]. In contrast, some similar research did not replicate these findings [47,48,49], even yielding opposite results [23]. Notably, the present study showed that serum UA levels significantly decreased in only MDD patients with suicide risk, and not in those without suicide risk. More recent evidence indicates that UA levels have a strong negative association with the severity of depressive symptoms [44, 50], while depression severity is one of most important risk factors for suicide [51, 52].
Thus, we speculated that the inconsistency with previous research might be related to serum UA levels specifically decreasing due to severe MMD. Another possible explanation for the discrepancy is the high heterogeneity between studies, especially between the sample demographics and clinical characteristics, such as age and BMI, which can significantly influence UA levels.
Several limitations of the present study are worth mentioning. First, in this cross-sectional study with a relatively small number of female subjects, clinical subtypes of MDD (such as psychotic, atypical or melancholic features and seasonal onset) were not considered- all of which may limit the generalization of findings. Our results need replication in a larger sample study with additional confounding factors considered and both male and female patients included. Second, although unified dietary patterns were provided in inpatients and healthy individuals were advised to consume a light diet the whole day before blood samples were drawn, the amount and type of dietary were possibly different among the groups. Third, any altered metabolic activity of purinergic system may be linked with abnormal UA levels, and we did not evaluate the levels of more purine metabolites, such as adenosine inosine, xanthine, and hypoxanthine. Additionally, genetic variations, including purinergic gene expression and single nucleotide polymorphisms (SNPs), for instance, P2X7 gene, have been found to show an association with increased risk for mood disorders [8]. Future studies should measure these related genetic variations and then explore their roles in contributing to abnormal UA levels. Fourth, it has been shown that impulsivity, i.e., one of the main clinical correlates of suicide, may have a positive association with serum UA [53]. However, we did not consider impulsivity levels in our study. Fifth, menstrual and premenopausal hormones affect UA levels, for example, after menopause and at the time of onset of menstruation, the concentration of uric acid in the plasma increases [54]. In our study, the effects of female menstrual and premenopausal hormones on UA levels were not considered. Last, Although M.I.N.I.’s suicidal module has been widely used in research [55,56,57], other special questionnaires of suicide assessment have been recommended, such as the Columbia–Suicide Severity Rating Scale (C-SSRS) [58].
Conclusions
Our study provides preliminary evidence of dysregulation of the purine cycle manifested as decreased levels of serum UA in MDD patients with suicide risk compared with those without suicide risk. Our results indicate that the purinergic imbalance might act as a characteristic biological marker of suicide risk in MDD patients, which might increase the better understanding of the physiological mechanisms of suicide and offer a potential target for effective therapeutic interventions and early preventions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Baxter AJ, Charlson FJ, Cheng HG, Shidhaye R, Ferrari AJ, Whiteford HA. Prevalence of mental, neurological, and substance use disorders in China and India: a systematic analysis. Lancet Psychiatry. 2016;3(9):832–41.
Chen Y, Bennett D, Clarke R, Guo Y, Yu C, Bian Z, et al. Patterns and correlates of major depression in Chinese adults: a cross-sectional study of 0.5 million men and women. Psychol Med. 2017;47(5):958–70.
Choi SB, Lee W, Yoon JH, Won JU, Kim DW. Risk factors of suicide attempt among people with suicidal ideation in South Korea: a cross-sectional study. BMC Public Health. 2017;17(1):579.
Carter G, Milner A, McGill K, Pirkis J, Kapur N, Spittal MJ. Predicting suicidal behaviours using clinical instruments: systematic review and meta-analysis of positive predictive values for risk scales. Br J Psychiatry. 2017;210(6):387–95.
Ribeiro FF, Xapelli S, Miranda-Lourenco C, Tanqueiro SR, Fonseca-Gomes J, Diogenes MJ, et al. Purine nucleosides in neuroregeneration and neuroprotection. Neuropharmacology. 2016;104:226–42.
Underwood MD, Arango V. Evidence for neurodegeneration and neuroplasticity as part of the neurobiology of suicide. Biol Psychiatry. 2011;70(4):306–7.
Cheffer A, Castillo ARG, Correa-Velloso J, Goncalves MCB, Naaldijk Y, Nascimento IC, et al. Purinergic system in psychiatric diseases. Mol Psychiatry. 2018;23(1):94–106.
Ortiz R, Ulrich H, Zarate CA Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57:117–31.
Karanovic J, Svikovic S, Pantovic M, Durica S, Brajuskovic G, Damjanovic A, et al. Joint effect of ADARB1 gene, HTR2C gene and stressful life events on suicide attempt risk in patients with major psychiatric disorders. World J Biol Psychiatry. 2015;16(4):261–71.
Karanovic J, Ivkovic M, Jovanovic VM, Svikovic S, Pantovic-Stefanovic M, Brkusanin M, et al. Effect of childhood general traumas on suicide attempt depends on TPH2 and ADARB1 variants in psychiatric patients. J Neural Transm (Vienna). 2017;124(5):621–9.
Zhang L, Su TP, Choi K, Maree W, Li CT, Chung MY, et al. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res. 2011;45(4):435–41.
Bartoli F, Clerici M, Carra G. Purinergic system and suicidal behavior: exploring the link between adenosine A2A receptors and depressive/impulsive features. Mol Psychiatry. 2018.
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14.
Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–62.
Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608–19.
Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19(4):1331–6.
Bartoli F, Crocamo C, Mazza MG, Clerici M, Carra G. Uric acid levels in subjects with bipolar disorder: a comparative meta-analysis. J Psychiatr Res. 2016;81:133–9.
Verhaaren BF, Vernooij MW, Dehghan A, Vrooman HA, de Boer R, Hofman A, et al. The relation of uric acid to brain atrophy and cognition: the Rotterdam scan study. Neuroepidemiology. 2013;41(1):29–34.
Wiener RC, Shankar A. Association between serum uric acid levels and sleep variables: results from the National Health and nutrition survey 2005-2008. Int J Inflam. 2012;2012:363054.
Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998;80(1):29–39.
Jimenez-Fernandez S, Gurpegui M, Diaz-Atienza F, Perez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76(12):1658–67.
Bartoli F, Trotta G, Crocamo C, Malerba MR, Clerici M, Carra G. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: a meta-analysis and meta-regression. Eur Arch Psychiatry Clin Neurosci. 2017.
Tao R, Li H. High serum uric acid level in adolescent depressive patients. J Affect Disord. 2015;174:464–6.
Wen S, Cheng M, Wang H, Yue J, Li G, Zheng L, et al. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. 2012;45(1–2):49–53.
Bartoli F, Crocamo C, Trotta G, Bava M, Capuzzi E, Castagna G, et al. Testing the role of the antioxidant uric acid as a biomarker of suicidal ideation in subjects with major affective disorders: an exploratory study. Gen Hosp Psychiatry. 2018;51:128–9.
Bartoli F, Crocamo C, Bava M, Castagna G, Di Brita C, Riboldi I, et al. Testing the association of serum uric acid levels with behavioral and clinical characteristics in subjects with major affective disorders: a cross-sectional study. Psychiatry Res. 2018;269:118–23.
Bernal M, Haro JM, Bernert S, Brugha T, de Graaf R, Bruffaerts R, et al. Risk factors for suicidality in Europe: results from the ESEMED study. J Affect Disord. 2007;101(1–3):27–34.
Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001;158(7):1091–8.
Bartoli F, Trotta G, Crocamo C, Malerba MR, Clerici M, Carra G. Antioxidant uric acid in treated and untreated subjects with major depressive disorder: a meta-analysis and meta-regression. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):119–27.
Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on plasma uric acid concentrations. Environ Health Prev Med. 2011;16(5):307–12.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33 quiz 4–57.
Si TMSL, Dang WM, Su YA, Chen JX, Dong WT, Kong QM, Zang WH. Evaluation of the reliability and validity of Chinese version of the mini-international neuropsychiatric interview in patients with mental disorders. Chin Ment Health J. 2009;23(7):493–503.
Pompili M, Rihmer Z, Akiskal H, Amore M, Gonda X, Innamorati M, et al. Temperaments mediate suicide risk and psychopathology among patients with bipolar disorders. Compr Psychiatry. 2012;53(3):280–5.
Wiener CD, de Mello FS, Pedrotti Moreira F, Bittencourt G, de Oliveira JF, Lopez Molina M, et al. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J Affect Disord. 2015;184:245–8.
Chen J, Chen H, Feng J, Zhang L, Li J, Li R, et al. Association between hyperuricemia and metabolic syndrome in patients suffering from bipolar disorder. BMC Psychiatry. 2018;18(1):390.
Yan D, Tu Y, Jiang F, Wang J, Zhang R, Sun X, et al. Uric acid is independently associated with diabetic kidney disease: a cross-sectional study in a Chinese population. PLoS One. 2015;10(6):e0129797.
Xu H, Zhang W, Wang X, Yuan J, Tang X, Yin Y, et al. Prevalence and influence factors of suicidal ideation among females and males in northwestern urban China: a population-based epidemiological study. BMC Public Health. 2015;15:961.
Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 2016;29:3–8.
Guo LF, Chen X, Lei SS, Li B, Zhang NY, Ge HZ, et al. Effects and mechanisms of Dendrobium officinalis six nostrum for treatment of Hyperuricemia with hyperlipidemia. Evid Based Complement Alternat Med. 2020;2020:2914019.
Lis AW, McLaughlin RK, McLaughlin DI. Urinary purine levels in suicide. Physiol Chem Phys. 1975;7(4):325–33.
Vargas HO, Nunes SO. Pizzo de Castro M, Bortolasci CC, Sabbatini Barbosa D, Kaminami Morimoto H, et al. oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J Affect Disord. 2013;150(3):923–30.
Krugel U. Purinergic receptors in psychiatric disorders. Neuropharmacology. 2016;104:212–25.
Lorenzi TM, Borba DL, Dutra G, Lara DR. Association of serum uric acid levels with emotional and affective temperaments. J Affect Disord. 2010;121(1–2):161–4.
Black CN, Bot M, Scheffer PG, Snieder H, Penninx B. Uric acid in major depressive and anxiety disorders. J Affect Disord. 2018;225:684–90.
Chaudhari K, Khanzode S, Dakhale G, Saoji A, Sarode S. Clinical correlation of alteration of endogenous antioxidant-uric acid level in major depressive disorder. Indian J Clin Biochem. 2010;25(1):77–81.
Kesebir S, Tatlidil Yaylaci E, Suner O, Gultekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord. 2014;165:131–4.
Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(5):1284–90.
Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22(2):67–73.
Wiener C, Rassier GT, Kaster MP, Jansen K, Pinheiro RT, Klamt F, et al. Gender-based differences in oxidative stress parameters do not underlie the differences in mood disorders susceptibility between sexes. Eur Psychiatry. 2014;29(1):58–63.
Gu Y, Han B, Wang L, Chang Y, Zhu L, Ren W, et al. Low serum levels of uric acid are associated with development of Poststroke depression. Medicine (Baltimore). 2015;94(45):e1897.
Wang YY, Jiang NZ, Cheung EF, Sun HW, Chan RC. Role of depression severity and impulsivity in the relationship between hopelessness and suicidal ideation in patients with major depressive disorder. J Affect Disord. 2015;183:83–9.
Capuzzi E, Bartoli F, Crocamo C, Malerba MR, Clerici M, Carra G. Recent suicide attempts and serum lipid profile in subjects with mental disorders: a cross-sectional study. Psychiatry Res. 2018;270:611–5.
Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, et al. Impulsivity is associated with uric acid: evidence from humans and mice. Biol Psychiatry. 2014;75(1):31–7.
Bartoli C, Berland-Benhaim C, Tuchtan-Torrents L, Kintz P, Leonetti G, Pelissier-Alicot AL. Suicide by medication overdose in prison: a study of three cases. J Forensic Sci. 2018;63(4):1316–20.
Baek JH, Kang ES, Fava M, Mischoulon D, Nierenberg AA, Yu BH, et al. Serum lipids, recent suicide attempt and recent suicide status in patients with major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;51:113–8.
Pedrotti Moreira F, Borges CJ, Wiener CD, da Silva PM, Portela LV, Lara DR, et al. Serum brain-derived neurotrophic factor levels in subjects with major depressive disorder with previous suicide attempt: a population-based study. Psychiatry Res. 2018;262:500–4.
Su YA, Lin JY, Liu Q, Lv XZ, Wang G, Wei J, et al. Associations among serum markers of inflammation, life stress and suicide risk in patients with major depressive disorder. J Psychiatr Res. 2020;129:53–60.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77.
Acknowledgements
The authors would like to thank Hong Mei Chen, Ning Wang, Ran-Li, Xuan Wang, Hong-Juan Li, and Hai-Ting Xu for all of their hard work and significant contributions to the study.
Funding
This study was supported by Beijing Council of Science and Technology (Z171100001017001), Beijing Municipal Hospital Training Programmer Foundation (PX2016067), the Capital Health Research and Development of Special (2018–3-2132) and the National Natural Science Foundation of China (No. 81771468).
The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conception of the study: J-XC, Y-AS, and Y-LT were involved in study design. J-XC, J-HF, L-GZ, YL, S-LW were responsible for recruiting the patients, performing the clinical rating, collecting all samples, and helping with statistical analysis and manuscript preparation. Y-AS, J-XC, J-HF and Y-LT were involved in interpreting the data, intellectual input, and writing and editing the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Beijing HuiLongGuan Hospital, and all participants provided written informed consent in accordance with National Health and Medical Research Council guidelines.
Consent for publication
All authors have agreed to the submission of the final manuscript.
Competing interests
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, JX., Feng, JH., Zhang, LG. et al. Association of serum uric acid levels with suicide risk in female patients with major depressive disorder: a comparative cross-sectional study. BMC Psychiatry 20, 477 (2020). https://doi.org/10.1186/s12888-020-02891-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-020-02891-8