Abstract
Background
To explore the prevalence, outcome and perinatal risks of neonatal hypoxemic respiratory failure (NRF) in a survey of all livebirths from a regional network of perinatal-neonatal care during the transition period after 5-year universal health insurance implemented in China.
Methods
Clinical data of all neonatal respiratory morbidities in Huai’an were retrospectively collected in the regional perinatal network database of all livebirths as vital statistics in 2015. NRF was defined as hypoxemia requiring continuous positive airway pressure (CPAP) and/or mechanical ventilation (MV) for at least 24 h. Mortality risks of antenatal and perinatal morbidities, major respiratory therapies and complications were analyzed by multivariable logistic regression model.
Results
There were 788 NRF cases identified in 9.9% (7960) hospitalized, or 13.3‰ (59056) livebirths, in which 6.7% received intensive care and 93.0% critical care. The major underlying morbidities were respiratory distress syndrome (RDS, 36.4%) and pneumonia/sepsis (35.3%), treated mainly by CPAP, MV and surfactant. Significantly improved outcomes by surfactant in RDS were in patients with birthweight (BW) < 1500 g or gestational age (GA) < 32 weeks. The overall mortality rate in NRF was 18.4% whereas for those of BW < 1000 g and GA < 28 weeks, 70% and 54%, respectively. The multivariable regression analysis showed the highest odds for NRF death among meconium aspiration syndrome, congenital anomalies, BW < 1500 g and necrotizing enterocolitis, whereas born in level III hospitals, cesarean delivery, CPAP and MV were associated with markedly reduced death odds.
Conclusions
The salient findings with associated risk estimates reflected efficiency of respiratory support as critical care in a prefectural regional network infrastructure for annual livebirths in 5.6 million inhabitants. It implicated the representativeness of contemporaneous perinatal-neonatal care standard at medium to medium-high level, in one/fourth of the population of China, aiming at saving more life of very critical and preterm infants for better survival.
Similar content being viewed by others
Introduction
In the past decade, the development of maternal-infant healthcare and modern perinatal-neonatal medicine has been in transition with universal health insurance implemented in China [1]. Almost all counties (about 2800 in number) have been establishing maternity and child healthcare hospitals/centers (more than 3000 in total), each serving for inhabitants from < 1/4 to > 1 million [2, 3]. Five to 15 of these counties consist of a sub-provincial metropolitan city, or prefectural region, and with a population from 1 to 2 million to 10 million. In nation-wide more than 300 such cities, there are > 500 level III, leading perinatal-neonatal care centers covering these cities/regions [3, 4].
Huai’an is such a city/region with 5.6 million inhabitants in 2015. Its gross domestic production (GDP, 44 billion USD equivalent, 1 USD =6.2 CNY), GDP per capita (9000 USD), and average disposable income (3300 USD) ranked 65th–70th, as medium or medium-high in ascending order, in all regional cities of the country [4, 5]. Its annual births were 50,000–60,000 in 2010–2019 [5]. We conducted a series of surveys to estimate incidences and risks of major outcomes in perinatal-neonatal care through Huai’an perinatal information system and perinatal-neonatal network. We have reported the rates of birth, livebirth, stillbirth, preterm birth, perinatal and neonatal mortality, including deaths at delivery, in hospital, and due to prematurity in Huai’an as vital statistic data in 2010 and 2015 [6, 7]. The database generated from these prospective surveys enabled integration of maternal obstetric information and in-hospital neonatal management and outcome, relevant for studies of various purposes [6,7,8,9,10,11,12]. For neonatal intensive and critical care, its availability and affordability constituted a foundation in view of equity, efficiency and equipoise from regional healthcare policy, facility, along with socioeconomic and sociocultural development. In this regard, outcome of neonatal patients requiring critical care, especially those of hypoxemic respiratory failure (NRF), tends to be a good focus [1].
The standard of neonatal critical care involves resuscitation at birth, transportation, prompt admission, life sign monitoring, catheterization, and respiratory support with advanced ventilation strategies and medications [1, 13,14,15]. During the transition period of implementation of universal health insurance, these therapeutic modalities were adopted to a variable extent in different regions of China, depending on the local economy, health policy, facilities which included number and competency of staffs [1, 2, 13,14,15,16]. In our previous studies, a series of prospective or retrospective surveys of NRF were carried out through nation-wide, provincial or trans-provincial networks of neonatal intensive care units (NICUs) as collaborative study groups [16,17,18,19,20,21,22]. It enabled geographical and longitudinal assessment of efficiency in respiratory support, ventilation mode, surfactant, inhaled nitric oxide in neonates of different lung development and pathologies. These facilities were mostly in tertiary hospitals in metropolitans or municipalities and the results may not be extrapolated for the regional care standard, as information in those treated outside of the central NICUs was not accessible.
For this issue, on the basis of the whole regional birth population survey in Huai’an in 2015 [7], we retrospectively conducted the first comprehensive clinical epidemiological study in the regional livebirth and in-hospital population with the same definition and protocol of NRF as in previous studies [16,17,18,19, 21,22,23]. Our purpose was to delineate efficiency of respiratory support as mainstay of critical care for NRF in the whole regional network of NICUs associated with local socioeconomic status (SES) as background [6,7,8,9,10,11,12], which coincided with the initial implementation of universal health insurance and adaptation within ensuing years [1]. We postulated that the results should facilitate estimation of incidence of major respiratory diseases, case fatality rate (CFR) and mortality rate by livebirths. By characterizing regional perinatal-neonatal care specific development, it may be further translated into nation-wide burden of neonatal respiratory morbidity and mortality, and efficiency of neonatal critical care in the era of universal health insurance as integrated.
Methods
Study design and data collection
The study design was on the assumption that the 2015 birth datafile provided comprehensive information regarding fetal deaths/stillbirths, livebirths, preterm births, in-hospital patients as well as maternal and perinatal morbidities, and overall perinatal and neonatal survival data [7, 9,10,11,12]. NRF cases were identified from all the in-hospital datafile of regional level II and III hospitals, through the collaborative network and research program based on previous experience and protocols [16, 17, 19, 21,22,23]. The definition of NRF was clinically and blood gas confirmed hypoxemia requiring continuous positive airway pressure (CPAP) and/or intratracheal mechanical ventilation (MV) for at least 24 h, or withdrawal of treatment/deaths within 24 h [16, 17, 19, 21,22,23]. Retrospective data collection and analysis were conducted in 2018–2021, and for transferred cases, the data were regarded as single hospitalization. Huai’an Maternal and Child Health Care Center (HMCHCC), as the major referral center in the region, acted as coordination center for this study. The ethics committee of HMCHCC and Children’s Hospital of Fudan University approved the study protocols, and the informed consent from parents/guardians was waived as no specific intervention was applied [9,10,11,12].
Data of maternal and perinatal morbidities, as well as neonatal outcomes were included, with the diagnostic definitions consistent with previous studies [6,7,8,9,10,11,12]. The major underlying diseases of NRF were defined as the primary cause and categorized as reported [12, 16, 19, 21, 22]. Briefly, underlying diseases were categorized as respiratory distress syndrome (RDS), meconium aspiration syndrome (MAS), temporary respiratory insufficiency of the newborn (TRIN) [24], pneumonia/sepsis, congenital anomalies (CA) and intraventricular hemorrhage (IVH, grades III and IV), with definitions in Additional file 1. The subsequent pathophysiological conditions developed from those underlying diseases were considered as complications, such as hospital acquired pneumonia/sepsis (ventilation-associated pneumonia, catheter-related sepsis and other healthcare-related severe infection after 48 h of hospitalization), persistent pulmonary hypertension of the newborn (PPHN), pulmonary hemorrhage, bronchopulmonary dysplasia (BPD, moderate to severe), neurological impairment (hypoxic ischemic encephalopathy, bilirubin encephalopathy, periventricular leukomalacia, hypoglycemic brain damage), patent ductus arteriosus (of hemodynamical significance), necrotizing enterocolitis (NEC, Bell’s stage II and III), retinopathy of prematurity (ROP, stage 3 and above) [10, 12, 25]. Information of respiratory support (CPAP, MV), surfactant, postnatal steroids, length of ventilation (LOV) including both CPAP and MV, length of hospital stay (LOHS), costs and survival at discharge, was also included. Score for neonatal acute physiology perinatal extension (SNAPPE) II was used for assessment of initial illness severity, and retrospectively determined based on routine items and score scales in the first 12 h after admission to the NICU based on the original in-hospital clinical records [26]. According to the severity of diseases and the treatment strength, care level in NICU was classified into intensive or critical care (Additional file 1) [27].
For the description of the clinical characteristics of NRF patients by maturity status, the gestational age (GA) was stratified into: GA, < 28 weeks (extreme preterm), 28–31 weeks (very preterm), 32–33 weeks (moderate preterm), 34–36 weeks (late preterm), 37–38 weeks (early term), 39–41 weeks (full term, as reference), ≥42 weeks (post-term); and birthweight (BW): < 1000 g (extremely low BW), 1000–1499 g (very low BW), 1500–2499 g (low BW), 2500–3999 g (normal BW, as reference), ≥4000 g. GA < 37 weeks and BW < 2500 g were deemed as preterm and low BW (LBW), respectively.
Statistical analysis
The statistical analysis was performed by software SPSS 23.0 (IBM, Chicago, IL). Continuous variables were presented as mean ± standard deviation (SD) or median [interquartile range (IQR)] or range. Categorical variables by GA or BW strata were presented as number and percentage (%) with comparisons by Chi-square test or Fisher’s exact test. Numbers needed to treat (NNT) was estimated by reciprocal (reverse ratio) of attributable risk difference of the survival rates for surfactant treatment. Mann-Whitney U test was applied for comparisons of continuous variables. Spearman rank correlation was used between SNAPPE-II in category of every 10-point increment and mortality rate, while Pearson correlation test was used for continuous variables. The prevalence or mortality rates and associated 95% confidence interval (CI) were estimated by a generalized linear Poisson regression model with empirical, robust standard errors but no explanatory variables [28, 29]. Factors associated with NRF deaths were identified through univariable logistic regression, and those with probability (P) < 0.10 were further analyzed by multivariable models with backward stepwise selection. The crude and adjusted odds ratio (OR) and 95%CI of the identified variables, as well as the Hosmer-Lemeshow test for goodness of fit, were estimated. P < 0.05 was considered statistically significant.
Results
There were 788 NRF cases, with corresponding prevalence of 13.3 and 95%CI of 12.4–14.3 per 1000 livebirth (n = 59,056), accounting for 9.9% total hospitalizations (n = 7960). As Table 1 lists, compared to non-NRF, NRF patients had lower median GA and BW, and were more prone to higher prevalence of maternal morbidities, more cesarean delivery, multiple births, preterm births, LBW, male, Apgar score at 5 min < 7 and delivery resuscitation. They also tended to have higher perinatal comorbidities, such as asphyxia, RDS, congenital pneumonia, early onset sepsis and CA. Moreover, 70.7% of NRF were born at, and 98.5% were admitted to, level III hospitals, and 99.7% in NICU, all higher than those of non-NRF patients (P < 0.001). Admission within 7 postnatal days (PND) accounted for 96.2% of total NRF, while 88.5% on PND 1. NRF required longer hospital stay days and higher costs compared to non-NRF. The overall CFR of NRF was 18.4% (n = 145), with the corresponding livebirth population-based mortality rate of 2.5‰ (95%CI 2.1‰, 2.9‰). For deaths in NRF, contributing to 86.3% of total in-hospital deaths (n = 168), 65.6% were within 7 PND, 93.1% within 28 PND, and 55.9% as withhold/withdrawal from critical care.
Morbidity and mortality
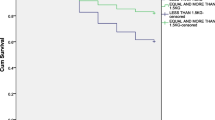
The main underlying morbidities of NRF were RDS (36.4%), pneumonia/sepsis (35.3%), TRIN (13.6%), CA (10.2%), MAS (1.6%) and IVH (2.9%), with corresponding CFR of each morbidity in NRF patients as 21.6%, 10.1%, 10.3%, 45.0%, 46.2% and 4.3%, respectively. In those extremely (BW < 1000 g or GA < 28 weeks) and very (BW < 1500 g or GA < 32 weeks) premature patients, RDS was diagnosed in approximately 100% and 70% with CFR of 50–70% and 40%, which contributed to 100% and 85% of NRF deaths, respectively (Table 2, Table S1, Fig. 1). The main complications were acquired pneumonia/sepsis (21.4%), neurological impairment (19.9%), while the occurrence of air leak, BPD, pulmonary hemorrhage, PPHN, NEC and ROP ranged from 4% to 10%.
As Table 2 and Table S1 list, the overall mortality rates of NRF were 70% or 53.8% in those of BW < 1000 g or GA < 28 weeks, while 30.1% or 20.7% of BW 1000–1499 g or GA 28–31 weeks, respectively. The median SNAPPE-II scores were highest in those with BW < 1000 g or GA 25–27 weeks. As Fig. 2 shows, SNAPPE-II was strongly correlated with mortality rate of NRF (r = 0.895, P < 0.001).
Respiratory support and surfactant use
The prevalence rates of CPAP, MV, surfactant and postnatal steroids use were 80.8%, 44.2%, 27.8% and 15.7%, respectively. In very premature patients, the utility rates of CPAP, MV and surfactant were nearly 90%, 50% and 60%, respectively (Table 2 and Table S1). The median values of LOV, LOHS, and costs of stay in the NRF survivors were 1.8, 6.3 and 3.1 times, respectively, of those deceased. The correlation coefficients of LOV and LOHS, LOV and costs, as well as LOHS and costs were 0.599, 0.623 and 0.754, respectively (all P < 0.001). The costs of rescuing a NRF patient were about 20,000 CNY, equivalent to a local adult’s annual disposable income. The financial burden was much higher in those of extremely or very preterm/LBW, about 80,000 CNY or 50,000 CNY, respectively. Table 3 depicts the overall efficiency of surfactant in RDS-related NRF patients. Surfactant improved survival rate mainly in the very and extremely preterm/LBW patients.
Perinatal-neonatal risks for death
The mortality odds evaluated by univariable logistic regression models (Table 4 and Table S2) showed that BW < 1000 g, pulmonary hemorrhage and PPHN were strongly (ORs > 6), while GA < 28 weeks, Apgar 5 min < 7, MAS, CA, NEC and MV were moderately (ORs 3–5), associated with mortality. In the multivariable models, BW < 1500 g, higher SNAPPE-II, MAS, CA, PPHN, NEC remained odds for death in NRF, with MAS and CA the highest (ORs > 30), followed by BW < 1500 g and NEC (ORs 7–8). The increment of SNAPPE-II (by every 10 point) was associated with a steady increase in mortality (OR = 2.29, P < 0.001). In contrast, BW ≥4000 g, born in level III hospitals, cesarean delivery, CPAP and MV were associated with the reduction of death odds.
Discussion
The results of prevalence and outcome of NRF in Huai’an in 2015 depicted the efficiency of respiratory care of the regional tertiary centers and associated facilities in the context of perinatal-neonatal care paradigm for pregnant morbidities with high risk of exposure to their offspring. The survival of critically ill newborns, especially very and extremely low GA and BW infants remained a big challenge, despite surfactant and non-invasive ventilation showing benefits in patients with RDS. By logistic regression analysis, the improved survival of NRF was associated with born in the tertiary hospital, cesarean delivery and respiratory support. It should reflect an integrated strategy for the prenatal, peripartum and early postnatal care. RDS, MAS and CA became the leading odds for increased NRF mortality, which indicates a shift of clinical priorities for reducing high death risk population under local perinatal-neonatal network system in transition. The study design, protocol and definition of NRF were similar to the previous multicenter studies in nation-wide, provincial or trans-provincial perspectives (mainly based on the tertiary NICU admission), which facilitated temporal and spatial comparisons regarding the overall and specific care efficiency.
Based on the current study, we have shown a prevalence of 13.3‰ and mortality of 2.5‰ of NRF in the whole regional livebirths. Although the data were close to NRF in United States and Italy with prevalence of 18–22‰, and mortality of 2.0–3.2‰, in the mid-1990s, the CFR (18.4%) in our study was still higher than in those countries (11.1–14.6%), denoting development of the care system still lagged at least two decades behind [30, 31]. The high proportion of NRF deaths due to withhold/withdrawal from critical care compromised the overall outcome. As there was a trend of reduced stillbirths and increased livebirths from the studied population [6, 7], we anticipate that the proportion of the very preterm infants may continue to grow in the subsequent years. Therefore, the survival rate and quality in early infancy for the very and extremely preterm infants, should be the focus in future investigation [32,33,34]. It was corroborated that respiratory support played a key role in the neonatal critical care for better survival as seen in the early 1990s [33, 35, 36].
Compared to those livebirths with very preterm or very LBW, the BW in reference range still accounted for 30–40% NRF in proportion, and contributed to nearly 50% of total deaths, despite that the prevalence of NRF in term livebirths was significantly low (< 1%). For those term infants, the perinatal morbidities underlying NRF were associated with substantial proportion of congenital pneumonia and early onset sepsis as at-risk population from all the hospitalized. However, their occurrence as primary diagnosis in those NRF of very and extremely low GA or BW was low or none (Table 2, Table S1). As there underwent expansion for both facility and technique implementation, the diagnostic criteria and definition of common neonatal diseases at level II and III hospitals may not be consistent. There was also a concern that the overdiagnosis and associated overuse of antibiotics of potential infection at birth when information from maternal aspect was missing or not linked efficiently, especially encountered when NICU admissions were through inter-hospital transportation. For the major complications of NRF, acquired pneumonia/sepsis was complicated in 50–70% of extremely, and 30–40% very preterm/LBW NRF patients (Table 2, Table S1), whereas postnatal steroids were applied to < 15% of them. As there was a concern of postnatal steroids related high risk of late onset sepsis [37], the use of steroids seemed restricted in the critical care of the region.
The other major morbidities in term patients were MAS and CA, with CFR around 50% and adjusted ORs as high as 30 by multivariable logistic regression analysis. The declining trend of prevalence of MAS compared to previous NRF studies [16, 17, 19, 22, 23] indicated the progress in prevention of fetal distress and delivery resuscitation for severe asphyxia-associated meconium aspiration and prompt caesarean delivery for post-term or macrosomia. However, the highest CFR still highlighted the limitation of local neonatal critical care in management of PPHN, severe sepsis, or CA with cardiopulmonary failure.
By comparing the previous NRF studies (2004–2012) [16, 17, 19, 22, 23] (Table 5), current study showed a declined overall CFR, from 32.1% in 2004 to 18.4% in 2015 (current study), with more applications of surfactant (from 16.6% to 27.8%) and CPAP (from 52.6% to 80.8%), and higher proportion of female, very and extremely preterm/LBW infants. The total benefit of surfactant treatment in RDS estimated by the net difference in survival increment was similar to the previous ones (with overall NNT around 10). However, the benefit turned to be more prominent in those of BW < 1500 g (NNT 3.0). As for the primary underlying diseases of NRF, RDS remained the first leading cause (around 35–60%) and pneumonia/sepsis the second (20–35%), while the proportion of MAS declined (from 9.5% in 2004 to 1.6% in 2015) and CA increased (from 3.0% in 2004 to 10.2% in 2015). The occurrence of acquired infection and neurological impairment increased, as well as for BPD and NEC as preterm-associated complications.
The region of Huai’an had been undergoing transition of the maternal-infant healthcare since 2010 with improvement in both care facility and the universal health insurance [6,7,8,9,10,11,12, 23], which was characterized by availability and affordability of advanced facility and medication, coverage of all critically ill newborn infants from birth with parental engagement including decision-making. These changes in association with altered benefit and risk factors impacted on the outcome of NRF, represented to a great extent the quality of practice of perinatal and neonatal care at medium to medium-high level of domestic regions [4, 5, 7, 9,10,11,12]. We therefore, speculate that some of the major findings may occur in subsequent years (2016–2025), in a great number of evolving regions with similar transition in SES and perinatal care. From geographic and socioeconomic point of view, we postulate that the overall results may be representative of, and relevant for, up to 20–25% of the population in the country, amounted to 250–350 million of domestic population [4]. Moreover, by assuming the perinatal-neonatal care of Huai’an in 2015 to be a paradigm of respiratory management, we attempt to estimate the prevalence of major morbidities and burden from all hospitalized patients and total livebirths. From an incidence of NRF in 13‰, 5‰ RDS, 10‰ requiring CPAP, 6‰ MV, and 4‰ surfactant, we deduce these figures corresponding to 195,000, 75,000, 150,000, 90,000 and 60,000, respectively, or more, in the contemporaneous whole country livebirths (15 million) [4]. Nevertheless, more studies are needed to validate the relevance for the > 300 nation-wide sub-provincial cities/regions with variable developmental stages in both maternal-infant healthcare and SES. This study indeed offered fundamental concept, methodology and datafile as strength.
The limitation, regarding the data reliability, may include under- or over-diagnosis and treatment, overt and occult, of major and minor respiratory morbidities. In the study design and data process, we managed to scrutinize clinical records in the retrospective data collection. Although we adjusted diagnosis according to the study protocol to balance the authenticity of original data with the diagnostic criteria and definitions, variations of the quality of practice among different facilities still existed. It revealed a real-world practice with respiratory support taking most of the major perinatal and neonatal morbidity and mortality into account. Even so, some risk factors were not controlled well from the data analysis due to sample size with stratification in the multivariable logistic regression models. Interpretation of the results should be cautious. Next, the mortality of NRF still had substantially high proportion of withhold/withdrawal of critical care though the health insurance was implemented. There was no socioeconomic and sociopsychological element engaged in the risk estimation. Further studies should include these factors into estimation for exposure risks and outcome in the perinatal-neonatal care in transition.
Conclusion
The salient findings revealed efficiency of neonatal critical care in the management of NRF by associated risk estimates delineating respiratory support and surfactant use among all the hospitalized patients from regional livebirths. It should enable a comprehension of current neonatal critical care status at sub-provincial region, and may be extrapolated to a large proportion of domestic population taking SES and transitional perinatal-neonatal care into account. It requires further studies to validate from different regions for those requiring critical care with adequate respiratory support and management, especially very and extremely preterm births, for better survival.
Availability of data and materials
The datasets generated during and analyzed during the current study are not publicly available due to other concurrent studies based on the datasets but are available from the corresponding author on reasonable request.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- BW:
-
Birthweight
- CA:
-
Congenital anomalies
- CFR:
-
Case fatality rate
- CI:
-
Confidence interval
- CNY:
-
Chinese Yuan
- CPAP:
-
Continuous positive airway pressure
- GDP:
-
Gross domestic production
- HFOV:
-
High frequency oscillatory ventilation
- HMCHCC:
-
Huai’an Maternal and Child Health Care Center
- IQR:
-
Interquartile range
- IVH:
-
Intraventricular hemorrhage
- LBW:
-
Low birthweight
- LOHS:
-
Length of hospital stay
- LOV:
-
Length of ventilation
- MAS:
-
Meconium aspiration syndrome
- MV:
-
Mechanical ventilation
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- NNT:
-
Numbers needed to treat
- NRF:
-
Neonatal hypoxemic respiratory failure
- OR:
-
Odds ratio
- PND:
-
Postnatal day
- PPHN:
-
Persistent pulmonary hypotension of the newborn
- ROP:
-
Retinopathy of prematurity
- SD:
-
Standard deviation
- SNAPPE-II:
-
Score for neonatal acute physiology perinatal extension II
- TRIN:
-
Temporary respiratory insufficiency of the newborn
References
Sun B, Shao X, Cao Y, Xia S, Yue H. Neonatal-perinatal medicine in a transitional period of China. Arch Dis Child Fetal Neonat Ed. 2013;98:440–4.
Wang Y, Li X, Zhou M, Luo S, Liang J, Liddell CA, et al. Under-5 mortality in 2851 Chinese counties, 1996-2012: a subnational assessment of achieving MDG 4 goals in China. Lancet. 2016;387:273–83.
Statistical communique on the development of national health in China. 2020. Available from: http://www.gov.cn/guoqing/2021-07/22/content_5626526.htm. Accessed 20 Aug 2021. (in Chinese).
National Bureau of Statistics of China. Available from: http://www.stats.gov.cn/. Accessed 20 August 2021. (in Chinese).
Statistical yearbook by Huai’an government. Available from: http://www.huaian.gov.cn/. Accessed 20 Aug 2021. (in Chinese).
Sun L, Yue H, Sun B, Han L, Qi M, Tian Z, et al. Estimation of birth population-based perinatal-neonatal mortality and preterm rate in China from a regional survey in 2010. J Matern Fetal Neonatal Med. 2013;26:1641–8.
Wang H, Yue H, Sun B, Zhu X, Niu H, Qi T, et al. Birth population survey in Huai'an in 2015: perinatal-neonatal mortality and preterm birth rate in emerging regions in China. J Matern Fetal Neonatal Med. 2020;33:838–46.
Sun L, Yue H, Sun B, Han L, Tian Z, Qi M, et al. Estimation of high risk pregnancy contributing to perinatal morbidity and mortality from a birth population-based regional survey in 2010 in China. BMC Pregnancy Childbirth. 2014;14:338.
Zhu X, Niu H, Wang H, Li X, Qi T, Ding W, et al. High risk pregnancy associated perinatal morbidity and mortality: a second birth population-based survey in Huai'an in 2015. BMC Pregnancy Childbirth. 2019;19(1):224.
Guo X, Li X, Qi T, Pan Z, Zhu X, Wang H, et al. A birth population-based survey of preterm morbidity and mortality by gestational age. BMC Pregnancy Childbirth. 2021;21(1):291.
Xu Y, Guo X, Pan Z, Zheng G, Li X, Qi T, et al. Perinatal risks of neonatal and infant mortalities in a sub-provincial region of China: a livebirth population-based cohort study. BMC Pregnancy Childbirth. 2022;22(1):338.
Xu Y, Zhu X, Wang H, Pan Z, Li X, Guo X, et al. Prevalence of major morbidities and outcome of all hospitalized neonates. A retrospective cohort study of Huai’an neonatal survivals. J Matern Fetal Neonatal Med. 2022; [Online ahead of print].
Li Q, Han T, Zhang Y, Zhang Q, Kong X, Yang Y, et al. A nationwide survey on neonatal medical resources in mainland China: current status and future challenges. BMC Pediatr. 2019;19(1):436.
Wang H, Dong Y, Sun B, Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Admission volume is associated with mortality of neonatal respiratory failure in emerging neonatal intensive care units. J Matern Fetal Neonatal Med. 2019;32(13):2233–40.
Sun B, Ma L, Liu X, Gao X, Ni L. Development of neonatal respiratory and intensive care: Chinese perspectives. Neonatology. 2012;101(2):77–82.
Ma L, Liu C, Wang Y, Li S, Zhai S, Gu X, et al. Mortality of neonatal respiratory failure related to socioeconomic factors in Hebei province of China. Neonatology. 2011;100(1):14–22.
Qian L, Liu C, Zhuang W, Guo Y, Yu J, Chen H, et al. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics. 2008;121(5):e1115–24.
Wang YF, Liu CQ, Gao XR, Yang CY, Shan RB, Zhuang DY, et al. Effects of inhaled nitric oxide in neonatal hypoxemic respiratory failure from a multicenter controlled trial. Chin Med J. 2011;124:1156–63.
Wang H, Gao X, Liu C, Yan C, Lin X, Yang C, et al. Morbidity and mortality of neonatal respiratory failure in China: surfactant treatment in very immature infants. Pediatrics. 2012;129:e731–40.
Jiang Q, Gao X, Liu C, Chen C, Lin X, Xia S, et al. Early inhaled nitric oxide in preterm infants <34 weeks with evolving bronchopulmonary dysplasia. J Perinatol. 2016;36:883–9.
Wang H, Gao X, Liu C, Yan C, Lin X, Dong Y, et al. Surfactant reduced the mortality of neonates with birth weight ≥1500 g and hypoxemic respiratory failure: a survey from an emerging NICU network. J Perinatol. 2017;37:645–51.
Zhang L, Qiu Y, Yi B, Ni L, Zhang L, Taxi P, et al. Mortality of neonatal respiratory failure from Chinese northwest NICU network. J Matern Fetal Neonatal Med. 2017;30(17):2105–11.
Pan ZJ, Ding SF, Gao ZB, Zhao YX, Han LR, Yue HN. A population-based epidemiological survey of neonatal respiratory failure in Huai’an City of Jiangsu Province, in 2010. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(11):1138–42 (in Chinese).
Agrawal V, David RJ, Harris VJ. Classification of acute respiratory disorders of all newborns in a tertiary care center. J Natl Med Assoc. 2003;95(7):585–95.
Martin RJ, Fanaroff AA, Walsh MC. Neonatal-perinatal medicine. Disease of the fetus and infant. 10th ed. Philadelphia: Elsevier; 2015.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–10.
Smithhart W, Wyckoff MH, Kapadia V, Jaleel M, Kakkilaya V, Brown LS, et al. Delivery room continuous positive airway pressure and pneumothorax. Pediatrics. 2019;144(3):e20190756.
Best KE, Rankin J, Dolk H, Loane M, Haeusler M, Nelen V, et al. Multilevel analyses of related public health indicators: the European Surveillance of Congenital Anomalies (EUROCAT) public health indicators. Paediatr Perinat Epidemiol. 2020;34(2):122–9.
Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6.
Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med. 2001;164(7):1154–60.
Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. A multicenter study on incidence and fatality rates of neonatal acute respiratory disorders according to gestational age, maternal age, pregnancy complications and type of delivery. Italian Group of Neonatal Pneumology. Biol Neonate. 1998;74(1):7–15.
Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–40.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–51.
Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321(12):1188–99.
Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345:e7976.
Larroque B, Bréart G, Kaminski M, Dehan M, André M, Burguet A, et al. Survival of very preterm infants: Epipage, a population based cohort study. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F139–44.
Stoll BJ, Temprosa M, Tyson JE, Papile L-A, Wright LL, Bauer CR, et al. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics. 1999;104(5):e63.
Acknowledgments
The authors thank the cooperation from the Huai’an perinatal-neonatal study group members; and contribution of Drs X. Guo, S. Luo and M. Chen in data collection is highly appreciated.
Huai’an Perinatal-Neonatal Study Group
Members:
Sufang Ding1; Zhaojun Pan1; Guofang Zheng1; Hui Wang3; Hongni Yue1,3; Xiaoqin Zhu3; Weijie Ding3; Xiaoqiong Li4; Tingting Qi4; Muling Zhang5; Zhaofang Tian5; Honghua Guan6; Juan Yang6; Yongjian Wu7; Tao Xu8; Chunhong Tang9; Maotian Dong9; Chunhua Zhang10; Chunqin Dong11; Sumei Zhou12; Yani Lei13; Shouzhong Li14; Keyan Zhu14; Xia Zhao15; Yaodong Yin16; Haijun Wang16; Bi Xue17; Zhaoxia Wang18; Shucheng Wang19; Hong Liu20; Zhou Xu20; Chuntao Yuan21; Xihui Cao22; Jianya Zhang22; Bu Xu23; Wenlong Lin23; Cui Gao24; Yongbo Heng24; Lei Wang25; Moqing Wang25.
Affiliations:
4Department of Obstetrics, Huai’an Maternal and Child Health Care Center, Huai’an, Jiangsu, China; 5Huai’an First People’s (General) Hospital, Huai’an, Jiangsu, China; 6Huai’an Second People’s (General) Hospital, Huai’an, Jiangsu, China; 7Huai’an Traditional Chinese Medicine Hospital, Huai’an, Jiangsu, China; 8PLA 82nd General Hospital (uniformed service), Huai’an, Jiangsu, China; 9Huai’an District Hospital, Huai’an, Jiangsu, China; 10Huai’an District Maternity Hospital, Huai’an, Jiangsu, China; 11Huai’an District Xinqu Hospital, Huai’an, Jiangsu, China; 12Huai’an City Xiehe Hospital, Huai’an, Jiangsu, China; 13The First Division of Huaian First People’s (General) Hospital, Huai’an, Jiangsu, China; 14Huaiyin District Hospital, Huai’an, Jiangsu, China; 15Huaiyin District Maternity Hospital, Huai’an, Jiangsu, China; 16Lianshui County People’s (General) Hospital, Huai’an, Jiangsu, China; 17Lianshui Second People’s (General) Hospital, Huai’an, Jiangsu, China; 18Lianshui County Third People’s (General) Hospital, Huai’an, Jiangsu, China; 19Lianshui County Traditional Chinese Medicine Hospital, Huai’an, Jiangsu, China; 20Hongze County Traditional Chinese Medicine Hospital, Huai’an, Jiangsu, China; 21Hongze County Maternity Hospital, Huai’an, Jiangsu, China; 22Xuyi County People’s (General) Hospital, Huai’an, Jiangsu, China; 23Xuyi County Traditional Chinese Medicine Hospital, Huai’an, Jiangsu, China; 24Jinhu County People’s (General) Hospital, Huai’an, Jiangsu, China; 25Jinhu County Traditional Chinese Medicine Hospital, Huai’an, Jiangsu, China.
Accordance
All methods were confirmed to perform in accordance with the relevant guidelines and regulations.
Funding
Supported by a grant from the Project of Maternal and Child Health Care by Jiangsu Provincial Commission of Health (F201402 [Yue H]).
Author information
Authors and Affiliations
Consortia
Contributions
DSF and XYL collected the data, proposed the first draft, conducted data analysis, interpretation and wrote the manuscript. WH engaged in data collection and analysis. YHN supervised and validated clinical data collection and analysis, reviewed manuscript, and funded this study. SB and PZJ conceptualized and designed the study, supervised data analysis, and critically revised manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and informed consent to participate
The ethics committee of Children’s Hospital of Fudan University approved the study design and protocol, and waived the need for informed consent (#2019–194). This approval was adopted and approved by Huai’an Maternal and Child Health Care Center and all participated hospitals, with the permissions and the names of the ethics committees and scientific committees of the major participating hospitals referring to reference [9].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The definitions of primary diagnosis as underlying disease of neonatal respiratory failure, and the criteria of care level of neonatal intensive care unit.
Additional file 2: Table S1.
Primary diagnosis, intervention and outcome of NRF in GA strata.
Additional file 3: Table S2.
The univariable logistic regression of other perinatal factors not included in the multivariable regression model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, S., Xu, Y., Wang, H. et al. Outcome of neonatal hypoxemic respiratory failure: a livebirth population-based retrospective survey. BMC Pediatr 22, 552 (2022). https://doi.org/10.1186/s12887-022-03603-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03603-9