Abstract
Background
People with severe mental illness have markedly reduced life expectancy; cardiometabolic disease is a major cause. Psychiatric hospital inpatients have elevated levels of cardiometabolic risk factors and are to a high degree dependent of the routines and facilities of the institutions. Studies of lifestyle interventions to reduce cardiometabolic risk in psychiatric inpatients are few. The current study aimed at assessing the feasibility and effects of a lifestyle intervention including Motivational Interviewing (MI) on physical activity levels, cardiometabolic risk status and mental health status in psychotic disorder inpatients.
Methods
Prospective naturalistic intervention study of 83 patients at long term inpatient psychosis treatment wards in South-Eastern Norway. Patients were assessed 3–6 months prior to, at start and 6 months after a life-style intervention program including training of staff in MI, simple changes in routines and improvements of facilities for physical exercise. Assessments were done by clinical staff and included level of physical activity, motivation, life satisfaction, symptom levels (MADRS, AES-C, PANSS, and GAF) as well as anthropometric and biochemical markers of cardiometabolic risk. A mixed model was applied to analyze change over time.
Results
A total of 88% of patients received MI interventions, with a mean of 2.5 MI interventions per week per patient. The physical activity level was not increased, but activity level was positively associated with motivation and negatively associated with positive symptoms. Triglyceride levels and number of smokers were significantly reduced and a significant decrease in symptom levels was observed.
Conclusions
The current results suggest that a simple, low cost life-style intervention program focusing on motivational change is feasible and may reduce symptoms and improve lifestyle habits in psychosis patients in long term treatment facilities. Similar programs may easily be implemented in other psychiatric hospitals.
Trial registration
ClinicalTrials.gov. NCT03528278, date of registration: 05/16/2018 (retrospectively registered).
Similar content being viewed by others
Background
People with severe mental disorders carry a significantly higher risk for premature deaths due to cardiovascular disease [1]. Some of this increased risk seems to be linked to the genetic susceptibility of the disease itself [2], but several unhealthy lifestyle habits are also possible causes [1]. Tobacco smoking is highly prevalent [3], and has well-known detrimental effects on health and mortality, also in schizophrenia [4]. Many people with severe mental illness have unhealthy diets, often driven by poor economy or loss of interest in personal maintenance [5]. Side effects of especially the second generation antipsychotics are regarded as an important contributor to the increased risk for the metabolic syndrome in schizophrenia [6,7,8].
A balanced diet may reduce cardiovascular disease (CVD) risk factors [9,10,11]. Physical exercise has beneficial effects on CVD risk factors such as body mass index (BMI), and blood lipids [12] and may reduce mortality [13]. Improvement of physical fitness seems likely to reduce the elevated mortality in this patient group [14]. Exercise programs are feasible and may also improve mental well-being and overall outcome among patients with schizophrenia [15]. Increased physical exercise or improvements in dietary habits are thus possible strategies for reducing the increased mortality in schizophrenia.
There are, however, many barriers to life-style changes in the general population, and in addition several obstacles for changes specifically among patients with severe mental illness [16]. Some of these, such as negative symptoms and reduced initiative, are often part of the mental disorder itself. Many patients are ambivalent to making a change in their lifestyle habits and need help in their motivational process [17]. An increased focus on facilitating life-style changes related to cardiometabolic risk in the mental health care system would be of great importance.
Different motivational methods have been used for facilitating behavioural change in psychiatric settings [18, 19], with positive results for increased physical exercise in schizophrenia patients in outpatient and day care settings [20,21,22,23]. Motivational interviewing (MI) was originally developed for facilitating behavioral change in substance use disorders [24], but has spread to other areas of medicine where behavior change is crucial [25, 26]. Despite the accumulated evidence, comprehensive interventions aiming to improve physical fitness among patients with mental illness have only to a small degree been implemented in psychiatric clinics, and research on feasibility and effects of training programs in a hospital setting is scarce. To our knowledge no previous study has addressed the usefulness of MI for increasing physical activity or other lifestyle changes in severe mental illness in an inpatient setting. There is a need for low cost interventions in this area which are feasible to implement in daily clinical routines.
A project for improvement of lifestyle factors associated with cardiometabolic risk was carried out at several long term inpatient psychosis treatment wards in Southeastern Norway. Comprehensive clinical data at baseline confirmed high levels of cardiometabolic risk factors including obesity, smoking and unhealthy eating habits [27]. The current project aimed to answer the following research questions: How feasible is it to implement a simple lifestyle improvement program based on MI interventions targeting cardiometabolic health? Will level of motivation be associated with interventions or lifestyle changes? Further, will the intervention be associated with increased levels of physical activity, reduced cardiometabolic risk factors or improved mental health status?
Methods
Setting
The current study was carried out at The Oslo University Hospital (OUH) and the private inpatient psychiatric care facility Skjelfoss Psychiatric Centre - Lukas Foundation (SPC). At the OUH the two participating departments were in three different buildings at Gaustad in Oslo Municipality and at two sites at Dikemark in Asker Municipality, west of Oslo. The SPC is located in Hobøl Municipality south of Oslo. All departments provide inpatient treatment for long term psychiatric patients serving catchment areas in the South-East Health Region of Norway. The departments have the capacity to administer compulsory measures and included high security wards. The total number of beds was 116.
Inclusion criteria
Inpatients, aged between 18 and 65 years and with DSM-IV diagnoses of schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic disorder NOS, bipolar I or bipolar disorder NOS. Exclusion criteria were established cognitive deficit (IQ < 70), brain damage, BMI < 17.5, inability to speak Scandinavian or English language. All participants had to be able and willing to give informed consent to participate in the study.
Design
A project for lifestyle change was conceived of by the local administration and clinicians involving changing clinical routines at all wards relying on existing resources, e.g. local personnel performing all tasks. Interventions should be clinically relevant and should be possible to be transformed into permanent changes of practice. Practical obstacles for establishing an adequate control condition and ethical considerations ruled out a randomized, controlled study. The current study is thus a prospective observational study of patient related conditions before and after the introduction of a lifestyle enhancement program targeting cardiometabolic health, following as many patients as possible through their course of inpatient treatment.
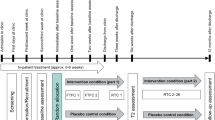
The intervention was preceded by Start assessments performed 0–4 weeks prior, and was followed by End assessments performed 6–7 months after the starting date for the interventions. The End assessments were compared to the assessments of the pre-intervention period (Fig. 1), which also included Baseline assessments performed 3–6 months prior, and thus served as a control condition.
Consecutive patients were included during a 12- month period which differed in onset between wards. Inclusion to the study at the first ward started in January 2013 and ended at the last ward in March 2015. Effort was made to have a continuous inclusion unbiased of patient related factors. The intervention was carried out at all wards and implemented at a date specified for each of the wards.
Intervention
The intervention aimed at a life style change to reduce cardiometabolic risk. A user representative and the clinical staff at all participating wards were involved in the planning of the intervention, which consisted of three components:
-
1)
Motivational interventions
Training in motivational interviewing (MI) was mandatory for all staff and was organized by certified instructors as a two-day intensive course with follow-up practical guidance. The course included lectures, discussion, role play and group guidance with the MI-expert. The first day of the course was held within a week before the defined starting day for interventions, the second within four weeks after. Patients were informed about the purpose and implications of the interventions. Motivational interventions for enhancing physical activity, dietary improvement and smoking cessation were applied in different settings: 1) Daily motivating interaction with patients was encouraged. All patients were offered at least 15 min structured MI per week, often performed during hikes in the countryside. In order to help adherence to protocol, staff used a daily recording form where type of MI-interventions, patients’ response to the MI-interventions and patients’ physical activity was recorded. This form is attached (in English translation) as supplementary material (Additional file 1). During all physical training activities, the staff focused on individual feedback and encouragement. 2) Adherence to treatment plans (see next component) would lead to instant positive verbal feedback and attention from staff (reinforcement), while adherence failure would receive neutral feedback (no reinforcement). 3) Staff would behave in a facilitating manner e.g. by changing to training clothes at times for assistance in physical activity. 4) Logbooks were given to every participant for personal logging of activities and results. 5) T-shirts with a logo and slogan were issued for promoting a sense of togetherness.
-
2)
Physical activity as part of individual treatment plans
Patients were offered a comprehensive physical training program and participants and staff together made weekly individual treatment plans including a goal of 30 min physical exercise three times per week as a minimum. The physical exercise consisted of any continuous physical activity, e.g. walking, running, ball-play, cycling, weight lifting or gymnastics.
-
3)
Establishment of a basic infrastructure for physical activity and improved diet
An infrastructure for physical activity enhancement was implemented by establishing a gym (weight lifting equipment and treadmill and/or stationary bicycles), a high intensity interval training group as well as low intensity walking/cycling groups at each site. The gym was manned by a physiotherapist at regular hours giving individual exercise advice. Programs for interval training were developed. Physiotherapists made regular visits to all wards in order to secure the quality of the programs and ensure implementation. Details about these programs are provided as an additional file (see Additional file 2). The infrastructure was made available at the intervention starting date. The intervention also included improvement in diet. During the project between-meals offerings of vegetables and fruit were introduced and sugar containing beverages were replaced with water or beverages with artificial sweeteners.
Assessments at baseline only
Data was gathered from interviews and medical charts. Time of admission, smoking, and use of current psychopharmacological medication were recorded. Height was measured. The participants were diagnosed with the Structural Clinical Interview for DSM-IV for axis I disorders (SCID-I) [28]. More details about data collection is reported elsewhere [27].
Assessments at baseline, start and end
Cardiometabolic risk factors were assessed according to established guidelines [29]. Weight was measured and Body Mass Index (BMI) was calculated. Waist circumference was measured with a horizontal tape measurement from the top of the right iliac crests. S-Glucose, HbA1c, Insulin, C-reactive Protein (CRP), vitamin D and fasting plasma lipids (Total Cholesterol, High Density Lipoprotein - LDL, Low Density Lipoprotein - LDL and Triglycerides –TG) were measured. Smoking habits were reported.
Level of physical activity was assessed as the product of the rating scores of the questions of frequency of sessions (never: 0, less than once a week: 0.5, weekly: 1, 2–3 times a week: 2.5, almost daily: 5), intensity of sessions (low effort: 1, moderate effort: 2, almost maximum effort: 3) and duration of sessions (less than 15 min: .10, 15–29 min: .38, 30–60 min: .75, more than 60 min: 1.0) of physical activity in the HUNT questionnaire [30,31,32]. Sedentary behavior was assessed by daily waking hours of inactivity.
We used the life satisfaction item from the HUNT study, categorizing life satisfaction on a 7-point scale from extremely discontent to extremely content. Self-esteem was assessed by the Rosenberg Self-Esteem Scale [33]. The level of psychotic symptoms was measured with The Positive and Negative Symptom Scale (PANSS) [34], depressive symptoms with the Montgomery Asberg Depression Rating Scale (MADRS) [35]. Global symptoms and psychosocial functioning were measured by the Global Assessment of Functioning Scale (GAF), the scores were split into scales of symptoms (GAF-S) and functioning (GAF-F) to improve psychometric properties [36]. Apathy was measured with the abridged clinical version of the Apathy Evaluation Scale (AES-C-Apathy) [37, 38].
Motivation was measured by applying two visual analog scales from 0 (minimum) to 10 (maximum) assessing perceived importance for change and readiness for change.
Weekly assessments during the intervention period
Recordings were made of number of MI session per patient per week.
Diagnostics and symptom assessments were done by medical doctors and psychologists who were part of the regular staff and had participated in a training course in SCID and PANSS assessments and regularly diagnostic consensus meetings led by clinically well experienced specialists.
Statistics
All analyses were done using the IBM Statistical Package for the Social Sciences (SPSS) version 25.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as frequencies, proportions, mean and median with range. Group comparisons were evaluated with independent sample t-tests and Chi square tests as appropriate. Bivariate analyses were performed with Pearson’s r and Spearman’s rho for normally distributed or skewed data, correspondingly.
Linear mixed model analyses were performed to estimate the fixed effects of time (Baseline, Start and End) on the different outcome (dependent) variables. Individual patients and time were set as random factors. No covariance structure was pre-specified (i.e. unstructured). After model fit, we estimated marginal means for time to explore differences between the time points. If significant changes by time were observed, a posteriori analyses were performed to explore the potential contribution of key variables with clinical interest. Thus, sex, BMI or use of antipsychotics was included as fixed effects, one at a time, in these analyses. Further, to explore the potential contribution of motivation, depressivity, positive and negative symptom status and institution for changes in physical activity, the following variables were included, one at a time, as fixed effects: motivation (importance and readiness), PANSS-positive symptoms, MADRS, AES-C Apathy and institution. A secondary analysis was performed, substituting physical activity by sedentary behavior, in order to study physical inactivity.
Results
Of a total of 114 patients who met the inclusion criteria, 83 patients (73%) signed informed consent. Mean age was 40.1 years (n = 80), 57 of 82 (69.5%) were male, 57 (76.0%) had schizophrenia, 7 (10.7%) had schizoaffective disorder and 10 (13.4%) had bipolar disorder or other psychotic disorder. Median admission time prior to the intervention was 24 months (range 1–240), n = 31. At Start 43 of 50 patients (86.0%) used antipsychotic medication. Some patients had attenuated or absent positive psychotic symptoms at the time of inclusion. Due to variable staff resources, time constraints and sometimes non-compliance with assessment protocol, full datasets at all time points could not be obtained. There were no significant differences in assessments at Start between patients with or without assessments at Baseline. Levels of physical activity and motivation at both Start and End were obtained for 16 patients. Data on smoking at both Start and End were obtained for 40 patients. Weekly MI reports were obtained for 79 patients (95%) at week 1 (Start), but declined to 50% at week 13. An additional figure shows this in more detail (see Additional file 3: Figure S1). A total of n = 73 (88%) of the participants received MI-sessions during the intervention period. Mean MI sessions per patient per week during the 6 months follow-up was 2.5 (SD 2.3). An additional figure shows this in more detail (see Additional file 4: Figure S2). Uncorrected post hoc analyses showed that mean number of MI sessions per patient per week was 1.4 (SD 1.3) at OUS wards and 5.3 (SD 2.0) at SPC wards (p < .001).
The levels of physical activity increased from Start to End in 38% (6/16). Motivation levels at End were higher in the group with increase in physical activity; importance: 8.0 (SD 2.1) vs 4.9 (3.8), p = .056; readiness: 6.7 (1.4) vs 4.2 (4.1), p = .048. Among the smokers at Start, 17% (2/12) had quitted smoking at End, while among 28 initial non-smokers none started smoking (p < .001).
The mixed linear model analyses showed no statistically significant changes in estimated marginal means (EMMs) for physical activity or inactivity over time. There was a nominal trend for a reduction in sedentary behavior over time, although not statistically significant (p = .473). The reduction from Start to End of depressive symptoms (MADRS) was statistically significant (p = .009). There was a significant decrease of PANSS positive symptoms from Baseline to End (p = .006). The increase in self-esteem was borderline significant from Start to End (p = .051). There was a non-significant nominal decrease in both measures for motivation from Start to End (Importance: p = .473; Readiness: p = .179), while for the AES-C and the PANSS negative there were no apparent changes (Table 1).
There was a significant decrease in blood triglyceride levels after intervention (p = .002). For other blood lipids levels and CRP there were no clear trends for the intervention period (Start to End). There was a significant increase in D-vitamin levels from Baseline to End (p = .010) (Table 1). Adjusting for sex, BMI or use of antipsychotics at Start in the linear mixed model did not affect main findings.
Adjusted linear mixed model analyses showed a highly significant positive effect of motivation at Baseline (importance, p < .001, readiness, p = .011) and a significant negative effect of positive symptoms at Baseline on changes in levels of physical activity (p = .016). The MADRS and the AES-C at Baseline showed no significant effects on physical activity (Table 2). Similar analyses of sedentary behavior at baseline showed no significant effects of any of these potential covariates.
Discussion
The current study assessed a naturalistic intervention for lifestyle improvement including motivational interviewing to reduce cardiometabolic risk in a naturalistic long term inpatient ward setting. The study was observational, and relied on the institutions’ own staff for all interventions and assessments. The implementation of this low cost intervention was fairly successful, with an adequate number of MI-sessions, particularly in the first phase of the interventions. We found a significant reduction in triglyceride level and smoking after intervention. We did not detect any significant increase in mean activity level, but observed a significant decrease in depressive symptom level. We found change in physical activity to be positively associated with motivation and negatively associated with positive symptoms. To the best of our knowledge, the current findings are unique and highly clinically relevant as studies of low cost lifestyle interventions are scarce among psychiatric inpatient populations.
The findings of incomplete implementation of parts of the program may be taken as an indication that feasibility depended on available clinical resources and manpower. However, an evaluation of the health care system was beyond the scope of the present study. Thus, we have no systematic measures of budgets, work load, available staff resources at the long-term wards during the project period. However, we note that the long-term hospital SPC, with rural location, low patient turnover and no acute admissions, seems to have more MI sessions than the larger hospital OUH, with a high number of acute ward admissions per year. The design of the study with implementation by the local staff in regular clinical service, led to missing data. We have no indications that missing collection of data affected the quality of the assessments, or resulted in systematic bias in the data collected. The failure to improve the mean activity level and the lack of association between MI sessions and change in physical activity may suggest that the intervention was too weak or non-targeted. Previous studies suggest that quite comprehensive regimens are needed to make changes in physical activity level, and often provide modest effects [39,40,41]. However, sedentary behavior is increasingly considered as a more important target for interventions [42] and is possibly easier to improve. In fact, we observed a declining pattern in the inactivity level which did not reach statistical significance. Thus, the current findings indicate that there is a potential of low-cost cardiometabolic interventions in chronic inpatient settings. However, to support the notion that the current lack of significant effect is a type II error, more studies are needed.
We found that higher level of motivation was associated with improvement in physical activity level, but mean levels of neither of these improved during the intervention. A possible interpretation of these results is that interventions for physical activity enhancement only succeeded in targeting a subgroup of motivated patients. A proportion of patients seemed to reduce motivation during the project period, although not significant. This seems to reason with non-systematic evaluation of the registered individual patients’ reports suggesting that some psychological “saturation” effects might have been at play. Unfortunately, due to low N it was not possible to explore changes in symptoms or motivation across groups with or without improvement in physical activity.
Two patients quitted smoking during the interventions, and none started. It is conceivable that this highly clinically meaningful life style change is attributable to the staff’s motivational efforts which included smoking cessation. Smoking cessation may reduce the metabolism of antipsychotics and thus increase the effect of medication. This can potentially affect both activity level and symptom load. However due to the low number we were not able to control for this possible association. Further, we found that triglyceride level was significantly reduced from Start to End independently of BMI or use of antipsychotics, and there was no increase in other lipid levels. However, the change in triglyceride level was not associated with change in activity level, and this result must be interpreted with caution. We could speculate that the change is related to improved diet, which is previously shown to improve lipid levels in schizophrenia patients [43]. Changing diet in a hospital setting depends on motivation both in patients and staff, which was a goal of the present intervention.
Level of depression was significantly decreased from Start to End, which suggests that it was independent of the general symptom improvement during hospitalization. Decreasing depression level after interventions for enhancement of physical activity has been shown in previous meta-analyses [39, 44]. As we did not detect any increase in mean activity level, it is possible that the observed reduction in the MADRS score could be associated directly with the motivational work [45] and a potential change in ward atmosphere [46]. It is of interest that improving mood is an important motivator for physical exercise in SMI [16]. A previous analysis of the Baseline data found high level of depression to be associated with low level of physical activity [27]. However, the level of depression was not associated with change in physical activity in the current intervention study.
The finding of a decrease in positive symptoms was only significant from Baseline to End and could hence not be associated with the intervention specifically. However, reduced level of positive symptoms was associated with an increase in physical activity. A reduction in positive symptoms during exercise interventions has been shown in other studies [39, 47]. This might have clinical importance.
The main strength of the study is the naturalistic design with comprehensive clinical descriptions and the assessments of changes in “real-life” psychosis-treatment hospital wards. We argue that this design has important advantages for representativity and translational value, and our methods and experiences could be useful for other lifestyle enhancement projects. However, as the design was not experimental with e.g. a control group, there are limitations in interpreting effects of the interventions. There were incomplete data in the time series due to instability in data gathering resources of the clinical staff in the project period. This gave a risk for type II errors and limited the testing of hypotheses in subgroups. Bias could be introduced if there were any systematic selection of patients for assessments or missing data. However, we have tested for differences associated with missing data, and found no clear bias. We have no indications that other selection mechanisms were at play, but bias cannot be ruled out. Site or ward specific differences could also affect the results, but we had not statistical power for evaluating site-dependent factors. The mixed model analyses made it possible to test hypotheses of datasets with incomplete time series with a high retention of statistical power. The study of change in a naturalistic design made it impossible to compare change in a control group in the same time period. We were thus not able to control for effects related to time, something that is a concern in a setting of active treatment. By comparing the change in the intervention period to the pre-intervention period, we were nevertheless able to make some assessments of the contributions of time as a factor. Further, it can be argued that dramatic changes over some months in the intervention period are not expected in this population of predominantly chronically ill patients where over 50% had more than 2 years duration of admission at the time of inclusion. We did not have records of the staff’s attitudes and behavior or ward atmosphere, hence, potential changes in these factors associated with the interventions could not be controlled for. Another limitation is that we have no objective measurements of level of physical activity.
Conclusions
In conclusion, the current study shows that a combined intervention for lifestyle change, with emphasis on motivational work is feasible in psychiatric inpatients settings, and may have positive effects on psychiatric symptom level as well as on lifestyle factors related to cardiometabolic risk. The study highlights both the importance of motivational factors in lifestyle change and the need for evidence based practices to guide milieu therapeutic interventions at inpatient hospital wards. The interventional program described in the current study relied on existing staff resources for the actual patient interventions and could easily be implemented elsewhere, provided motivated staff with necessary resources.
Viewing a psychiatric hospital as a place of retention is still a reality in minds, routines and legislations, and staffing of wards will often be more focused on avoiding unacceptable risks than on the improvement of clinical practice. A psychiatric hospital environment, however, has opportunities for the involvement of both users and staff in the planning and provision of better services with potential positive impact on highly important aspects of health.
Abbreviations
- AES-C Apathy:
-
Apathy Evaluation Scale
- BMI:
-
Body Mass Index (BMI)
- CVD:
-
Cardiovascular disease
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- EMM:
-
Estimated Marginal Means
- GAF:
-
Global Assessment of Functioning
- HbA1C:
-
Glycosylated Hemoglobin
- HDL:
-
High Density Lipoprotein Cholesterol
- LDL:
-
Low Density Lipoprotein Cholesterol
- MADRS:
-
Montgomery Asberg Depression Rating Scale
- MI:
-
Motivational Interviewing
- NOS:
-
Not Otherwise Specified
- OUH:
-
Oslo University Hospital
- PANSS:
-
Positive And Negative Syndrome Scale
- S.E:
-
Standard Error
- SCID:
-
Structured Clinical Interview for DSM-IV Axis I Disorders
- SPC:
-
Skjelfoss Psychiatric Centre - Lukas Foundation
References
Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease - a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. 2014;5:137.
Petersen L, Sorensen TI. Studies based on the Danish adoption register: schizophrenia, BMI, smoking, and mortality in perspective. Scand J Public Health. 2011;39(Suppl 7):191–5.
McCreadie R. Use of drugs, alcohol and tobacco by people with schizophrenia: case--control study. British J Psychiatry. 2002;181(4):321–5.
Beary M, Hodgson R, Wildgust HJ. A critical review of major mortality risk factors for all-cause mortality in first-episode schizophrenia: clinical and research implications. J Psychopharmacol. 2012;26(Suppl 5):52–61.
McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. British J Psychiatry. 2003;183(6):534–9.
Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93.
Tenback D, Pijl B, Smeets H, Os J, Harten P. All-cause mortality and medication risk factors in schizophrenia: a prospective cohort study. J Clin Psychopharmacol. 2012;32(1):31–5.
Kahl KG, Westhoff-Bleck M, Krüger THC. Effects of psychopharmacological treatment with antipsychotic drugs on the vascular system. Vasc Pharmacol. 2018;100:20–5.
Mann JI. Nutrition recommendations for the treatment and prevention of type 2 diabetes and the metabolic syndrome: an evidenced-based review. Nutr Rev. 2006;64(9):422–7.
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18.
Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288(20):2569–78.
Pagels P, Raustorp A, Archer T, et al. Influence of moderate, daily physical activity upon body composition and blood lipid profile in Swedish adults. J Phys Act Health. 2011;9(6):867–74.
Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373–83.
Wildgust HJ, Beary M. Are there modifiable risk factors which will reduce the excess mortality in schizophrenia? J Psychopharmacol. 2010;24(Suppl 4):37–50.
Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45(7):1343–61.
Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med. 2016;46(14):2869–81.
Soundy A, Stubbs B, Probst M, Hemmings L, Vancampfort D. Barriers to and facilitators of physical activity among persons with schizophrenia: a survey of physical therapists. Psychiatr Serv. 2014;65(5):693–6.
Cohen R, Florin I, Grusche A, Meyer-Osterkamp S, Sell H. The introduction of a token economy in a psychiatric ward with extremely withdrawn chronic schizophrenics. Behav Res Ther. 1972;10(1):69–74.
O'Brien F, Azrin NH, Henson K. Increased communications of chronic mental patients by reinforcement and by response priming. J Appl Behav Anal. 1969;2(1):23–9.
Beebe LH, Smith K, Burk R, McIntyre K, Dessieux O, Tavakoli A, et al. Effect of a motivational intervention on exercise behavior in persons with schizophrenia spectrum disorders. Community Ment Health J. 2011;47(6):628–36.
Daumit GL, Dalcin AT, Jerome GJ, Young DR, Charleston J, Crum RM, et al. A behavioral weight loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obes. 2011;35:1114-23.
Ganguli R. Behavioral therapy for weight loss in patients with schizophrenia. J Clin Psychiatry. 2007;68(Suppl 4):19–25.
Methapatara W, Srisurapanont M. Pedometer walking plus motivational interviewing program for Thai schizophrenic patients with obesity or overweight: a 12-week, randomized, controlled trial. Psychiatry Clin Neurosci. 2011;65(4):374–80.
Miller WR, Rollnick S. Motivational interviewing: helping people change. New York: Guilford press; 2012.
Knight KM, McGowan L, Dickens C, Bundy C. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol. 2006;11(2):319–32.
Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29(4):283–93.
Ringen PA, Faerden A, Antonsen B, Falk RS, Mamen A, Rognli EB, et al. Cardiometabolic risk factors, physical activity and psychiatric status in patients in long-term psychiatric inpatient departments. Nordic J Psychiatry. 2018; https://doi.org/10.1080/08039488.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental illness. Fourth edition ed. Washington DC: APA; 1994.
De Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller H-J. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009; 24(6):412–424.
Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag health study (HUNT 2). Eur J Epidemiol. 2007;22(6):379–87.
Nes BM, Janszky I, Aspenes ST, et al. Exercise patterns and peak oxygen uptake in a healthy population: the HUNT study. Med Sci Sports Exerc. 2012;44(10):1881–9.
Kurtze N, Rangul V, Hustvedt B-O, et al. Reliability and validity of self-reported physical activity in the Nord-Trøndelag health study — HUNT 1. Scandinavian Journal of Public Health. 2008;36(1):52–61.
Rosenberg M. Society and the adolescent self-image. Princeton, NJ: Princeton University Press; 1965.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the global assessment of functioning-split version. Compr Psychiatry. 2007;48(1):88–94.
Faerden A, Nesvåg R, Barrett EA, Agartz I, Finset A, Friis S, et al. Assessing apathy: The use of the Apathy Evaluation Scale in first episode psychosis. Eur Psychiatry. 2008; 23(1):33–9.
Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38(2):143–62.
Dauwan M, Begemann MJH, Heringa SM, Sommer IE. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):588–99.
Kilbourne AM, Barbaresso MM, Lai Z, Nord KM, Bramlet M, Goodrich DE, et al. Improving physical health in patients with chronic mental disorders: 12-month results from a randomized controlled collaborative care trial. J Clin Psychiatry. 2017;78(1):129–37.
Speyer H, Brix C, Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry. 2016;15(2):155–65.
Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. 2017;16(3):308–15.
Urhan M, Ergün C, Aksoy M, Ayer A. Effects of weight loss diet therapy on anthropometric measurements and biochemical variables in schizophrenic patients. Nordic J Psychiatry. 2015;69(5):323–30.
Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(9):964–74.
Baker AL, Richmond R, Kay-Lambkin FJ, Filia SL, Castle D, Williams JM, et al. Randomised controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders: outcomes to 36 months. Aust N Z J Psychiatry. 2018;52(3):239–52.
Beazley P, Gudjonsson G. Motivating inpatients to engage with treatment: the role of depression and ward atmosphere. Nordic J Psychiatry. 2011;65(2):95–100.
Tarpada SP, Morris MT. Physical activity diminishes symptomatic decline in chronic schizophrenia: a systematic review. Psychopharmacol Bull. 2017;47(4):41–52.
Acknowledgements
The authors like to thank the participating patients, staff and administration.
Funding
This work was supported by the Research Council of Norway under Grant 223273. The Research Council of Norway had no role in the design of the study or in the collection, analysis, or interpretation of data or in writing the manuscript. The study received funding and resources from the Division of Mental Health and Addiction, Oslo University Hospital, and from the Skjelfoss Psychiatric Center. The study were led and carried out by employees of these institutions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
AF, AM, EBR, EWM, OAA and PAR designed the study. AF, AM, BA, DKS, EBR and PAR participated in the practical implementation of the study. AF, BA, DKS and PAR participated in the data collection. PAR and RSF analyzed and interpreted the patient data. PAR and RSF were major contributors in writing the manuscript, while all authors read, contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
Petter Andreas Ringen, MD, PhD: Chief consultant psychiatrist and researcher at the Oslo University Hospital. Special interest in somatic health and substance use in severe mental illness.
Ragnhild S. Falk, MSc PhD: Researcher and senior statistician at the Oslo Centre for Biostatistics and Epidemiology.
Ann Færden, MD, PhD: Psychiatrist and researcher at the Oslo University Hospital. Expertise in in negative symptoms and reduced motivation in severe mental illness.
Bjørnar Antonsen, M.D., Ph.D: Clinical doctor at the Department of Psychiatry at Lovisenberg Diaconal Hospital. Main focus of research has been long term outcome from psychotherapy for patients with personality disorders.
Asgeir Mamen, PhD: Associate Professor at Kristiania University College. Exercise physiologist with expertise in the use of physical activity in rehabilitation of substance use disorder subjects.
Eline B. Rognli, PhD: Clinical psychologist and research advisor at the Oslo University Hospital. Her area of research is substance use and psychosis. Expertise in Motivational Interviewing.
Dag K. Solberg, MD: Specialist in psychiatry and clinical pharmacology. Head of department, Center for Psychopharmacology, Diakonhjemmet Hospital, Oslo, and senior psychiatrist at Skjelfoss Psychiatric Center.
Egil W. Martinsen, MD, PhD: Professor in psychiatry, University of Oslo. Expertise in the relation between physical activity and mental health in psychiatric populations.
Ole A. Andreassen, MD, PhD: Professor in Psychiatry, University of Oslo and attending psychiatrist Oslo University Hospital. Expertise in somatic comorbidity in mental illness.
Ethics approval and consent to participate
All participants signed informed consent to participation. The study was approved by the Regional Ethics committee in South-Eastern Norway, reference number 2012/2266.
Competing interests
Prof. Ole A. Andreassen received speakers’ honoraria from Lundbeck. The other authors report no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Daily entry form intervention period. (DOCX 15 kb)
Additional file 2:
Detailed description of the 4X4 high intensity (HIT) training and the “Hill Run”. (DOCX 17 kb)
Additional file 3:
Figure S1. Recordings of MI (n) per week after intervention start at each ward. Legend: x-axis: weeks counting from start of intervention. (DOCX 107 kb)
Additional file 4:
Figure S2. Mean MI interventions per participant per week after intervention start at each ward. Legend: x-axis: weeks counting from start of intervention. (DOCX 101 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ringen, P.A., Falk, R.S., Antonsen, B. et al. Using motivational techniques to reduce cardiometabolic risk factors in long term psychiatric inpatients: a naturalistic interventional study. BMC Psychiatry 18, 255 (2018). https://doi.org/10.1186/s12888-018-1832-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-018-1832-6